Abstract
Objective:
Fresh osteochondral allograft transplantation (OCA) is an increasingly available option for patients with damage to the bone-cartilage complex of the distal femur. This study prospectively assesses osseous integration and early clinical results following fresh OCA with single or multiple cylindrical grafts to the femoral condyle.
Design:
Patients with grade 4 International Cartilage Repair Society (ICRS) defects of the distal femur were treated with OCA. Outcome measures were collected preoperatively and postoperatively at 6, 12, and 24 months. Computed tomography (CT) scans obtained at 6 months were used to assess degree of osseous incorporation regionally.
Results:
Thirty-four patients, with a mean age of 34.5 years (range, 15-61), with a mean femoral osteochondral lesion of 5.7 cm2 (range, 1.5-15.0) due to focal osteoarthritis, osteochondritis dissecans, and avascular necrosis, are reported. Statistically significant (P < 0.05) mean improvement in outcome scores at 2 years included Knee Injury and Osteoarthritis Outcomes Score (KOOS) pain, sports and recreation, quality of life, and International Knee Documentation Committee (IKDC). CT imaging indicated grafts implanted to direct weightbearing regions had >75% incorporation (20/26 grafts) compared to <50% incorporation in the indirect weightbearing regions (8/14 grafts). A greater degree of incorporation and earlier outcome improvement were found after single (n = 23) compared to multiple (n = 11) grafts.
Conclusion:
CT scans were used to assess osseous incorporation of fresh osteochondral allografts in a cohort that showed significant improvements after 2 years. Single-graft implantation is associated with stable incorporation of a greater percentage of the graft. Lesser incorporation appears more frequently with grafts in posterior indirect weightbearing regions of the condyle and multiple contiguous grafts.
Keywords: allograft, articular cartilage, bone, knee
Introduction
Osteochondral allograft transplantation (OCA) is an increasingly available option for treating symptomatic osteochondral lesions.1-3 Application of this technique, originally used principally as an oncological salvage procedure,4 has evolved to include osteochondral defects resulting from a variety of pathologies including osteochondritis, traumatic articular fractures with substance loss, avascular necrosis, and focal cystic osteoarthritis.5,6 The spectrum of techniques designed to apply these grafts ranges from entire condyle replacement for large osteochondral destruction to smaller cylindrical grafts for contained or focally stable defects.7-14 Traditionally, regardless of application technique and size of defect, rehabilitation after OCA has focused on an extended period of limited weightbearing or nonweightbearing on the affected joint.10,15-19 Although several recent studies suggest earlier weightbearing and accelerated rehabilitation may enhance clinical outcomes after matrix-assisted autologous chondrocyte implantation, to our knowledge, similar early weightbearing studies have not been reported in patients after OCA.20,21
Osteochondral allografts transplanted to the knee are generally associated with good long-term survivability and overall outcome. However, the incidence of graft “failure” is considerable. Although some studies report 95% graft “survival” at 5 years, up to 80% at 10 years, 76% at 15 years, and even 66% at 20 years,10,15-18 functional outcomes and mechanism of failure are less well characterized. Williams et al. suggested fragmentation or collapse as a cause of failure in 21%.19 Others report similar failure rates and mechanisms between 8% to 20%.6,10,15-18 These failure mechanisms have been associated with stresses to the graft in the early weightbearing period. Graft location and rehabilitative joint loading have been theorized to contribute to graft failure.19,22
In this study, patients with essentially contained defects were treated with press-fit osteochondral allograft(s) followed by a rehabilitation protocol that considered concomitant procedures in dictating weightbearing status. Validated knee outcomes measures—International Knee Documentation Committee (IKDC) and Knee Injury and Osteoarthritis Outcomes Score (KOOS)—were applied to assess knee function, pain, and improvement in quality of life. Osseous graft incorporation to host bone was evaluated by computed tomography (CT) scan. Two subgroups, single versus multiple graft dowels, were compared, and the region of transplant was considered in analyses. We report findings from this case series at 6 months, 1 year, and 2 years postoperatively.
