Abstract
Objective. Four days of blood exposure leads to irreversible cartilage damage in vitro. In contrast, intermittent intra-articular blood injections twice a week during 4 weeks (mimicking micro-bleeds) in a canine model resulted in transient damage only. In this study, it was evaluated whether acute joint bleeds are more harmful than micro-bleeds in a canine model of knee arthropathy. Design. Seven dogs received 4 sequential daily intra-articular blood injections twice in 2 weeks (mimicking 2 acute 4-day joint bleeds). Seven other dogs received the same blood load but in a total of 8 injections intermittently over the 4-week period with at least 1 day in between (mimicking micro-bleeds over the same timespan). Contralateral knees served as controls. Ten weeks after the last injection cartilage matrix turnover and synovial inflammation were evaluated. Results. Only after the acute joint bleeds the release of newly formed and total (resident) cartilage matrix glycosaminoglycans were increased (P = 0.04 and P = 0.01, respectively). Furthermore, in animals with the acute joint bleeds cartilage glycosaminoglycan content was decreased (P = 0.01) and not in animals with micro-bleeds. Mild synovial inflammation was observed in both groups (both P < 0.0001) but was not different between groups. Conclusions. In contrast to micro-bleeds, 2 acute joint bleeds lead to prolonged cartilage damage independent of the level of synovial inflammation. This model suggests that micro-bleeds are less devastating than acute joint bleeds with respect to joint damage, which might be of relevance to treatment of joint bleeds in clinical practice.
Keywords: hemarthrosis, joint damage, micro-bleeds, acute joint bleed, cartilage
Introduction
Recurrent joint hemorrhages due not only to trauma or major joint surgery but also to a bleeding disorder like hemophilia, lead to inflammation, damage of articular cartilage, and eventually to destruction of the whole joint.1,2 Even in the absence of evident acute joint bleeds, joint damage is observed in hemophilia patients. It has been hypothesized that this is due to chronic micro-bleeds causing deterioration of the joints without a clinically apparent acute joint bleed.3 Natural evacuation of blood from the joint cavity results in deposition of iron (hemosiderin) in the synovial tissue.4 This causes proliferation and hypertrophy of the synovium, fibrosis, and neovascularization.5,6 As a response, infiltration of synovial tissue with lymphocytes results in an inflammatory reaction, contributing to cartilage damage.4,7
In vitro studies have shown that harmful effects of joint bleeds on cartilage independent of synovial inflammation are also evident. The combination of monocytes/macrophages and red blood cells, as present in whole blood, leads to long-lasting disturbance of cartilage matrix turnover.8 A proposed mechanism for this irreversible damage is the conversion of hydrogen peroxide and catalytic iron, supplied by damaged red blood cells, into hydroxyl radicals.9 Hydrogen peroxide is produced by chondrocytes under the influence of interleukin-1 formed by activated monocytes/macrophages. Hydroxyl radicals cause chondrocyte apoptosis resulting in permanent cartilage damage,10 since the chondrocyte is the only cell type of cartilage and responsible for maintenance of the cartilage matrix. It is unclear whether these mechanisms take place to the same extent as a result of recurrent distinct acute joint bleeds and frequently occurring subclinical micro-bleeds.
In humans, it is anticipated from clinical practice that after an acute clinical joint bleed blood reaches volume ratios close to 100% volume/volume (v/v) because the amount of synovial fluid is generally negligible compared with the volume of blood entering the joint. Blood is estimated to be cleared within less than a week assuming the bleed stops. This assumption is supported by rat and rabbit in vivo studies using intra-articular injections of blood.11,12 As such, several in vitro studies have used a 4-day blood exposure of 50% v/v to mimic an acute joint bleed and to evaluate the effects and mechanisms of blood-induced cartilage damage. It appeared that the presence of this blood load for such a time period results in long-lasting damage of both human and canine cartilage in vitro.8,10,13,14
In a canine in vivo model of blood-induced joint damage, 2 injections of autologous blood into their knees at day 0 and 2 caused an impaired cartilage matrix turnover as observed directly after blood exposure (day 4).15 However, the effects appeared transient: Blood injections given twice a week for 4 weeks did not result in long-term cartilage tissue damage (analyzed 10 weeks after the last blood injection).13 A possible explanation for the discrepancy between in vitro long-term cartilage damage and the absence of prolonged damage in vivo could be a difference in the actual exposure load (duration and concentration) of cartilage to blood. In follow-up experiments, it appeared that blood is cleared from a canine knee much faster than anticipated originally: Blood concentrations decreased within 2 days to less than 5% v/v.16 This is much faster than the clearance rate observed in humans as well as in rats and rabbits.11 As such, in previous experiments, canine cartilage was exposed to much lower blood loads in vivo than the acute joint bleeds mimicked in vitro with human and canine cartilage. Therefore, lower concentrations of blood (mimicking micro-bleeds) might be less harmful.
