Abstract
Objective:
We have developed an ultrapurified low endotoxin alginate (UPLE alginate), which can drastically reduce endotoxin levels. Our purposes were to examine the effects of UPLE alginate administration on osteoarthritis (OA) progression and to determine the adequate molecular weight of the UPLE alginate for therapeutic effects.
Design:
To induce knee OA, 35 Japanese White rabbits underwent anterior cruciate ligament transection. Intra-articular injections of 0.3 mL solution of each material were started at 4 weeks postoperatively for a total of 5 weekly injections. Seventy knees were divided into the following groups: AL430 (430 kDa molecular weight UPLE alginate), AL1000 (1,000 kDa), AL1700 (1,700 kDa), HA (hyaluronan), and NS (normal saline). At 9 weeks postoperatively, all knees were assessed macroscopically, histologically, and mechanically.
Results:
Macroscopically, the UPLE alginate groups exhibited milder cartilage degradation compared to that of the NS and HA groups. Histological findings of the UPLE alginate groups showed an obvious reduction in the severity of OA. The histological scores of Kikuchi et al. were superior in the alginate treatment groups compared to the NS group. The friction coefficient of the AL1000 group was significantly lower than that of the NS and HA groups.
Conclusion:
This study indicates that our UPLE alginates, especially AL1000, have promising potential as an effective agent in preventing OA progression.
Keywords: alginate, osteoarthritis, cartilage, molecular weight, intra-articular injection
Introduction
The diagnosis of osteoarthritis (OA) is frequently made in an advanced stage with severe pain and functional limitations in the affected joint. The current options for therapeutic intervention are limited due to a lack of capacity for self-repair of articular cartilage. Therefore, it is important to develop novel therapeutic strategies for preventing or delaying the disease progression. Intra-articular injection of a therapeutic agent is one possible strategy. To date, elastoviscous hyaluronan (HA) solutions and HA derivatives have been widely used as intra-articular injection agents for knee and shoulder OA.1-8 Hyaluronan, which is a high molecular weight polymer and a main component of the articular cartilage matrix and synovial fluid, has been reported to have some positive effects on the maintenance of cartilage matrix integrity during the development of experimental OA.9-13 Clinically, however, the therapeutic effects of HA injection on OA are still debated. Recent clinical studies have demonstrated no therapeutic effects of HA injection on preventing the disease progression.14-16 Therefore, a novel therapeutic agent for intra-articular administration must be developed.
Alginate is a naturally abundant and anionic polysaccharide with homopolymeric blocks of (1-4)-linked β-D-mannuronate and its C-5 epimer α-L-gluronate residues. This polysaccharide is known as an HA-like biocompatible polymer often used in biomaterials science because it contains uronic acid as a main sugar residue of the repeating unit. In addition, alginate is considered to be a good mimic for cartilage glycosaminoglycans. A number of studies have demonstrated that alginate is one of the most suitable materials as a cell carrier or a scaffold for cartilage tissue engineering.17-20 Previous in vitro studies have also shown that alginate enhances chondrogenesis of stem and progenitor cells.21-23 Based on these characteristics and the chondrogenic potential of alginate, we considered the usage of this naturally occurring material as an intra-articular injection agent for the treatment of OA. On the other hand, alginate includes mitogenic and cytotoxic impurities that induce a foreign body reaction or heavy pericapsular fibrosis in a living body. These impurities in alginate have limited its clinical application. To overcome this drawback, the authors developed a highly purified biocompatible alginate material, which can drastically reduce the endotoxin level.21 Using rabbit and canine osteochondral defect models, we clarified that the novel ultrapurified alginate enhanced cartilage regeneration without any macroscopically or histologically inflammatory findings indicating foreign body reaction in living joints.21,24 These previous results support the potential of our novel alginate material for clinical application.
The effects of intra-articular injection of alginate on OA cartilage have not been clarified. In the present study, we hypothesized that our novel ultrapurified low endotoxin alginate (UPLE alginate) could exhibit therapeutic effects on experimental OA. To test this hypothesis, we examined the antiarthritic activity of intra-articular administrations of the UPLE alginate in rabbits with knee OA induced by anterior cruciate ligament transection (ACLT) and investigated the effects of its molecular weight on this activity.