Materials and Methods
Procedure
This study was approved by the Institutional Review Board at our institution. Between October 2006 and January 2008, 43 patients received osteochondral allograft transplants to repair grade 4 International Cartilage Repair Society (ICRS) articular cartilage defects of the femoral condyle. Three patients were excluded from analysis because they required compressive fixation for graft stability at the time of surgery. Additionally, 4 patients were excluded because baseline questionnaire data were not available, and 2 patients were excluded because no questionnaire data were available postoperatively. All grafts were obtained from the Joint Restoration Foundation (Centennial, CO), a tissue bank approved by the American Association of Tissue Banks, and stored at 4 °C. The mean time between donor acquisition and implantation of the allograft was 21.1 days (range, 16-26) (Table 1). Donor and recipient condyles were size matched by plain radiographs. Patients were evaluated arthroscopically and underwent dowel graft OCA using the press-fit technique described by Williams et al.19 Patients underwent additional concurrent procedures as indicated (Table 1). Postoperatively, patients were allowed full open-chain passive range of motion and immediate weightbearing. An indwelling femoral nerve block catheter was used for postoperative pain control in all patients, and a hinged unloader brace was used for a minimum of 1 week to protect against quadriceps inhibition. Longer periods of bracing were used in those patients who had associated procedures including concurrent meniscal transplantation or high tibial osteotomy.
Table 1.
Patient Demographics
| Overall | Single graft | Multiple grafts | |
|---|---|---|---|
| No. of patients | 34 | 23 | 11 |
| Age, y | 34.5 (15-61) | 32.3 (16-61) | 38.2 (15-60) |
| Male, n (%) | 25 (74%) | 16 (70%) | 9 (82%) |
| BMI, kg/m2 | 26.9 (19.7-39.1) | 26.3 (19.7-39.1) | 27.8 (22-35) |
| Lesion size,a cm2 | 5.7 (1.5-15) | 3.9 (1.5-12.5) | 10.2 (5.0-15.0) |
| Graft age, d | 21.1 (16-26) | 21.0 (16-26) | 21.6 (17-26) |
| Diagnosis | |||
| Avascular necrosis | 2 | 1 | 1 |
| Osteochondritis dissecans | 20 | 12 | 8 |
| Osteochondral injury/degenerative | 15 | 10 | 5 |
| Previous procedures | |||
| Meniscectomy | 12 | 6 | 6 |
| ACL reconstruction | 4 | 3 | 1 |
| Microfracture | 4 | 4 | 0 |
| Fixation/drilling of OCA lesion | 5 | 1 | 4 |
| Concurrent procedures | |||
| ACL reconstruction | 1 | 1 | 0 |
| Tibial osteotomy | 3 | 0 | 3 |
| MPFL reconstruction/lateral release | 1 | 1 | 0 |
| Meniscus transplant | 4 | 3 | 1 |
Note: Values are means with ranges in parentheses. BMI = body mass index; ACL = anterior cruciate ligament; OCA = osteochondral allograft transplantation; MPFL = medial patellofemoral ligament.
Statistically significant (P < 0.001) in single versus multiple grafts.
Clinical Assessment
The operating surgeon performed standard follow-up examinations at 10 days, 6 weeks, and 3, 6, 12, and 24 months postoperatively. Patient-reported functional outcomes were collected using validated subjective scoring systems: IKDC and KOOS. Outcome questionnaires were collected at 4 time points: preoperatively and post-OCA at 6, 12, and 24 months.
Radiographic Assessment
Radiographs were obtained preoperatively for donor matching and to assess overall alignment and postoperatively to evaluate graft incorporation. CT scans of the knee joint were obtained for patients at 6 months postoperatively. Sagittal and coronal views of CT scans were used to determine the position of the graft on the femoral condyle using anatomic zones as defined by Cahill and Berg (Fig. 1).23 The same images were used to grade the amount of graft osseous incorporation and recorded as a percentage of the original graft size. Grafts were considered fully integrated if there was no cystic change in the subchondral bone. Cystic changes and lack of osseous bridging have been shown to be associated with graft resorption.24 Therefore, evidence of either characteristic was considered indicative of incomplete incorporation of the transplanted graft.
Figure 1.
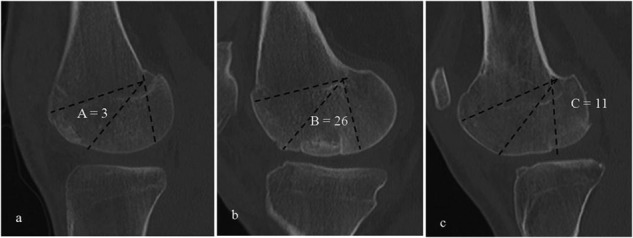
Anatomic zones A (A), B (B), and C (C), as described by Cahill and Berg4 represented by computed tomography (CT) images. Zone B is considered the direct weightbearing zone, while zones A and C are regarded as indirect weightbearing areas. Each image contains one well-incorporated graft in the respective zone. Also shown is the number of grafts that were transplanted to each zone.