Human in vitro studies have shown that there is a threshold for the blood load (duration and concentration) to which cartilage has to be exposed before cartilage damage becomes irreversible. A minimal blood exposure of at least 2 days with 10% v/v blood is needed to cause long-term cartilage damage,17 suggesting that micro-bleeds do not cause irreversible direct breakdown of cartilage. In the present study, the effect of 2 acute 4-day joint bleeds with 2-week interval was compared with the effect of the same blood load, but given intermittently over the 4-week period to mimic micro-bleeds with the change in cartilage proteoglycan content between the experimental and control joints as primary outcome.
Materials and Methods
Animals
Fourteen skeletally mature Beagle dogs were divided in 2 groups of 7 animals each (6/8 male/female; mean age = 2.1 ± 0.1 years, weight 9-15 kg; all equally distributed). Animals were obtained from the animal laboratory of the Utrecht University, the Netherlands. They were housed in groups of 2 to 3 dogs, and were let out on a patio for at least 2 hours daily. They were fed a standard commercial diet with water ad libitum. According to strict European regulations, the Utrecht Medical Ethical Committee for animals approved the study.
Experimental Design
During the experimental injection procedures, dogs were given short-term anesthesia (Dormitor/Antisedan). In all dogs, left knees were injected intra-articularly with on average 1.8 ± 0.1 mL freshly collected autologous blood. This is the average maximum amount of blood that can be injected intra-articularly in a canine knee of this size. A vena puncture was performed using a 23G needle and Luer lock syringe. Approximately 5 mL of blood was collected, the needle was replaced with a clean one, and immediately the blood was injected into the joint until resistance was felt. This procedure was performed within one minute for each animal. Random checks on accurate injection were made by placing a second needle at the other side of the joint. In all cases, a blood flow from the injected joint was demonstrated, indicating that the joint was completely filled with blood. After removal of the needles, pressure was put on the puncture holes until the bleed was stopped, thereby preventing leakage of blood outside the joint cavity.
Seven dogs received 4 blood injections in 4 successive days (Fig. 1; acute bleeds). This was repeated after 2 weeks. Based on previous studies,16 this results in 2 periods of at least 4 days continuous blood exposure with at least 20% v/v blood, mimicking 2 sequential clinically evident acute joint bleeds. Left knees of these dogs were injected at the same time points with a similar volume of saline.
Figure 1.
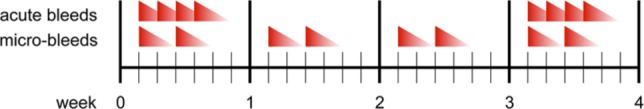
Schedule of blood injections for acute and micro-bleeds. To mimic 2 successive clinically evident joint bleeds in 4 weeks, left knees of Beagles were injected with autologous blood for 4 subsequent days twice in 4 weeks. To mimic subclinical micro-bleeds over a same time period with a same overall blood load, animals were injected in their left knee twice a week during 4 weeks with at least 1 day in between the injections. As a control, right knees were either injected according to the same injection scheme with an equal volume of saline (acute bleeds) or not injected (micro-bleeds).
In the other 7 other dogs, the same blood load was given by 8 injections with at least 1 day in between mimicking subclinical micro-bleeds over a same time period (Fig. 1; micro-bleeds) using the same procedure as described above. Right knees of these dogs were left untouched. Together with the saline injected joints of the other group these joints provided a control for the possible inflammatory responses due to the repeated injections.
