Abstract
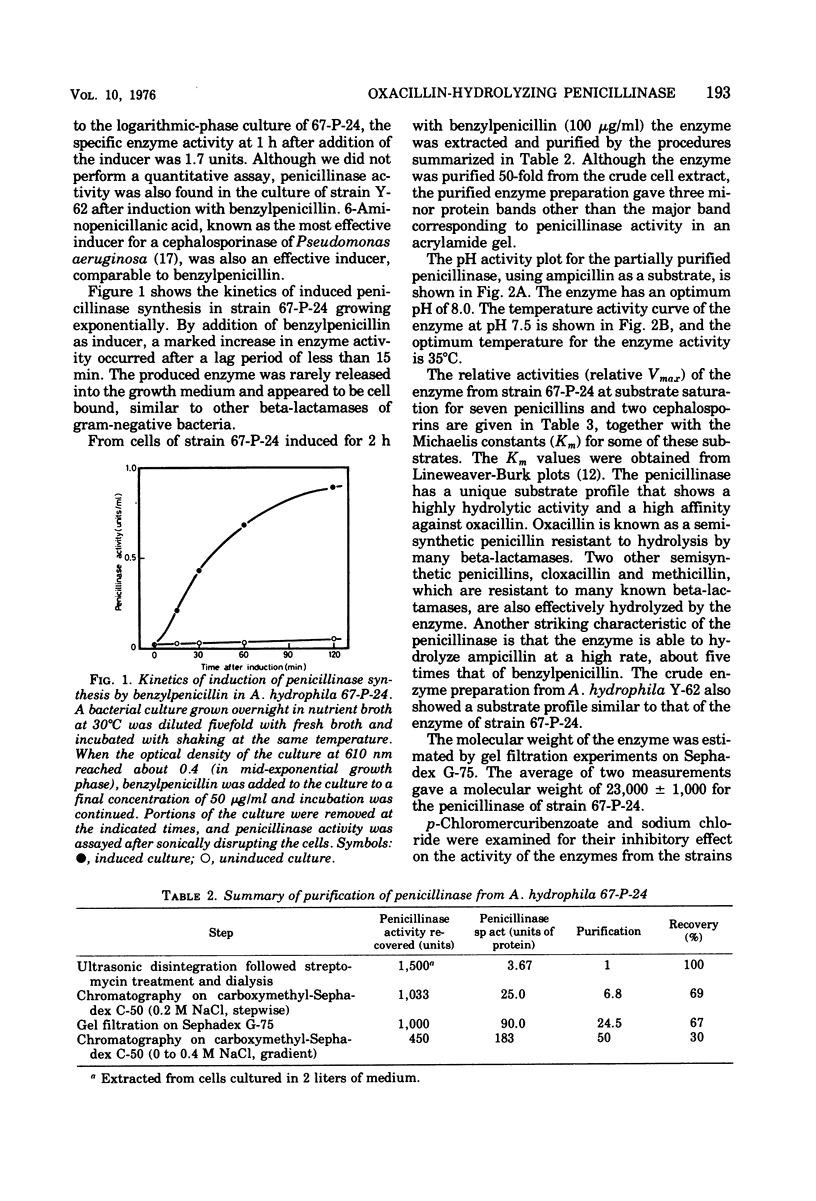
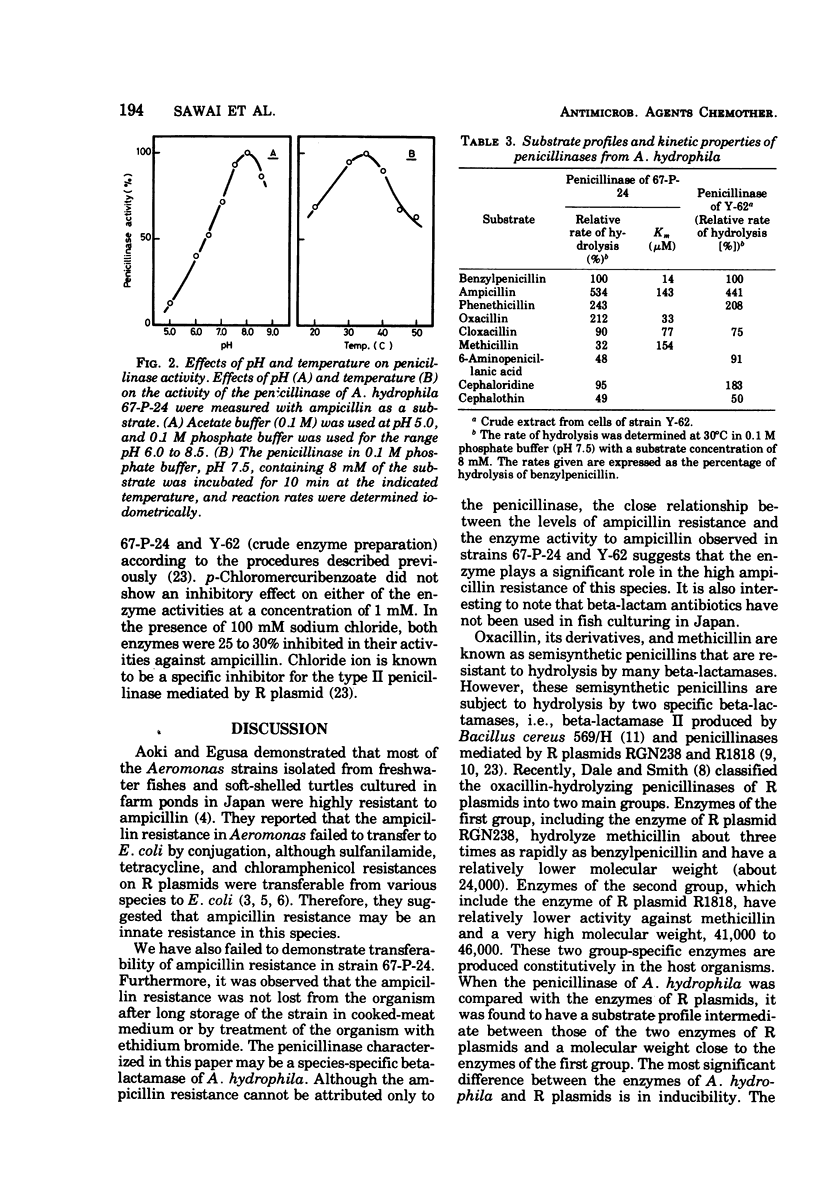
An inducible penicillinase was shown to be present in a strain of Aeromonas hydrophila subsp. hydrophila isolated from freshwater fish. Enzyme induction was observed with benzylpenicillin or 6-aminopenicillanic acid, and the enzyme was cell bound. The penicillinase was purified 50-fold from a crude cell extract. The molecular weight was estimated to be 23,000 by gel filtration. The pH and temperature optima for the enzyme activity were 8.0 and 35°C, respectively. The penicillinase showed a unique substrate profile by hydrolyzing oxacillin about twice as rapidly as benzylpenicillin. The enzyme activity was weakly inhibited by sodium chloride but was not affected by p-chloromercuribenzoate. The property of penicillinase production by the A. hydrophila strain could not be transferred to Escherichia coli and also could not be eliminated from the bacteria by ethidium bromide treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON E. S., DATTA N. RESISTANCE TO PENICILLINS AND ITS TRANSFER IN ENTEROBACTERIACEAE. Lancet. 1965 Feb 20;1(7382):407–409. doi: 10.1016/s0140-6736(65)90004-8. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Ogata Y., Watanabe T. Detection of resistance factors in fish pathogen Aeromonas liquefaciens. J Gen Microbiol. 1971 Mar;65(3):343–349. doi: 10.1099/00221287-65-3-343. [DOI] [PubMed] [Google Scholar]
- Aoki T., Egusa S., Yada C., Watanabe T. Studies of drug resistance and R factors in bacteria from pond-cultured salmonids. I. Amago (Oncorhynchus rhodurus macrostomus) and Yamame (Oncorhynchus masou ishikawae). Jpn J Microbiol. 1972 May;16(3):233–238. doi: 10.1111/j.1348-0421.1972.