Materials and Methods
Materials
We utilized brown seaweed (Lessonia) as the raw material for alginate. The alginate in seaweed was extracted first by conversion to water-soluble sodium alginate. Caustic soda was added to extract this sodium alginate from the seaweed. The aqueous alginate solution was then isolated by our original separation procedure. The seaweed extraction was filtrated to separate sodium alginate solution from fibrous seaweed residue. To isolate alginate from this sodium alginate solution, an acid was added. In this acidic system, insoluble alginate was precipitated and isolated. This method, referred to as an acid precipitation method, is particularly suitable for the production of the purest alginate because it reduces calcium concentration. The viscosity of alginate highly correlates to its molecular weight. Based on this property of alginate, 3 different molecular weight UPLE alginates were prepared as follows: AL430 (average molecular weight of 430 kDa), AL1000 (1,000 kDa), and AL1700 (1,700 kDa) (Mochida Pharmaceutical Co. Ltd., Tokyo, Japan). We evaluated the molecular weight using size exclusion chromatography (SEC). All materials had quite a low endotoxin level of 5.76 EU/g according to a commercial limulus amebocyte lysate assay (Limulus Color KY Test Wako, Wako Pure Chemical Industries Ltd., Osaka, Japan). All kinds of alginate were sterilized by freeze drying and packaged in a sterilized vial. The mannuronate/guluronate ratio of the UPLE alginates was 1.0:1.4. The dynamic viscosities were measured using a cone-and-plate rotational viscometer (TVE-20LT, Toki Sangyo Co. Ltd., Tokyo, Japan). The share rate for the measurement was 50 seconds−1. The value of each material solution was as follows: less than 100 mPa/s of AL430, 100 to 200 mPa/s of AL1000, and 400 to 600 mPa/s of AL1700. For comparison, sodium HA (average molecular weight of 660 kDa, dynamic viscosity of 850-900 mPa/s) (ARTZ, Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) was used in this study.
Experimental OA Model
Japanese White rabbits weighing 2.8 to 3.0 kg purchased from a professional breeder (Japan SLC Inc., Hamamatsu, Japan) were used for this study according to established ethical guidelines approved by the local animal care committee. Animals were appropriately anesthetized with 0.05 mg/kg of pentobarbital intravenous injection, followed by isoflurane in oxygen gas anesthesia. Then, both knees in each rabbit were shaved, prepared, and draped in a sterile fashion. To induce OA in both knees, ACLT was performed through a medial parapatellar approach.25 The patella was dislocated laterally, and then, the anterior cruciate ligament was transected with a sharp blade. The knee joints were irrigated with sterile normal saline and closed with running 3-0 nylon sutures in layers. Positive manual anterior drawer test and Lachman test findings were confirmed to provide anterior instability in all knees. All animals were maintained in individual cages and were allowed to move freely.
Treatment Protocols
Using a 26-gauge tuberculin syringe, intra-articular injections of 0.3 mL solution of 1% of each material were performed through the patella tendon in a sterile fashion under intravenous anesthesia of 0.05 mg/kg of pentobarbital. The injection of each material started at 4 weeks after surgery, for a total of 5 weekly injections. According to the injection materials, 70 knees of 35 rabbits were randomly divided into 5 treatment groups as follows: AL430, AL1000, AL1700, HA, and normal saline (NS) groups. For each assessment, all animals were euthanized at 9 weeks postoperatively by an intravenous overdose of anesthesia.
Morphological Assessment
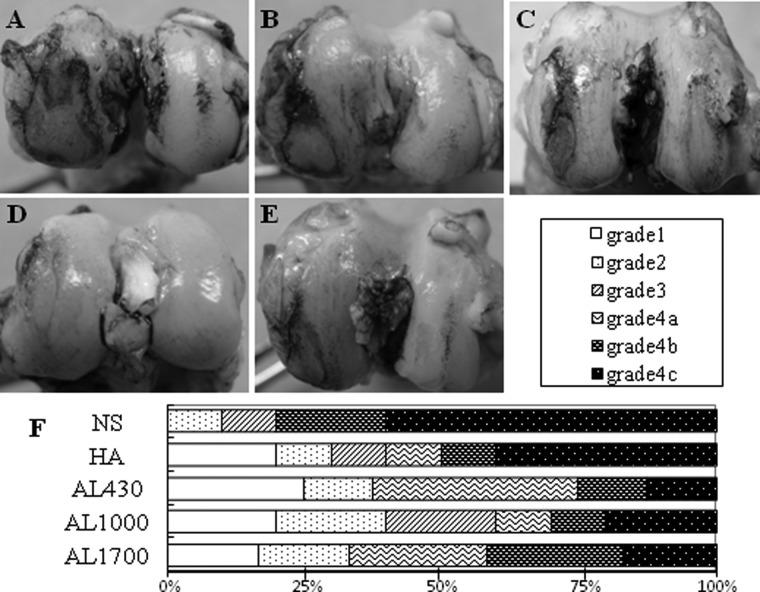
Gross morphological assessment of the knee (10 knees in each group) was performed in 4 compartments, including the medial femoral condyle (MFC), lateral femoral condyle (LFC), medial tibial plateau (MTP), and lateral tibial plateau (LTP). The articular cartilage surface was stained with a solution of India ink diluted with phosphate buffered saline (1:5). The stained surfaces were photographed with a high-resolution digital camera. Macroscopic findings were assessed according to the following criteria: grade 1 (intact surface), surface is normal in appearance and does not retain India ink; grade 2 (minimal fibrillation), surface retains India ink as elongated specks or light gray patches; grade 3 (overt fibrillation), areas are velvety in appearance and retain India ink as intense black patches; and grade 4 (erosion), loss of cartilage exposing the underlying bone.26 Grade 4 was further divided into the following 3 subgrades: grade 4a, erosion <2 mm; grade 4b, erosion ≥2 mm and <5 mm; and grade 4c, erosion ≥5 mm.