In all cases, dogs regained normal function and use of the experimental joints shortly after awaking from anesthesia. Function was not scored subjectively as this was not part of the present study and because it is known from previous experiments that this is unreliable and needs accurate force-plate analyses to provide objective reliable data.18 All animals were sacrificed 10 weeks after the last injection. The dogs were euthanized using intravenous injection of Euthesate (Na-Pentobarbital). Both hind limbs were amputated and high-resolution photographs were taken from the synovial tissue and from the tibial and femoral cartilage surfaces. Within 3 hours after death of the animals, cartilage of femoral condyles and tibial plateau of both knee joints was collected. The cartilage was cut as thick as possible, excluding the underlying bone, and cut into square pieces (mean weight 3.3 ± 0.1 mg). Subsequently, the cartilage tissue samples were put in 96-well round-bottomed microtiter plates with 200 µL culture medium. Culture medium consisted of Dulbecco’s modified Eagle medium (Invitrogen, Carlsbad, CA) supplemented with glutamine (2 mM), penicillin (100 IU/mL), streptomycin sulfate (100 µg/mL; all PAA, Pasching, Austria), ascorbic acid (85 µM; Sigma, St. Louis, MO), and 10% heat-inactivated pooled Beagle serum.
Cartilage Analysis
Cartilage damage was evaluated macroscopically on high-resolution digital photographs of tibia and femur by 3 blinded observers. Severity of the damage was graded on a scale from 0 to 4 according to the OsteoArthritis Research Society International (OARSI) histopathology criteria for canine osteoarthritic cartilage.19
Biochemistry of 8 cartilage explants of predefined fixed locations from femoral condyles and tibial plateau was determined and compared with identical locations at the contralateral joint. As a measure of proteoglycan synthesis rate, the ex vivo radioactive sulfate incorporation rate of glycosaminoglycans (GAGs) was determined. Cartilage explants were placed in fresh equilibrated (37 °C, 5% CO2 in air, 95% humidity) culture medium and 740 kBq per well Na235SO4 (NEX-041-H carrier free; DuPont, Dordrecht, the Netherlands) was added. After 4 hours of pulse labeling, the cartilage samples were washed and incubated fresh culture medium without radioactive label for 3 additional days. Culture medium and cartilage explants that were washed twice in ice-cold phosphate-buffered saline were stored at −20 °C. Thawed cartilage samples were digested for 2 hours at 65 °C with 2% papain (Sigma, St. Louis, MO) in a buffered solution containing 50 mM phosphate buffer (pH 6.5; Baker), 2 mM Na2-EDTA, and 2 mM N-acetylcysteine (both Sigma). GAGs were precipitated from the cartilage digest with 0.1% Alcian Blue (Sigma) and dissolved in 2% sodium dodecyl sylfate (Sigma). The amount of radioactivity was measured by liquid scintillation analysis. Radioactive counts were normalized to the specific activity of the medium, labeling time, and wet weight of cartilage (nmoL/h/g).
As a measure of proteoglycan content, the amount of stained GAGs was quantified by absorptiometry at 620 nm using chondroitin sulfate (Sigma) as a reference. Results were expressed as milligrams GAG per wet weight of cartilage tissue (mg/g).
As a measure of retention of newly synthesized proteoglycans in the cartilage matrix, the amount of Na235SO4-labeled GAGs in the 3-day culture medium was assessed by precipitating the total GAG amount with Alcian Blue. The release of labeled GAGs as a measure of newly formed proteoglycans is normalized to GAG synthesis rate and expressed as percentage release of labeled GAGs in the 3 days of culture (% newly formed GAG release). The total release of proteoglycans is expressed as a percentage of the original cartilage GAG content (% GAG release).
Synovial Tissue Analysis
Inflammation of synovial tissue was evaluated macroscopically on high-resolution digital photographs by 3 blinded observers. Severity of the inflammation was graded on a scale from 0 to 5 according to the OARSI histopathology initiative developed for dogs.19
Statistical Analyses
All cartilage explants were handled individually and the average result of 8 samples was taken as representative of that joint surface. The primary outcome was the changein proteoglycan content between experimental (blood-injected) and contralateral control joints. This change was compared by an unpaired t test (as no clear skewing of data was observed in either group; data of individual animals are provided) between the “acute bleeds” group and the “micro-bleeds” group. In addition to this primary outcome, additional predefined secondary questions (cartilage proteoglycan synthesis, release and retention of newly formed proteoglycans as well as synovial inflammation) were statistically tested. Paired Student’s t test was used to compare data of the experimental and contralateral control joints within each group to answer the question whether “acute bleeds” or “micro-bleeds” have any effect. All changes pointed to the same direction as the primary outcome; there were no contradictory changes. Thus the secondary outcomes supported the results of the primary outcome (although not always statistically significant). This allowed multiple t testing. Data were analyzed using SPSS 15.0 software and differences were considered statistically significant when P < 0.05.