Statistical Analysis
Descriptive statistics including means, standard deviations, and ranges were included when appropriate. The paired Student t test was utilized to compare the mean KOOS and IKDC scores to their baseline at measured time points. Single-graft and multiple-graft patient populations were also compared. To identify potential bias resulting from attrition, drop-out analysis was performed. Patients lost to follow-up at each time point were compared to those who remained in the study for analysis, and significant differences were noted.
Results
Demographics
Thirty-four patients (45 grafts) were evaluated. The average age was 34.5 years (range, 15-61). Twenty-five patients were male (74%). The average body mass index (BMI) was 26.9 kg/m2 (range, 19.7-39.1) (Table 1). Diagnoses included 2 patients (5%) with avascular necrosis, 11 (31%) with osteochondritis dissecans, and 23 (64%) with focal osteoarthritic defects (idiopathic and posttraumatic types). On average, patients underwent less than one (0.70) previous surgery. These procedures included prior OCA (n = 1), meniscectomy (n = 9), anterior cruciate ligament reconstruction (n = 2), microfracture (n = 5), and transcondylar drilling or fixation of osteochondritis lesions (n = 8) (Table 1).
Intraoperative Results
The average lesion size was 5.7 cm2 (range, 1.5-15.0). Twenty-three (68%) patients required one cylindrical graft to fill the damaged region. Three patients had a single plug placed in each condyle. In these cases, the mean lesion size was 3.9 cm2 (range, 1.5-12.5). The other 11 (32%) patients had a larger lesion averaging 10.2 cm2 (range, 5.0-15). This group of patients required 2 grafts to fill the damaged region. In all of these patients, the plugs were adjacent and continuous. Nine patients (26%) had concurrent procedures including anterior cruciate ligament reconstruction (n = 1), tibial realignment osteotomy (n = 5), medial patellofemoral ligament reconstruction (n = 1), and meniscus transplant (n = 2) (Table 1).
Clinical Assessment
Overall improvements in KOOS (pain, activities of daily living [ADLs], sports and recreation, quality of life [QoL]) and IKDC clinical outcomes were observed at 6 months and were maintained at 1 year and 2 years (Table 2; Fig. 2). Compared to baseline, statistically significant improvements were observed in all KOOS domains and the IKDC at the 6-month follow-up. Significance was maintained at the 1-year follow-up for all measures except the KOOS symptoms domain. At the 2-year follow-up, KOOS pain (P = 0.028), sports/recreation (P = 0.005), and QoL (P < 0.001), as well as IKDC (P < 0.001), maintained statistically significant gains over baseline measures. All domains were numerically improved (Table 3).
Table 2.
Change from Baseline in Subjective Outcome Scores
| Overall | Baseline | 6 months | 1 year | 2 years | Baseline versus 2 years |
|---|---|---|---|---|---|
| Outcomes system | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | P value |
| KOOS | (n = 34) | (n = 32) | (n = 26) | (n = 24) | |
| Pain | 59 ± 17 | 79 ± 17 | 77 ± 18 | 74 ± 22 | 0.028 |
| Symptoms | 58 ± 16 | 69 ± 20 | 69 ± 21 | 70 ± 20 | 0.172 |
| ADLs | 69 ± 21 | 85 ± 16 | 84 ± 16 | 83 ± 23 | 0.058 |
| Sports/recreation | 37 ± 26 | 65 ± 27 | 59 ± 23 | 57 ± 30 | 0.005 |
| QoL | 23 ± 17 | 47 ± 21 | 49 ± 24 | 48 ± 22 | <0.001 |
| IKDC | 45 ± 11 | 57 ± 14 | 59 ± 15 | 62 ± 20 | <0.001 |
| Single graft | Baseline | 6 months | 1 year | 2 years | Baseline versus 2 years |
| Outcomes system | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | P value |
| KOOS | (n = 23) | (n = 22) | (n = 16) | (n = 16) | |
| Pain | 60 ± 19 | 84 ± 12 | 80 ± 16 | 73 ± 21 | 0.129 |
| Symptoms | 62 ± 15 | 74 ± 18 | 72 ± 21 | 68 ± 19 | 0.634 |
| ADLs | 71 ± 23 | 90 ± 12 | 87 ± 16 | 82 ± 23 | 0.249 |
| Sports/recreation | 40 ± 22 | 68 ± 23 | 63 ± 20 | 56 ± 33 | 0.090 |
| QoL | 26 ± 17 | 52 ± 19 | 56 ± 22 | 49 ± 24 | 0.005 |
| IKDC | 48 ± 11 | 60 ± 11 | 65 ± 17 | 62 ± 22 | 0.018 |
| Multiple grafts | Baseline | 6 months | 1 year | 2 years | Baseline versus 2 years |
| Outcomes system | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | P value |
| KOOS | (n = 11) | (n = 10) | (n = 10) | (n = 8) | |
| Pain | 56 ± 14 | 68 ± 23 | 73 ± 20 | 77 ± 23 | 0.054 |
| Symptoms | 52 ± 18 | 59 ± 21 | 64 ± 21 | 75 ± 22 | 0.072 |
| ADLs | 66 ± 18 | 76 ± 19 | 80 ± 17 | 86 ± 25 | 0.052 |
| Sports/recreation | 30 ± 33 | 58 ± 35 | 52 ± 27 | 63 ± 24 | 0.009 |
| QoL | 15 ± 15 | 37 ± 21 | 39 ± 25 | 46 ± 19 | 0.003 |
| IKDC | 39 ± 9 | 50 ± 18 | 51 ± 15 | 63 ± 18 | 0.004 |
Note: Statistically significant (bold and italic). KOOS = Knee Injury and Osteoarthritis Outcomes Score; ADLs = activities of daily living; QoL = quality of life; IKDC = International Knee Documentation Committee.