Histological Assessment
For histological analysis, the distal femur and the proximal tibia from each knee (8 knees in each group) were fixed with 4% phosphate buffered paraformaldehyde for 24 hours, decalcified with 10% ethylenediamine tetra-acetic acid (EDTA, pH 7.4) for 1 week, and embedded in paraffin. A 5-μm-thickness sagittal section was obtained from the center of the MFC and of the tibial plateau in each sample. The sections were stained with Safranin-O. All sections were randomly examined by one observer. To avoid observer bias, slides were coded prior to the examination. Cartilage degeneration in each sample was quantitatively assessed using the scoring system described by Kikuchi et al. (Table 1).9
Table 1.
Scoring System for Histological Evaluation of Cartilage Degeneration9
| Points | |
|---|---|
| Loss of superficial layer | |
| <Slight | 1 |
| Moderate | 2 |
| Focally severe | 3 |
| Extensively severe | 4 |
| Erosion of cartilage | |
| <Detectable | 1 |
| Moderate | 2 |
| Focally severe | 3 |
| Extensively severe | 4 |
| Fibrillation and/or fissures | |
| <Noticeable (<1 very small) | 1 |
| Moderate (1 small) | 2 |
| Marked (2 small or 1 medium) | 3 |
| Extensive (3 small, 2 medium, or 1 large) | 4 |
| Loss of proteoglycan | |
| <Paler stain than control | 1 |
| Moderate loss of Safranin-O stain | 2 |
| Marked loss of Safranin-O stain | 3 |
| Total loss of Safranin-O stain | 4 |
| Disorganization of chondrocytes | |
| Noticeable | 1 |
| Moderate, with some loss of columns | 2 |
| Marked loss of columns | 3 |
| No recognizable organization | 4 |
| Loss of chondrocytes | |
| <Noticeable decrease in cells | 1 |
| Moderate decrease in cells | 2 |
| Marked decrease in cells | 3 |
| Very extensive decrease in cells | 4 |
| Exposure of subchondral bone | |
| <Focal exposure of bone | 1 |
| Moderate exposure of bone | 2 |
| Fairly extensive exposure of bone | 3 |
| Very extensive exposure of bone | 4 |
| Cluster formation | |
| <3-4 small or 1-2 medium | 1 |
| 5-6 small, 3-4 medium, or 1-2 large | 2 |
| 7 or more medium or 5-6 large | 3 |
| 7 or more small, 5-6 medium, or 3-4 large | 4 |
Friction Tests
Thirty knees (6 knees in each group) were prepared for friction testing. These samples were only used for this test. The effect of the intra-articular injection on the joint lubrication was assessed using a pendulum friction tester designed by our laboratory for small samples. The knees were resected at the proximal end of the femoral shaft and at the distal end of the tibia and then secured to polyethylene tubes with bone cement. Except for the joint capsule, the tendons, and the ligaments around the knee, all soft tissues were removed from the joint. The tubes were attached to a pendulum friction tester (Fig. 1). During the experiments, the joints were kept moist with an NS injection. The total mass of the pendulum weight and the frame of the pendulum was 40 N. The displacement was measured by a sensor (Keyence, Tokyo, Japan) mounted on the upper plate of the frame (Fig. 1). Based on the damping oscillation curve of the pendulum, the frictional coefficient μ was calculated by the following equation: μ = LΔθ/4r, where r is the radius of the rabbit femoral condyle, L is the distance between the center of gravity and the center of rotation, and Δθ is the average decrease in amplitude of the pendulum swing. The measurement for each sample was repeated 3 times, and then, the obtained values were averaged.
Figure 1.