Results
Acute Joint Bleeds Cause Cartilage Degradation
Macroscopic cartilage damage was still minimal after blood exposure according to the OARSI criteria of canine cartilage damage and did not increase because of an acute joint bleed (control 0.34 ± 1.00, experimental 0.59 ± 0.11, P = 0.18) or because of a micro-bleed (control 0.44 ± 1.00, experimental 0.36 ± 0.10, P = 0.66). The differences of the control and experimental joints between the acute bleed and micro-bleed group were also not statistically significantly different (P = 0.68 and P = 0.40, respectively).
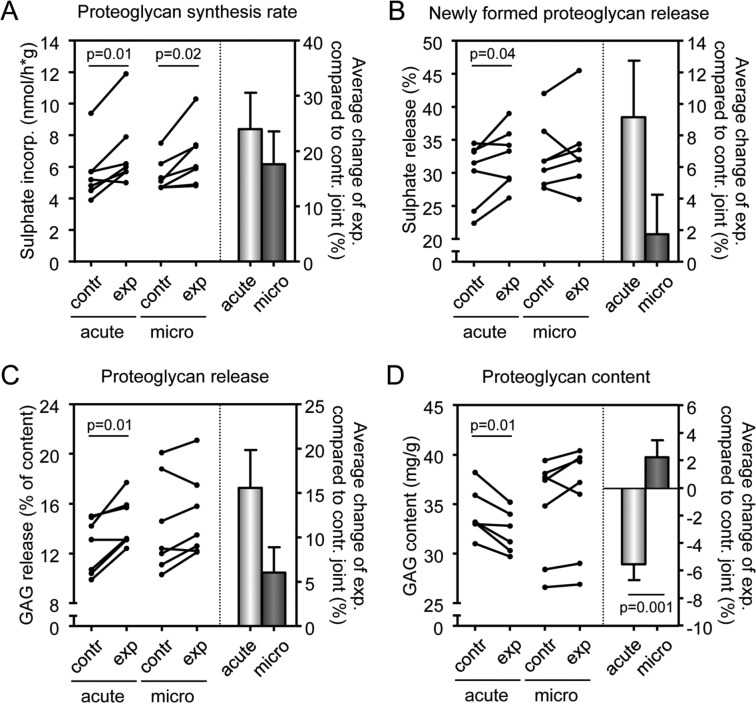
Changes in proteoglycan turnover, a much more sensitive measurement, revealed a cartilage degenerative process. Blood injections according to the acute bleeds injection scheme, as well as the micro-bleeds scheme, caused an increase in proteoglycan synthesis rate compared with the contralateral control joint (Fig. 2A; P = 0.01 and P = 0.02, respectively). This effect appeared to be slightly more outspoken for the acute joint bleeds than for the micro-bleeds (24% vs. 18% increase; right panel Fig. 2A), although this difference was not statistically significant.
Figure 2.
Parameters of cartilage damage on acute and micro-bleeds. Proteoglycan synthesis rate (A), newly formed proteoglycan release (B), total proteoglycan release (C), and proteoglycan content (D) were measured 10 weeks after the last acute joint bleed (n = 7) or after the last micro-bleed (n = 7). All these parameters are also expressed as change compared to control leg (right panel of each graph) with bars representing means ± standard error of the mean. Contr = control; exp = experimental, that is, blood-injected; acute = acute joint bleeds (white bar); micro = micro-bleeds (gray bar). P values are given in case they are less than 0.05, otherwise differences were not statistically significant.
An increased proteoglycan synthesis rate is the expression of initiated (ineffective) repair activity characteristic of early degenerative (osteoarthritic) cartilage.20 This increased proteoglycan synthesis rate was indeed ineffective, but only in the knees exposed to acute joint bleeds, since the release of newly formed GAGs (indicating a decreased retention of newly formed proteoglycans) was only statistically significantly increased after acute bleeds (Fig. 2B; 9%, P = 0.04). After micro-bleeds, change of 2% was observed (not significant, ns). The total release of proteoglycans, primarily resident proteoglycans in addition to newly formed proteoglycans, was increased with 16% in case of an acute bleed (Fig. 2C; P = 0.01), but only with 6% in case of micro-bleeds (ns).