Figure 2.
Average outcome scores at each time point for all groups: overall, single-graft, and multiple-graft patient populations. Standard error bars are shown.
Table 3.
Outcomes Comparison between Single-Graft (SG) and Multiple-Graft (MG) Patient Populations
| Baseline | 1 year | P value | 2 years | P value | |
|---|---|---|---|---|---|
| KOOS | |||||
| Pain | |||||
| Overall | 59 ± 17 | 77 ± 18 | 0.001 | 74 ± 22 | 0.028 |
| SG | 60 ± 19 | 80 ± 16 | 0.010 | 73 ± 21 | 0.129 |
| MG | 56 ± 14 | 73 ± 20 | 0.028 | 77 ± 23 | 0.054 |
| SG versus MG | 0.562 | 0.333 | 0.648 | ||
| Symptoms | |||||
| Overall | 58 ± 16 | 69 ± 21 | 0.084 | 70 ± 20 | 0.172 |
| SG | 62 ± 15 | 72 ± 21 | 0.195 | 68 ± 19 | 0.634 |
| MG | 52 ± 18 | 64 ± 21 | 0.128 | 75 ± 22 | 0.072 |
| SG versus MG | 0.120 | 0.372 | 0.493 | ||
| ADLs | |||||
| Overall | 69 ± 21 | 84 ± 16 | 0.013 | 83 ± 23 | 0.058 |
| SG | 71 ± 23 | 87 ± 16 | 0.081 | 82 ± 23 | 0.249 |
| MG | 66 ± 18 | 80 ± 17 | 0.031 | 86 ± 25 | 0.052 |
| SG versus MG | 0.540 | 0.281 | 0.686 | ||
| Sports/recreation | |||||
| Overall | 37 ± 26 | 59 ± 23 | 0.001 | 57 ± 30 | 0.005 |
| SG | 40 ± 22 | 63 ± 20 | 0.012 | 56 ± 33 | 0.090 |
| MG | 30 ± 33 | 52 ± 27 | 0.013 | 63 ± 24 | 0.009 |
| SG versus MG | 0.340 | 0.247 | 0.742 | ||
| QoL | |||||
| Overall | 23 ± 17 | 49 ± 24 | <0.001 | 48 ± 22 | <0.001 |
| SG | 26 ± 17 | 56 ± 22 | <0.001 | 49 ± 24 | 0.005 |
| MG | 15 ± 15 | 39 ± 25 | 0.010 | 46 ± 19 | 0.003 |
| SG versus MG | 0.078 | 0.078 | 0.739 | ||
| IKDC | |||||
| Overall | 45 ± 11 | 59 ± 15 | <0.001 | 62 ± 20 | <0.001 |
| SG | 48 ± 11 | 65 ± 17 | 0.001 | 62 ± 22 | 0.018 |
| MG | 39 ± 9 | 51 ± 15 | 0.026 | 63 ± 18 | 0.004 |
| SG versus MG | 0.038 | 0.031 | 0.957 |
Note: Statistically significant (bold and italic). KOOS = Knee Injury and Osteoarthritis Outcomes Score; ADLs = activities of daily living; QoL = quality of life; IKDC = International Knee Documentation Committee.