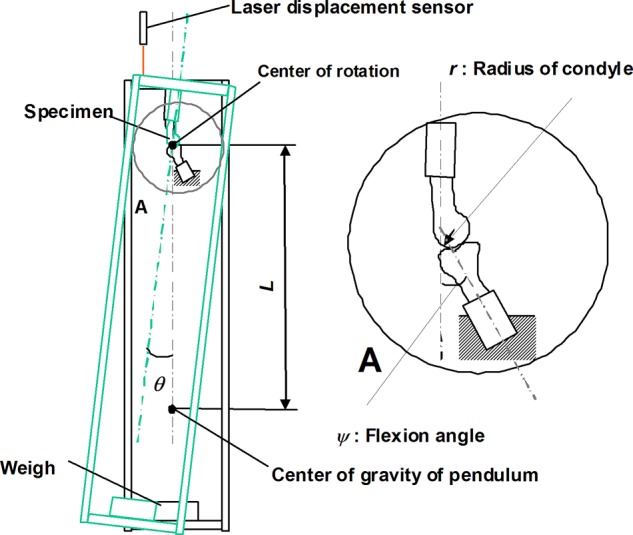
Schematic illustration of the pendulum-typed friction tester used in this study. The frictional coefficient μ was calculated by the equation μ = LΔθ/4r, where r is the radius of the rabbit femoral condyle, L is the distance between the center of gravity and the center of rotation, and Δθ is the average decrease in amplitude of the pendulum swing.
Statistical Analysis
Data are represented as mean ± standard error. Significant differences in histological scores among the groups were assessed by a Kruskal-Wallis test followed by Steel-Dwass post hoc tests. Statistical comparisons of the friction coefficient were performed using a 1-way ANOVA followed by Scheffe post hoc tests. P values less than 0.05 were considered statistically significant.
Results
Perioperative Conditions
All operations were uneventful with no perioperative complications such as infection or joint contracture. At euthanasia, no significant difference in the body weights of the animals was found among any of the experimental groups. Complete transection of the anterior cruciate ligament was macroscopically confirmed.
Gross Morphological Findings
At 9 weeks after ACLT, all knee joints were stained with India ink and graded according to the scoring system described above.26 All knees exhibited some degree of mild to severe degenerative changes and joint effusion with synovial fluid (Fig. 2A-F). The degenerative changes were more severe in the femoral condyle than in the tibial plateau. The NS and HA groups showed extensive cartilage erosion mainly at the MFC (Fig. 2A and 2B). The alginate injection groups exhibited milder cartilage degradation than the HA and NS groups (Fig. 2C-E). Regarding the macroscopic grading at the MFC, the most severe changes of grade 4c (erosion of cartilage >5 mm) were found between 10% to 20% in the alginate treatment groups, 40% in the HA group, and 60% in the NS group (Fig. 2F). At other joint components, no lesions of grade 4c were exhibited in any alginate treatment groups (Fig. 2C-E).
Figure 2.
Macroscopic findings of femoral cartilage stained with India ink at 9 weeks after anterior cruciate ligament transection (ACLT) of (A) the normal saline (NS) group, (B) the hyaluronan (HA) group, (C) the AL430 group, (D) the AL1000 group, and (E) the AL1700 group. The retained intense black patches of ink indicate articular cartilage degeneration in the medial condyle. (F) Gross morphological grading of cartilage degeneration at the medial femoral condyle (MFC) in each group.
Histological Findings
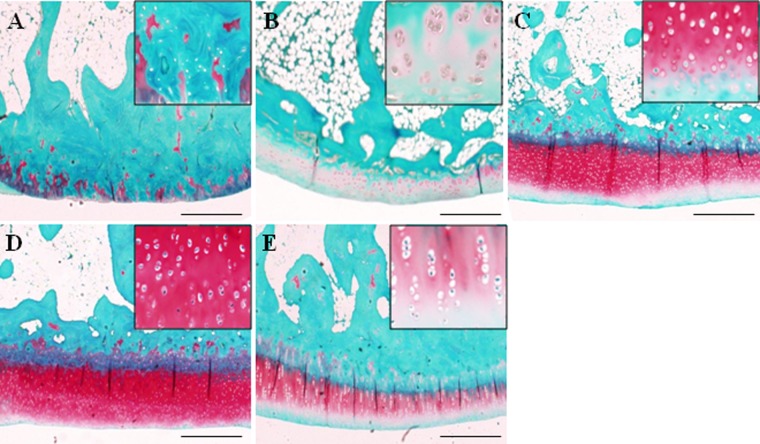
In the NS group, loss of the superficial layer, fibrillation/fissures, and cleft extended into the junction of calcified cartilage and subchondral layers were observed in the MFC (Fig. 3A). The samples of the HA group showed fibrillation, fissures, and loss of proteoglycan mainly in the MFC (Fig. 3B). The AL1700 group revealed mild erosion compared to the NS and HA groups (Fig. 3E). In the AL1000 group, there was an obvious reduction in the severity of cartilage lesions compared to other groups. Only superficial irregularity, slight fibrillation, and loss of Safranin-O staining were observed in the AL1000 samples (Fig. 3D). The loss of Safranin-O staining at the MFC was milder in the AL1000 group than the AL430 group (Fig. 3C and 3D). The overall histological scores (8 = normal to 32 = the most severe OA) of the alginate groups were significantly lower than those of the NS group (P < 0.05) (Table 2). This indicates that the alginate groups suppressed the cartilage degradation by ACLT. No significant difference in the scores was found between the NS and the HA group. When the histological scores among the treatment groups were compared, the scores tended to be lower in the alginate groups than in the HA group. Although the value of the AL1000 group tended to be lower than other alginate treatment groups, there were no significant differences in the values among the 3 groups. For each category in the scoring system, the score of cartilage fibrillation in the alginate treatment groups was significantly lower than that in the NS group (P < 0.01, v. AL430 and AL1000; P < 0.05, v. AL1700) (Table 2). The AL1000 and AL1700 groups showed a significant decrease in the scores assigned to a decline in the superficial layer and in chondrocytes compared to the NS group (P < 0.05) (Table 2). The score of proteoglycan loss was significantly lower in the AL1000 group than in the NS group (P < 0.05) (Table 2).