Ineffective synthesis and enhanced release led to a statistically significant decrease in proteoglycan content (the primary outcome parameter) in case of acute joint bleeds (Fig. 2D; P = 0.01) and not in case of micro-bleeds. There was even a slight increase of 2% in proteoglycan content compared to contralateral control joints in the micro-bleeds-exposed joints, whereas there was a clear decrease of 6% in the joints exposed to acute joint bleeds when compared with contralateral control joints. This difference between both groups was statistically significant (right panel, Fig. 2D; P = 0.001). Importantly, the results of the 3 cellular parameters (synthesis, release, and retention) all supported the primary outcome on cartilage tissue structure, namely proteoglycan loss.
Synovial Inflammation due to Acute Bleeds and Micro-Bleeds
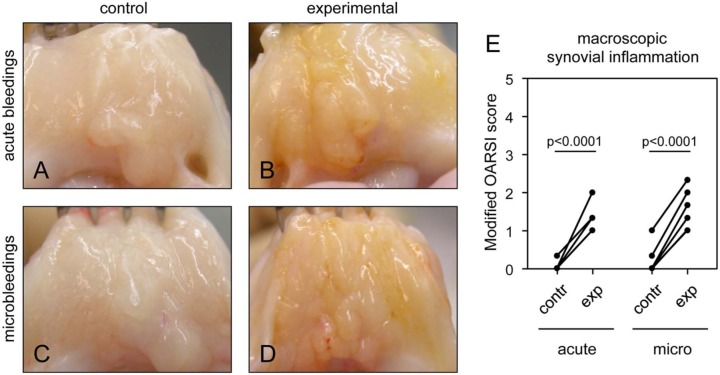
Mild synovial inflammation was observed due to acute bleeds and micro-bleeds when compared with the control knee (an increase of 1.2 vs. 1.1 points on the OARSI scale, respectively, on a total scale of 5), statistically significant for both (Fig. 3E; both P < 0.0001). There was no difference in synovial inflammation between both groups. Representative photographs demonstrate this slight increase in inflammation, which is similar for both groups (Fig. 3A-D). This absence of a difference confirms that the discrepancy in cartilage damage between both groups is because of direct effects of blood on cartilage largely independent of synovial inflammation.
Figure 3.
Macroscopic changes of the synovial tissue as a result of acute and micro-bleeds. Beagle left knee joints were injected according to the acute bleeds protocol (A and B; n = 7) or the micro-bleeds protocol (C and D; n = 7). Representative pictures of control (A and C) and experimental (blood-injected) synovial tissue (B and D) 10 weeks after the last injection are shown. Acute joint bleeds and micro-bleeds caused synovial inflammation according to the modified OARSI score (scale 0-5) (E). Macroscopy was scored by 3 blinded observers and averaged. Contr = control; exp = experimental, that is, blood-injected; OARSI = OsteoArthritis Research Society International.
Intra-Articular (Saline) Injections Do Not Cause Joint Damage
There was no clear difference in cartilage and synovial tissue parameters between the control joints of both groups (Figs. 2A-D and 3E). Although groups were small for unpaired statistical comparison with significant inter-animal variations, there was no sign of a systematic difference between the saline-injected joints and the untouched joints of both groups. As such, it may be concluded that the repeated injections in the knee joint with saline did not cause alteration of cartilage biochemistry or synovial inflammation compared to knees that were not injected at all.
Discussion
The present study demonstrates that exposure to blood for at least 4 days twice in 4 weeks, representing 2 clinically evident acute joint bleeds, leads to long-lasting cartilage damage. In contrast, a similar blood load applied intermittently over the same time span, representing subclinical micro-bleeds, does not result in long-term cartilage damage. Blood exposure, both by acute bleeds and micro-bleeds, results in equal synovial inflammation, which on its own does not induce long-lasting cartilage damage in this model.
Several animal models with different outcomes are used to investigate blood-induced joint damage. A single intra-articular injection with freshly collected autologous blood in rabbit ankle or knee joints does not lead to persistent cartilage damage or loss of joint function.12,21 A single intra-articular blood injection in a rat knee results in joint damage, although not permanent.22 In contrast, in joints of dogs that are exposed to high levels of blood (6 intra-articular injections per week during 12-18 weeks) cartilage changes are observed.23 Also a canine model using high-pressure hemarthrosis causes cartilage damage up to 8 weeks postoperatively.24 Indirectly, it is shown that blood has harmful effects in the joint of a canine model using anterior cruciate ligament transection to induce osteoarthritis. When joint bleeds are prevented during surgery, less synovitis, iron deposits, and cartilage degeneration are found.25
Additionally, several hemophilic animal models have been developed to investigate joint damage due to bleeds. Hemophilic mice possess a relatively mild bleed phenotype; they mostly only bleed after trauma. A joint bleed evoked by a blunt trauma results in joint swelling because of the bleed and inflammation.26 In a more sophisticated model, a single knee puncture causes a joint bleed and eventually results in cartilage degradation in the knee joint.27 Hemophilic dogs28 and sheep29 tend to bleed spontaneously, which leads to symptoms more closely mimicking humans with severe hemophilic arthropathy. However, these models are unpredictable regarding timing and total blood load.