In subgroup analysis, the single-graft and multiple-graft patient populations were similar in age (P = 0.09) and BMI (P = 0.29). The size of the osteochondral injury was significantly different, 3.9 cm2 and 10.2 cm2, respectively (P < 0.001). Baseline KOOS scores were not significantly different between the 2 groups; however, the multiple-graft group had significantly lower IKDC scores at baseline (Table 3). Overall improvement in clinical outcome at 6 months favored the single-graft transplantation. By 1 year, both single-graft and multiple-graft patient populations demonstrated statistically significant improvement compared to baseline in IKDC and all KOOS domains, except KOOS ADL in the single-graft group and KOOS symptoms in both groups. At the 2-year follow-up, the single-graft group maintained statistically significant gains over baseline scores for the KOOS QoL domain (P = 0.005) and the IKDC (P = 0.018). The multiple-graft subgroup remained statistically improved for KOOS sports/recreation (P = 0.009), KOOS QoL (P = 0.003), and IKDC (P = 0.004) (Table 3). In comparing the single-graft group to the multiple-graft group, IKDC scores favored single grafts (mean, 65 v. 51; P = 0.031) at 1 year. At all other time points, there was no statistical difference in outcomes scores between the 2 groups.
Drop-out Analysis
A statistical comparison was made between patients who were lost to follow-up at each time point and those who remained in the study for analysis. Thirty-four patients completed baseline questionnaires and had at least a 6-month follow-up required for inclusion in the study. We collected follow-up data for 32 patients at 6 months (94%), 26 patients at 1 year (76%), and 24 patients at 2 years (71%). Three patients moved out of state, one patient was converted to total knee arthroplasty, and 6 patients were lost for unknown reasons. Among all patients, those lost to follow-up between 6 months and 1 year had statistically higher IKDC scores than those who remained in the study (67 v. 53; P = 0.012). Between the 1-year and 2-year follow-up, patients who were lost to follow-up had statistically lower KOOS pain (80 v. 61; P = 0.05), KOOS symptoms (53 v. 75; P = 0.012), KOOS QoL (54 v. 23; P = 0.017), and IKDC (63 v. 42; P = 0.014) scores than those who were retained. In subgroup analysis, patients with single grafts who were lost between 1 and 2 years had lower KOOS symptoms scores (77 v. 39; P = 0.013) than those who were retained. In patients with multiple grafts, the one patient who was lost between 6 months and 1 year had higher scores in all outcomes measures than the 9 patients with multiple grafts who were retained for analysis. Between 1 year and 2 years, the 2 patients who were lost to follow-up had statistically lower scores than retained patients in several KOOS domains (pain, symptoms, ADLs, and QoL) as well as IKDC. Although the numbers are small, patients lost at each time point represent a heterogeneous group, and no clear statistical trend is apparent.
Radiographic Assessment
Plain radiographs were obtained at 12 weeks, and CT scans were obtained at 6 months postoperatively. CT scans were used to evaluate 40 grafts in 30 patients. Of these patients, 21 had lesions that were treated with single grafts. In those patients, 17 of 22 grafts were transplanted primarily within the direct weightbearing region (zone B), and 5 grafts were transplanted to primarily indirect weightbearing zones: 2 within zone A and 3 within zone C. Nine patients had larger osteochondral lesions and required multiple grafts. Of those with multiple contiguous graft transplants, 7 had one graft principally in weightbearing zone B and a second graft more posteriorly in the indirect weightbearing zone C. One patient had both grafts in zone B, and 1 patient had both grafts in zone C.
Overall assessment of graft incorporation is reported as a percentage of incorporation based on CT images. The mean level of incorporation of all grafts was grade 2 (50%-75%). Twenty of 26 total grafts transplanted to the direct weightbearing region (zone B) were noted to have more than 75% incorporation. Two of 3 grafts transplanted to the indirect anterior weightbearing region (zone A) had greater than 75% incorporation. In the posterior indirect weightbearing region (zone C), 8 of 11 transplanted grafts had less than 50% incorporation (Tables 4 and 5).
Table 4.
Grade of Incorporation by Zone
Grade 1: 76%-100%; grade 2: 51%-75%; grade 3: 26%-50%; grade 4: 0%-25%.
Table 5.