Figure 3.
Histological appearance of cartilage from the medial condyle of (A) the normal saline (NS) group, (B) the hyaluronan (HA) group, (C) the AL430 group, (D) the AL1000 group, and (E) the AL1700 group (Safranin-O stained). The bars indicate 500 μm.
Table 2.
Mean Histological Degenerative Score of the Medial Femoral Condyle at 9 Weeks after Anterior Cruciate Ligament Transection (ACLT) in Each Group
| Category | NS | HA | AL430 | AL1000 | AL1700 |
|---|---|---|---|---|---|
| Loss of superficial layer | 2.75 ± 0.19 | 2.25 ± 0.29 | 1.88 ± 0.26 | 1.38 ± 0.30a | 1.88 ± 0.14a |
| Erosion of cartilage | 2.63 ± 0.43 | 2.25 ± 0.36 | 1.38 ± 0.21 | 1.38 ± 0.21 | 1.63 ± 0.21 |
| Fibrillation and/or fissures | 3.13 ± 0.14 | 2.38 ± 0.37 | 1.88 ± 0.26b | 1.62 ± 0.21b | 1.75 ± 0.29a |
| Loss of proteoglycan | 2.88 ± 0.14 | 2.25 ± 0.19 | 2.50 ± 0.31 | 2.00 ± 0.22a | 2.38 ± 0.21 |
| Disorganization of chondrocytes | 2.75 ± 0.29 | 2.13 ± 0.14 | 2.25 ± 0.19 | 1.75 ± 0.19 | 2.25 ± 0.29 |
| Loss of chondrocytes | 3.25 ± 0.29 | 2.25 ± 0.19 | 2.38 ± 0.21 | 2.00 ± 0.22a | 2.00 ± 0.22a |
| Exposure of subchondral bone | 1.75 ± 0.42 | 1.88 ± 0.34 | 1.38 ± 0.21 | 1.25 ± 0.19 | 1.75 ± 0.19 |
| Cluster formation | 2.25 ± 0.29 | 2.00 ± 0.00 | 1.25 ± 0.19c | 1.25 ± 0.19c | 1.75 ± 0.36 |
| Overall score | 21.38 ± 1.66 | 17.38 ± 1.48 | 14.88 ± 1.10a | 12.63 ± 1.09a | 15.38 ± 1.11a |
Note: Mean ± standard error (SE). NS = normal saline; HA = hyaluronan.
P < 0.05 versus the NS group.
P < 0.01 versus the NS group.
P < 0.05 versus the HA group.
Frictional Test Findings
In preliminary testing, friction coefficients at 30°, 45°, and 60° flexion angle of the knee were examined in each group. Friction coefficients increased in the following order: 60°, 45°, and 30° (data not shown). Statistical comparisons among the experimental groups were based on results at 30° flexion of the knee. Significant increase in friction coefficients was found in the ACLT knees compared to the normal knees. Among the ACLT knees, there was no significant difference in the value between the HA group and the NS group. On the other hand, the coefficients of the AL1000 group and the AL430 group were significantly lower than those of the NS group (P < 0.005, 0.0294 ± 0.0012 in the NS group v. 0.0122 ± 0.0041 in the AL1000 group; P < 0.01, 0.0148 ± 0.003 in the AL430 group) (Fig. 4). The AL1000 group also significantly decreased the value compared to the HA group (P < 0.05, v. 0.0235 ± 0.0074 in the HA group) (Fig. 4).
Figure 4.