In our model, we injected coagulating blood intra-articularly. This represents a joint bleed due to trauma rather than a joint bleed of a hemophilia patient. However, in developed countries, many hemophilia patients receive clotting factor replacement therapy and as such this model can also represent these types of joint bleeds. Nevertheless, compared with noncoagulating blood this damage might be a bit overestimated, since blood coagulation aggravates blood-induced joint damage.30
It is advantageous to provoke controlled blood-induced joint damage models in larger animals because they allow us to investigate the pathology of blood-induced joint damage in more detail under controlled conditions. Furthermore, biochemical properties of cartilage can be evaluated more easily in larger animal models, since there is more cartilage tissue available to analyze. A reason to choose for a canine model is that canine cartilage is clearly more similar to human cartilage with respect to cartilage thickness, number of chondrocytes compared with the extracellular matrix, and the anatomy of the knee when compared to smaller rodent models.31-34
Our canine blood-induced cartilage damage model shows that clinically evident acute joint bleeds and subclinical micro-bleeds result in equal synovial inflammation. This confirms that the difference in cartilage damage between both models is due to direct effects of blood on cartilage, independent of synovial inflammation. Despite the direct effects of blood on cartilage, it is known that inflammation of synovial tissue also plays a major role in blood-induced arthropathy. Deposition of iron in the synovial tissue ultimately causes proliferation, hypertrophy, fibrosis, and neovascularization.5,6 As such, inflamed synovial inflammation contributes to cartilage damage at a later stage.4,7 Because of that, micro-bleeds can also deteriorate cartilage of an affected joint, but probably only over a prolonged period of time.
Cartilage damage present after 2 successive acute joint bleeds in vivo is still mild compared to the irreversible in vitro damage after exposure to 50% whole blood for 4 days.13 A possible explanation for this discrepancy could be that cubic cartilage explants cultured in vitro are exposed to blood at all 6 sides, compared with only the articular side that is exposed to blood in vivo. The superficial layer of cartilage has a lower permeability when compared to the deeper layers because of higher collagen content.35 Furthermore, the articular surface is covered with a thin anionic protein layer representing a charge and size barrier.36 This provides protection under healthy conditions to articular cartilage. Blood exposure to cartilage that is not protected by its superficial layer is probably the reason of more cartilage damage in vitro than in vivo. In addition, synovial tissue, which is not present in the in vitro culture system, could possibly produce neutralizing factors (e.g., transforming growth factor-β1, interleukin-10, and interleukin-1 receptor antagonist37,38) that could limit cartilage damage after exposure to blood. These conditions are restricted during in vitro circumstances.
In conclusion, this study confirms that in a canine in vivo model, 2 clinically evident acute joint bleeds within 4 weeks lead to direct cartilage damage, being a process independent of inflammation early in the degenerative process. Furthermore, this model suggests that subclinical micro-bleeds do not cause this direct cartilage degeneration but add to joint degeneration by inflammation in the long term. The direct devastating effects of clinical acute bleeds in contrast to subclinical micro-bleeds may need special attention regarding treatment of these specific bleeds (e.g., direct evacuation of blood from the joint).
Footnotes
Acknowledgments and Funding: The authors would like to thank Dr. P. Welsing for expert assistance in statistical analyses. This study was financially supported by an unrestricted grant from Baxter (to GR).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by our institutional review board.