Grade of Incorporation by Zone: Comparison of Single-Graft (SG) and Multiple-Graft (MG) Patients
| Graft | Region | Grade 1a | Grade 2a | Grade 3a | Grade 4a |
|---|---|---|---|---|---|
| SG (n = 22) | A | 2 | 0 | 0 | 0 |
| B | 12 | 4 | 1 | 0 | |
| C | 1 | 0 | 2 | 0 | |
| MG (n = 18) | A | 0 | 1 | 0 | 0 |
| B | 8 | 1 | 0 | 0 | |
| C | 2 | 0 | 1 | 5 |
Grade 1: 76%-100%; grade 2: 51%-75%; grade 3: 26%-50%; grade 4: 0%-25%.
Subsequent Procedures
Nine patients underwent a subsequent procedure believed to be directly related to the osteochondral allograft transplant. At the time of subsequent surgery, transplanted grafts were evaluated arthroscopically. Three patients had grafts that were pristine or demonstrated minimal fraying. One patient was noted to have fibrosis that impinged on the adjacent meniscus during flexion and extension. Two patients had ICRS grade 2/3 changes in the transplanted graft cartilage. Three patients were noted to have significant fragmentation or delamination of the graft with associated loose bodies. In one of these, an obese patient who had previously failed OCA, the fragmented failed revision grafting ultimately required a staged total knee arthroplasty.
Five additional patients underwent subsequent procedures that were unrelated to the transplanted OCA. Diagnostic arthroscopy was performed in 2 patients undergoing removal of symptomatic osteotomy hardware. One of these patients was noted to have an intact graft, and the other was found to have some progression of disease adjacent to the transplanted cartilage. One patient sustained a traumatic meniscal tear in the opposite compartment from their osteochondral allograft transplant. The graft was intact; however, the opposing cartilage on the tibial plateau demonstrated grade 4 ICRS changes. One patient sustained an anterior cruciate ligament tear and was found to have ICRS grade 3 changes on their osteochondral allograft transplant. An intact graft was observed in one patient who injured the contralateral knee but underwent diagnostic arthroscopy to evaluate the transplanted osteochondral allograft.
Five of the 9 patients requiring subsequent procedures related to their graft were in the single-graft subgroup. Injury to these grafts was limited to mild fraying and ICRS grade 2/3 changes. Four patients requiring subsequent procedures related to their graft were in the multiple-graft subgroup. Three of these were found to have significant delamination and/or graft fragmentation associated with loose bodies. It was observed that the posterior grafts were more likely to fail in the patients with multiple grafts. An initial debridement of the patient who ultimately required total knee arthroplasty revealed a stable zone B graft; however, the posterior zone C graft required extensive debridement and removal of loose bodies. Another patient who required loose body removal from his failed posterior graft ultimately underwent femoral unloading osteotomy for valgus malalignment after skeletal maturity.
Graft Success
Ninety-three percent of patients evaluated by CT scan had successful graft incorporation with no evidence of fragmentation. Two patients (7%) included in the analysis had loose bodies as a result of significant graft fragmentation or cartilage delamination. Both patients had multiple contiguous grafts, which included one graft in zone B and the other graft in zone C. One patient had a previously failed OCA. One patient was found to have good osseous incorporation of both grafts but substantial delamination of the posterior press-fit plug in zone C. Both patients had arthroscopic evaluation and were noted to have a stable, incorporated anterior graft; however, the more posterior zone C grafts exhibited delamination or evidence of graft fragmentation generating the symptomatic loose bodies.
Discussion
Full-thickness osteochondral lesions are challenging to treat and increase the risk of degenerative joint disease.22,25 OCA is an increasingly available option to replace the damaged bone-cartilage unit. We prospectively assessed the risk of early clinical failure by outcomes measures and CT scan criteria.
In our study, we observed consistent graft incorporation in the vast majority of cases. One patient failed, and 2 others required secondary procedures related to loss of graft integrity. In all cases, these circumstances were associated with application of multiple contiguous grafts placed in the indirect weightbearing area of the distal femora. Grafts placed more posteriorly on the femoral condyle trended toward lesser degrees of osseous integration as evaluated by CT scan. It has been shown that strains created by loading and motion may stimulate growth of callus and ultimately bone healing.26,27 Posterior grafts likely endure compressive, torsional, and/or shear forces different than those in direct weightbearing zones. Further biomechanical analysis, including zonal force analysis of OCAs, might shed light on this question. Additionally, patient-specific factors may play a role in the incorporation or failure of the graft. No preoperative assessment of patients’ baseline muscle strength or gait mechanics was undertaken in this study.