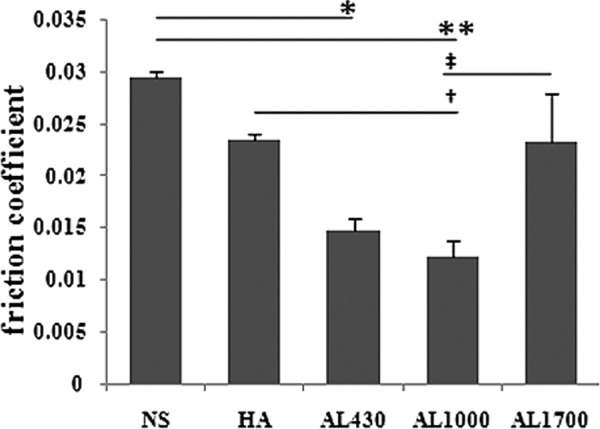
Results of the pendulum friction test. Values are the mean ± standard error (SE) friction coefficients. *P < 0.01 versus the normal saline (NS) group. **P < 0.005 versus the NS group. †P < 0.05 versus the hyaluronan (HA) group. ‡P < 0.05 versus the AL1700 group.
Discussion
The present study examined the antiarthritic effects of intra-articular administrations of a novel UPLE alginate on knee OA induced by ACLT in rabbits. To our knowledge, this is the first study to examine the influence of alginate materials on OA progression in vivo. The current OA model (NS group) showed significant degenerative changes in articular cartilage, similar to those observed in humans, at 9 weeks after surgery.
The histological results using the current OA model demonstrate a significant inhibition of OA progression by intra-articular administrations of UPLE alginate. Among the alginate treatment groups, the AL1000 group tended to inhibit the progression of OA changes compared to other groups. This result indicates that repeated intra-articular administrations of UPLE alginate have a preventive effect on OA progression. Regarding the molecular weight, AL1000 (1,000 kDa) is histologically more beneficial in preventing OA progression compared to AL430 (430 kDa) and AL1700 (1,700 kDa).
In terms of joint lubrication, the friction test using our system showed that the average friction coefficient of rabbit knees significantly increased from 0.0109 in the intact knee to 0.0294 in the OA knee induced by ACLT (NS group). The coefficient in the intact knee is consistent with values reported previously.27 This indicates that the current system was reliable for measuring the friction coefficient. The obtained results showed that the administrations of AL430 and AL1000 alginates significantly improved the friction coefficients of the ACLT knee. Especially, the AL1000 treatment significantly decreased the value compared to the HA treatment. By contrast, low molecular HA injection did not change the value. Kawano et al.27 demonstrated that both high molecular HA (2,000 kDa) and low molecular HA (800 kDa) injections did not significantly decrease the friction coefficient of instability-induced OA rabbits. Our result is consistent with this previous one.
A significant finding is that UPLE alginate administrations, especially AL1000, showed favorable effects on OA knees in both the histological grading and the friction test. Synovial joint lubrication comprises 2 elements, including fluid lubrication and boundary lubrication.28,29 Among the 2 elements, boundary lubrication helps to protect the articular cartilage against degenerative changes.27 In the present study, we lack definite information on the mechanism of influence of UPLE alginate administrations on joint lubrication. To confirm this point, further studies will be performed to measure the alteration in alginate concentration in joint fluid and in its layer thickness onto joint surfaces after intra-articular injection of different molecular weight UPLE alginates.
The current study indicates that the therapeutic effects of intra-articular alginate administrations on preventing OA progression are dependent on its molecular weight. Because no studies have shown such data, little is known regarding the mechanism of this molecular weight dependency. There is evidence obtained from experimental studies on intra-articular HA therapy to speculate on this mechanism. Although in vitro studies have generally suggested that high molecular weight HA is more biologically active than lower molecular weight HA,30-32 in vivo studies using experimental OA models treated with HA preparations have shown results that contrast with the results generated by those in vitro studies.32,33 Ghosh and Guidolin32 stated that this discrepancy was partly explained by the enhanced penetration of the lower molecular weight HA through the extracellular matrix of tissues, such as the synovium, thereby maximizing its concentration and facilitating its interaction with target cells. Our in vitro study showed that AL1700 had excellent chondrogenic potential.21 However, this high molecular weight alginate may not penetrate the extracellular matrix of cartilage and synovium to interact with chondrocytes, mesenchymal stromal cells, and synovial cells in the extracellular matrix.