References
- 1. Rodriguez-Merchán EC. Pathogenesis, early diagnosis, and prophylaxis for chronic hemophilic synovitis. Clin Orthop Relat Res. 1997;(343):6-11. [PubMed] [Google Scholar]
- 2. Jansen NW, Roosendaal G, Lafeber FP. Understanding haemophilic arthropathy: an exploration of current open issues. Br J Haematol. 2008;143:632-40. [DOI] [PubMed] [Google Scholar]
- 3. Manco-Johnson MJ, Abshire TC, Shapiro AD, Riske B, Hacker MR, Kilcoyne R, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357:535-44. [DOI] [PubMed] [Google Scholar]
- 4. Roosendaal G, Vianen ME, Wenting MJ, van Rinsum AC, van den Berg HM, Lafeber FP, et al. Iron deposits and catabolic properties of synovial tissue from patients with haemophilia. J Bone Joint Surg Br. 1998;80:540-5. [DOI] [PubMed] [Google Scholar]
- 5. Madhok R, York J, Sturrock RD. Haemophilic arthritis. Ann Rheum Dis. 1991;50:588-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roy S, Ghadially FN. Synovial membrane in experimentally-produced chronic haemarthrosis. Ann Rheum Di. 1969;28:402-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ovlisen K, Kristensen AT, Jensen AL, Tranholm M. IL-1 beta, IL-6, KC and MCP-1 are elevated in synovial fluid from haemophilic mice with experimentally induced haemarthrosis. Haemophilia. 2009;15:802-10. [DOI] [PubMed] [Google Scholar]
- 8. Roosendaal G, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Cartilage damage as a result of hemarthrosis in a human in vitro model. J Rheumatol. 1997;24:1350-4. [PubMed] [Google Scholar]
- 9. Hooiveld MJ, Roosendaal G, van den Berg HM, Bijlsma JW, Lafeber FP. Haemoglobin-derived iron-dependent hydroxyl radical formation in blood-induced joint damage: an in vitro study. Rheumatology (Oxford). 2003;42:784-90. [DOI] [PubMed] [Google Scholar]
- 10. Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol. 2003;162:943-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Niibayashi H, Shimizu K, Suzuki K, Yamamoto S, Yasuda T, Yamamuro T. Proteoglycan degradation in hemarthrosis. intraarticular, autologous blood injection in rat knees. Acta Orthop Scand. 1995;66:73-9. [DOI] [PubMed] [Google Scholar]
- 12. Safran MR, Johnston-Jones K, Kabo JM, Meals RA. The effect of experimental hemarthrosis on joint stiffness and synovial histology in a rabbit model. Clin Orthop Relat Res. 1994;(303):280-8. [PubMed] [Google Scholar]
- 13. Hooiveld M, Roosendaal G, Vianen M, van den Berg M, Bijlsma J, Lafeber F. Blood-induced joint damage: long-term effects in vitro and in vivo. J Rheumatol. 2003;30:339-44. [PubMed] [Google Scholar]
- 14. Roosendaal G, Vianen ME, Marx JJ, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a human in vitro study. Arthritis Rheum. 1999;42:1025-32. [DOI] [PubMed] [Google Scholar]
- 15. Roosendaal G, TeKoppele JM, Vianen ME, van den Berg HM, Lafeber FP, Bijlsma JW. Blood-induced joint damage: a canine in vivo study. Arthritis Rheum. 1999;42:1033-9. [DOI] [PubMed] [Google Scholar]
- 16. Jansen NW, Roosendaal G, Wenting MJ, Bijlsma JW, Theobald M, Hazewinkel HA, et al. Very rapid clearance after a joint bleed in the canine knee cannot prevent adverse effects on cartilage and synovial tissue. Osteoarthritis Cartilage. 2009;17:433-40. [DOI] [PubMed] [Google Scholar]
- 17. Jansen NW, Roosendaal G, Bijlsma JW, Degroot J, Lafeber FP. Exposure of human cartilage tissue to low concentrations of blood for a short period of time leads to prolonged cartilage damage: an in vitro study. Arthritis Rheum. 2007;56:199-207. [DOI] [PubMed] [Google Scholar]
- 18. Frost-Christensen LN, Mastbergen SC, Vianen ME, Hartog A, DeGroot J, Voorhout G, et al. Degeneration, inflammation, regeneration, and pain/disability in dogs following destabilization or articular cartilage grooving of the stifle joint. Osteoarthritis Cartilage. 2008;16:1327-35. [DOI] [PubMed] [Google Scholar]
- 19. Cook JL, Kuroki K, Visco D, Pelletier JP, Schulz L, Lafeber FP. The OARSI histopathology initiative—recommendations for histological assessments of osteoarthritis in the dog. Osteoarthritis Cartilage. 2010;18(Suppl 3):S66-79. [DOI] [PubMed] [Google Scholar]