One limitation of this study is that a control group of patients undergoing protected weightbearing was not available. Importantly, we did not observe a higher failure rate than that which has been previously reported.6,10,15-18 One study of note completed by McCulloch et al. included a similar patient population with a postoperative rehabilitation protocol that included touchdown weightbearing with crutches for 6 weeks. As in our study, patients were allowed unrestricted passive range of motion. Similar outcome scores (KOOS and IKDC) were seen in both our immediate weightbearing-as-tolerated group and the protected weightbearing group reported by McCulloch et al.6 Although indirect evidence, it may be safe to allow accelerated weightbearing, particularly for small grafts. Further investigation including a direct prospective, randomized controlled trial is planned.
All graft failures in this study occurred in multiple-graft transplants with lesions extending into the posterior zone C region. Despite a trend toward decreased graft incorporation and increased graft-related complications in patients with multiple grafts, especially those in zone C, these patients still reported significant clinical improvement across several domains. Among patients with single grafts, we observed that grafts in zone C showed inferior incorporation compared to zones A and B. This could be attributed to several possibilities. Limited direct exposure to the posterior femoral condyle during surgery may decrease the surgeon’s ability to create a congruent osteochondral graft. This may contribute to increased shear force on the graft as well as altered biomechanical loading during weightbearing. Additionally, grafts in this zone may be more likely to require hardware fixation for stability. In fact, 3 patients were excluded from analysis in this study because they required compressive hardware fixation to secure their grafts. Special consideration is likely necessary for those patients requiring posterior zone C region treatment as well as those with large defects requiring multiple continuous grafts.
Despite the cases of graft failure, mean group improvement was seen in all clinical measures. When comparing parameters between patients requiring a single graft versus multiple grafts, early outcome scores generally favored patients with lesser pathology requiring less grafting. In addition, use of multiple grafts was associated with a trend towards lesser improvement in most outcomes scores. On the other hand, the scores for the multiple-graft subgroup continue to show improvement at each time point, whereas single-graft patients appear to make their most significant gains in the first 6 months and then plateau. Another limitation of this study was the rate of attrition. This investigation was performed at a regional referral center serving an expansive catchment area. Additionally, it should be noted that participation in the study was voluntary and potentially represented a significant financial hardship for some patients. We speculate that travel distance and economic hardship may explain some of our loss to follow-up. One patient was lost after conversion to a total knee arthroplasty, and at least 3 patients are known to have moved out of state. Drop-out analysis suggests that in several clinical domains, those patients who were lost to follow-up between 1 and 2 years, especially those in the multiple-graft group (n = 2), had lower outcomes scores when compared to the patients who remained in the study (n = 8). Although we cannot offer an explanation for this trend, it may contribute to inflation of the mean outcomes scores for the remaining population. The number of patients in some of these subgroups is small and heterogeneous, which likely precludes us from drawing any meaningful conclusions about them.
We know of no other study that has utilized CT scans to assess graft incorporation after OCA. Although the routine use of CT scans to evaluate patients after OCA is likely not clinically indicated, we believe our study contributes to a further understanding of the appropriate use of allograft in the treatment of osteochondral lesions in the knee. Most grafts demonstrate stable osseous incorporation at 6 months, particularly single osteochondral grafts located primarily in the direct weightbearing zones of the femoral condyle. Similarly, we recommend careful consideration be paid to grafts in more posterior, indirect weightbearing zones as well as those grafts requiring compression fixation. Modifications in postoperative weightbearing and rehabilitation protocols might be appropriate in those patients. Future areas of study might aim to further elucidate the specific biological and mechanical factors that affect osteochondral allograft survival. Additionally, it would be valuable to identify specific patient factors and/or rehabilitation protocols that might contribute to optimal graft incorporation, especially in the difficult indirect weightbearing zones of the distal femur.