Currently, when OA advances to the stage of severe pain and functional impairment, total joint arthroplasty is the sole remaining treatment. However, total joint arthroplasty is a cost-intensive procedure and still has unsolved limitations, such as implant loosening, postoperative infection, fat embolism, or deep vein thrombosis. Therefore, efficacious therapies for preventing the disease progression are needed. Intra-articular HA injections have been widely used as such a treatment for OA. Despite a long history of its clinical usage, the efficacy of intra-articular HA injections in the treatment of OA is still controversial. Large placebo-controlled randomized trials showed no measurable efficacy in treating symptomatic OA compared with placebo treatments.14-16 The current study shows the potential for intra-articular injections of UPLE alginate, especially AL1000, to be more effective than that of HA in preventing OA progression. Alginate materials can maintain the phenotype of chondrocytes and enhance the chondrogenesis of stem or progenitor cells.21-23 Therefore, alginate materials have been widely applied to scaffold or cell carrier biomaterials for cartilage tissue engineering.18,19 In addition, the authors clarified that the current UPLE alginate had superior chondrogenic potential compared to other commercial alginates.21 These favorable properties of alginate are supportive rationale for the application of this biomaterial as an intra-articular injection agent for the treatment of OA. Furthermore, our ultrapurified alginate with extremely low endotoxin levels can assure safe clinical usage.
There were several limitations to this study. First, our results were mainly derived from a rabbit OA model. In order to consider clinical application, we should obtain more histological and biomechanical information from a large animal OA model. Second, we assessed each knee sample at 9 weeks postoperatively. The data based on samples at a longer postoperative period will be required for the clinical application of the current material. Finally, biological effects of different molecular weight alginates on synovium need to be elucidated. Finally, the metabolism of intra-articular–injected UPLE alginates was not clarified. The elucidation of the metabolism in living joints will help establish an adequate treatment protocol, including the material dose and interval and frequency of injection, for UPLE alginate injection into OA joints.
In conclusion, our findings suggest that intra-articular administrations of UPLE alginate are effective in preventing articular cartilage degeneration and improving joint lubrication of OA knees induced by ACLT. In terms of the molecular weight dependency, AL1000 (1,000 kDa) has more therapeutic effects on OA progression compared to AL430 (430 kDa), AL1700 (1,700 kDa), and HA. Based on our results, we may reasonably conclude that our novel alginate material has promising potential as an effective agent in the treatment of OA.
Acknowledgments
The authors thank Mr. M. Hamilton (Tokai University) for help in article preparation and Mr. S. Shimizu and Mr. N. Ohzawa for assistance in material preparations.
Footnotes
This study was funded by the “Development of a Novel Injectable Material for the Treatment of Joint Disorders” Science and Technology Incubation Program in Advanced Regions, Japan Science and Technology Agency.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1. Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86-A:538-45. [DOI] [PubMed] [Google Scholar]
- 2. Goldberg VM, Buckwalter JA. Hyaluronans in the treatment of osteoarthritis of the knee: evidence for disease-modifying activity. Osteoarthritis Cartilage. 2005;13:216-24. [DOI] [PubMed] [Google Scholar]
- 3. Cubukcu D, Ardic F, Karabulut N, Topuz O. Hylan G-F 20 efficacy on articular cartilage quality in patients with knee osteoarthritis: clinical and MRI assessment. Clin Rheumatol. 2005;24:336-41. [DOI] [PubMed] [Google Scholar]
- 4. Listrat V, Ayral X, Patarnello F, Bonvarlet JP, Simonnet J, Amor B, et al. Arthroscopic evaluation of potential structure modifying activity of hyaluronan (Hyalgan) in osteoarthritis of the knee. Osteoarthritis Cartilage. 1997;5:153-60. [DOI] [PubMed] [Google Scholar]
- 5. Altman RD, Moskowitz R. Intraarticular sodium hyaluronate (Hyalgan) in the treatment of patients with osteoarthritis of the knee: a randomized clinical trial. Hyalgan Study Group. J Rheumatol. 1998;25:2203-12. [PubMed] [Google Scholar]
- 6. Lohmander LS, Dalen N, Englund G, Hamalainen M, Jensen EM, Karlsson K, et al. Intra-articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Ann Rheum Dis. 1996;55:424-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puhl W, Bernau A, Greiling H, Kopcke W, Pforringer W, Steck KJ, et al. Intra-articular sodium hyaluronate in osteoarthritis of the knee: a multicenter, double-blind study. Osteoarthritis Cartilage. 1993;1:233-41. [DOI] [PubMed] [Google Scholar]