- 20. Lafeber FP, van Roy H, Wilbrink B, Huber-Bruning O, Bijlsma JW. Human osteoarthritic cartilage is synthetically more active but in culture less vital than normal cartilage. J Rheumatol. 1992;19:123-9. [PubMed] [Google Scholar]
- 21. Tan AH, Mitra AK, Chang PC, Tay BK, Nag HL, Sim CS. Assessment of blood-induced cartilage damage in rabbit knees using scanning electron microscopy. J Orthop Surg (Hong Kong). 2003;12:199-204. [DOI] [PubMed] [Google Scholar]
- 22. Ravanbod R, Torkaman G, Esteki A. Biotribological and biomechanical changes after experimental haemarthrosis in the rabbit knee. Haemophilia. 2010;17:124-33. [DOI] [PubMed] [Google Scholar]
- 23. Hoaglund FT. Experimental hemarthrosis. the response of canine knees to injections of autologous blood. J Bone Joint Surg Am. 1967;49:285-98. [PubMed] [Google Scholar]
- 24. Sancho FG. Experimental model of haemophilic arthropathy with high pressure haemarthrosis. Int Orthop. 1980;4:57-62. [DOI] [PubMed] [Google Scholar]
- 25. Myers SL, Brandt KD, O’Connor BL, Visco DM, Albrecht ME. Synovitis and osteoarthritic changes in canine articular cartilage after anterior cruciate ligament transection. Effect of surgical hemostasis. Arthritis Rheum. 1990;33:1406-15. [DOI] [PubMed] [Google Scholar]
- 26. Valentino LA, Hakobyan N, Kazarian T, Jabbar KJ, Jabbar AA. Experimental haemophilic synovitis: rationale and development of a murine model of human factor VIII deficiency. Haemophilia. 2004;10:280-7. [DOI] [PubMed] [Google Scholar]
- 27. Hakobyan N, Enockson C, Cole AA, Rick Sumner D, Valentino LA. Experimental haemophilic arthropathy in a mouse model of a massive haemarthrosis: gross, radiological and histological changes. Haemophilia. 2008;14:804-9. [DOI] [PubMed] [Google Scholar]
- 28. Ovlisen K, Kristensen AT, Tranholm M. In vivo models of haemophilia - status on current knowledge of clinical phenotypes and therapeutic interventions. Haemophilia. 2008;14:248-59. [DOI] [PubMed] [Google Scholar]
- 29. Porada CD, Sanada S, Long CR, Wood JA, Desai J, Frederick N, et al. Clinical and molecular characterization of a re-established line of sheep exhibiting hemophilia A. J Thromb Haemost. 2009;8:276-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Meegeren ME, Roosendaal G, Barten-van Rijbroek AD, Schutgens RE, Lafeber FP, Mastbergen SC. Coagulation aggravates blood-induced joint damage in dogs. Arthritis Rheum. 2012;64:3231-9. [DOI] [PubMed] [Google Scholar]
- 31. Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142-6. [PubMed] [Google Scholar]
- 32. Griffith CJ, Laprade RF, Coobs BR, Olson EJ. Anatomy and biomechanics of the posterolateral aspect of the canine knee. J Orthop Res. 2007;25:1231-42. [DOI] [PubMed] [Google Scholar]
- 33. Mastbergen SC, Lafeber FP. Animal models of osteoarthritis—why choose a larger model? US Musculoskeletal Rev. 2009;4:11-4. [Google Scholar]
- 34. Stockwell RA. The interrelationship of cell density and cartilage thickness in mammalian articular cartilage. J Anat. 1971;109:411-21. [PMC free article] [PubMed] [Google Scholar]
- 35. Muir H, Bullough P, Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970;52:554-63. [PubMed] [Google Scholar]
- 36. Stanescu R, Leibovich SJ. The negative charge of articular cartilage surfaces. An electron microscopic study using cationized ferritin. J Bone Joint Surg Am. 1982;64:388-98. [PubMed] [Google Scholar]
- 37. Bucht A, Larsson P, Weisbrot L, Thorne C, Pisa P, Smedegard G, et al. Expression of interferon-Gamma (IFN-γ), IL-10, IL-12 and transforming growth factor-beta (TGF-β) MRNA in synovial fluid cells from patients in the early and late phases of rheumatoid arthritis (RA). Clin Exp Immunol. 1996;103:357-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Firestein GS, Berger AE, Tracey DE, Chosay JG, Chapman DL, Paine MM, et al. IL-1 receptor antagonist protein production and gene expression in rheumatoid arthritis and osteoarthritis synovium. J Immunol. 1992;149:1054-62. [PubMed] [Google Scholar]