Footnotes
Acknowledgments and Funding: This research project was completed at Oregon Health & Science University, Portland, Oregon, and supported by AlloSource Inc., Centennial, Colorado.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;391S:S318-27. [DOI] [PubMed] [Google Scholar]
- 2. Lattermann C, Romine SE. Osteochondral allografts: state of the art. Clin Sports Med. 2009;28:285-301. [DOI] [PubMed] [Google Scholar]
- 3. Williams SK, Amiel D, Ball ST, Allen RT, Wong VW, Chen AC, et al. Prolonged storage effects on the articular cartilage of fresh human osteochondral allografts. J Bone Joint Surg Am. 2003;85:2111-20. [DOI] [PubMed] [Google Scholar]
- 4. Enneking WF. An abbreviated history of orthopaedic oncology in North America. Clin Orthop Relat Res. 2000;374:115-24. [DOI] [PubMed] [Google Scholar]
- 5. Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67-75. [DOI] [PubMed] [Google Scholar]
- 6. McCulloch PC, Kang RW, Sobhy MH, Hayden JK, Cole BJ. Prospective evaluation of prolonged fresh osteochondral allograft transplantation of the femoral condyle. Am J Sports Med. 2007;35:411-20. [DOI] [PubMed] [Google Scholar]
- 7. Chu CR, Convery FR, Akeson WH, Meyer M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Relat Res. 1999;360:159-68. [PubMed] [Google Scholar]
- 8. Gross AE, Silverstein EA, Falk J, Falk R, Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975;108:7-14. [DOI] [PubMed] [Google Scholar]
- 9. Gross AE, McKee NH, Pritzker KP, Langer F. Reconstruction of skeletal deficits at the knee: a comprehensive osteochondral transplant program. Clin Orthop Relat Res. 1983;174:96-106. [PubMed] [Google Scholar]
- 10. Gross AE, Shasha N, Aubin P. Long-term followup of fresh femoral osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79-87. [DOI] [PubMed] [Google Scholar]
- 11. Locht RC, Gross AE, Langer F. Late osteochondral allograft resurfacing for tibial plateau fractures. J Bone Joint Surg Am. 1984;66:328-35. [PubMed] [Google Scholar]
- 12. McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts: long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;197:96-102. [PubMed] [Google Scholar]
- 13. Parrish FF. Allograft replacement of all or part of the end of a long bone following excision of a tumor: report of twenty-one cases. J Bone Joint Surg Am. 1973;55:1-22. [PubMed] [Google Scholar]
- 14. Volkov M. Allotransplantation of joints. J Bone Joint Surg Br. 1970;52:49-53. [PubMed] [Google Scholar]
- 15. Emmerson BC, Gortz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907-14. [DOI] [PubMed] [Google Scholar]
- 16. Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts of post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008-13. [DOI] [PubMed] [Google Scholar]
- 17. Jamali AA, Hatcher SL, You Z. Donor cell survival in a fresh osteochondral allograft at twenty-nine years. J Bone Joint Surg Am. 2007;89:166-9. [DOI] [PubMed] [Google Scholar]
- 18. Maury AC, Safir O, Herad FL, Pritzker KPH, Gross AE. Twenty-five-year chondrocyte viability in fresh osteochondral allograft. J Bone Joint Surg Am. 2007;89:159-65. [DOI] [PubMed] [Google Scholar]
- 19. Williams RJ, Ranawat AS, Potter HG, Carter T, Warren R. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89:718-26. [DOI] [PubMed] [Google Scholar]
- 20. Della Villa S, Kon E, Filardo G, Richi M, Vincentelli F, Delcogliano M, et al. Does intensive rehabilitation permit early return to sport without compromising the clinical outcome after arthroscopic autologous chondrocyte implantation in highly competitive athletes? Am J Sports Med. 2009;38:68-77. [DOI] [PubMed] [Google Scholar]
- 21. Wondrasch B, Zak L, Welsch G, Marlovits S. Effect of accelerated weightbearing after matrix-associated autologous chondrocyte implantation on the femoral condyle on radiographic and clinical outcome after 2 years: a prospective, randomized controlled pilot study. Am J Sports Med. 2009;37:88S-96S. [DOI] [PubMed] [Google Scholar]
- 22. Koh JL, Wirsing K, Lautenschlager E, Zhang L. The effect of graft height mismatch on contact pressure following osteochondral grafting. Am J Sports Med. 2004;32:317-20. [DOI] [PubMed] [Google Scholar]
- 23. Cahill BR, Berg BC. 99m-Technietium phosphate compound joint scintigraphy in the management of juvenile osteochondritis dissecans of the femoral condyles. Am J Sports Med. 1983;11:329-35. [DOI] [PubMed] [Google Scholar]
- 24. von Rechenberg B, Akens MK, Nadler D, Bittman P, Zlinsky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing: an experiment in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11:265-77. [DOI] [PubMed] [Google Scholar]
- 25. Evans RC, Quinn TM. Dynamic compression augments interstitial transport of a glucose-like solute in articular cartilage. Biophys J. 2006;91:1541-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailon-Plaza A, van der Meulen MCH. Beneficial effects of moderate, early loading and adverse effects of delayed or excessive loading on bone healing. J Biomech. 2003;36:1069-77. [DOI] [PubMed] [Google Scholar]
- 27. Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998;355S:S41-55. [DOI] [PubMed] [Google Scholar]