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Dale J. W., Smith J. T. R-factor-mediated beta-lactamases that hydrolyze oxacillin: evidence for two distinct groups. J Bacteriol. 1974 Aug;119(2):351–356. doi: 10.1128/jb.119.2.351-356.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Kuwabara S., Abraham E. P. Some properties of two extracellular beta-lactamases from Bacillus cereus 569/H. Biochem J. 1967 Jun;103(3):27C–30C. doi: 10.1042/bj1030027c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsumoto H., Sawai T., Tazaki T., Yamagishi S., Mitsuhashi S. Characterization of the chromosomally mediated penicillinase in Klebsiella pneumoniae. Jpn J Microbiol. 1972 May;16(3):169–176. doi: 10.1111/j.1348-0421.1972.tb00645.x. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Rosselet A., Zimmermann W. Mutants of Pseudomonas aeruginosa with impaired -lactamase inducibility and increased sensitivity to -lactam antibiotics. J Gen Microbiol. 1973 Jun;76(2):455–457. doi: 10.1099/00221287-76-2-455. [DOI] [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Drug resistance of enteric bacteria. XIV. Comparison of beta-lactamases in gram-negative rod bacteria resistant to alpha-aminobenzylpenicillin. Jpn J Microbiol. 1968 Dec;12(4):423–434. doi: 10.1111/j.1348-0421.1968.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai T., Yamagishi S., Mitsuhashi S. Penicillinases of Klebsiella pneumoniae and their phylogenetic relationship to penicillinases mediated by R factors. J Bacteriol. 1973 Sep;115(3):1045–1054. doi: 10.1128/jb.115.3.1045-1054.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma S., Sawai T., Ono H., Yamagishi S., Mitsuhashi S. Biochemical properties of a cephalosporin beta-lactamase from Pseudomonas aeruginosa. Jpn J Microbiol. 1973 Mar;17(2):141–149. doi: 10.1111/j.1348-0421.1973.tb00718.x. [DOI] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]