- 8. Strauss EJ, Hart JA, Miller MD, Altman RD, Rosen JE. Hyaluronic acid viscosupplementation and osteoarthritis: current uses and future directions. Am J Sports Med. 2009;37:1636-44. [DOI] [PubMed] [Google Scholar]
- 9. Kikuchi T, Yamada H, Shimmei M. Effect of high molecular weight hyaluronan on cartilage degeneration in a rabbit model of osteoarthritis. Osteoarthritis Cartilage. 1996;4:99-110. [DOI] [PubMed] [Google Scholar]
- 10. Amiel D, Toyoguchi T, Kobayashi K, Bowden K, Amiel ME, Healey RM. Long-term effect of sodium hyaluronate (Hyalgan) on osteoarthritis progression in a rabbit model. Osteoarthritis Cartilage. 2003;11:636-43. [DOI] [PubMed] [Google Scholar]
- 11. Wenz W, Breusch SJ, Graf J, Stratmann U. Ultrastructural findings after intraarticular application of hyaluronan in a canine model of arthropathy. J Orthop Res. 2000;18:604-12. [DOI] [PubMed] [Google Scholar]
- 12. Hulmes DJ, Marsden ME, Strachan RK, Harvey RE, McInnes N, Gardner DL. Intra-articular hyaluronate in experimental rabbit osteoarthritis can prevent changes in cartilage proteoglycan content. Osteoarthritis Cartilage. 2004;12:232-8. [DOI] [PubMed] [Google Scholar]
- 13. Armstrong S, Read R, Ghosh P. The effects of intraarticular hyaluronan on cartilage and subchondral bone changes in an ovine model of early osteoarthritis. J Rheumatol. 1994; 21:680-8. [PubMed] [Google Scholar]
- 14. Lo GH, LaValley M, McAlindon T, Felson DT. Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. JAMA. 2003;290:3115-21. [DOI] [PubMed] [Google Scholar]
- 15. Felson DT, Anderson JJ. Hyaluronan sodium injections for osteoarthritis. Arch Intern Med. 2009;162:245-7. [DOI] [PubMed] [Google Scholar]
- 16. Richette P, Ravaud P, Conrozier T, Euller-Ziegler L, Mazieres B, Maugars Y, et al. Effect of hyaluronic acid in symptomatic hip osteoarthritis: a multicenter, randomized, placebo-controlled trial. Arthritis Rheum. 2009;60:824-30. [DOI] [PubMed] [Google Scholar]
- 17. Mierisch CM, Cohen SB, Jordan LC, Robertson PG, Balian G, Diduch DR. Transforming growth factor-beta in calcium alginate beads for the treatment of articular cartilage defects in the rabbit. Arthroscopy. 2002;18:892-900. [DOI] [PubMed] [Google Scholar]
- 18. Mierisch CM, Wilson HA, Turner MA, Milbrandt TA, Berthoux L, Hammarskjold ML, et al. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85-A:1757-67. [DOI] [PubMed] [Google Scholar]
- 19. Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277-97. [DOI] [PubMed] [Google Scholar]
- 20. Iwasaki N, Yamane S, Majima T, Minami A, Harada K, Nonaka S, et al. Feasibility of polysaccharide hybrid materials for scaffolds in cartilage tissue engineering: evaluation of chondrocyte adhesion to polyion complex fibers prepared from alginate and chitosan. Biomacromolecules. 2004;5:828-33. [DOI] [PubMed] [Google Scholar]
- 21. Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res A. 2010;94:844-55. [DOI] [PubMed] [Google Scholar]
- 22. Steinert A, Weber M, Dimmler A, Julius C, Schutze N, Noth U, et al. Chondrogenic differentiation of mesenchymal progenitor cells encapsulated in ultrahigh-viscosity alginate. J Orthop Res. 2003;21:1090-7. [DOI] [PubMed] [Google Scholar]
- 23. Paige KT, Cima LG, Yaremchuk MJ, Schloo BL, Vacanti JP, Vacanti CA. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg. 1996; 97:168-78, discussion 179-80. [DOI] [PubMed] [Google Scholar]
- 24. Igarashi T, Iwasaki N, Kawamura D, Kasahara Y, Abe R, Izumisawa Y, et al. Repair of articular cartilage defects with a novel injectable in-situ forming material in a canine model. Trans Annu Meet Orthop Res Soc. 2009;55:287. [DOI] [PubMed] [Google Scholar]
- 25. Matsuhashi T, Iwasaki N, Nakagawa H, Hato M, Kurogochi M, Majima T, et al. Alteration of N-glycans related to articular cartilage deterioration after anterior cruciate ligament transection in rabbits. Osteoarthritis Cartilage. 2008;16:772-8. [DOI] [PubMed] [Google Scholar]
- 26. Yoshioka M, Shimizu C, Harwood FL, Coutts RD, Amiel D. The effects of hyaluronan during the development of osteoarthritis. Osteoarthritis Cartilage. 1997;5:251-60. [DOI] [PubMed] [Google Scholar]
- 27. Kawano T, Miura H, Mawatari T, Moro-Oka T, Nakanishi Y, Higaki H, et al. Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis. Arthritis Rheum. 2003;48:1923-9. [DOI] [PubMed] [Google Scholar]
- 28. Linn FC, Radin EL. Lubrication of animal joints, 3: the effect of certain chemical alterations of the cartilage and lubricant. Arthritis Rheum. 1968;11:674-82. [DOI] [PubMed] [Google Scholar]
- 29. Walker PS, Dowson D, Longfield MD, Wright V. Lubrication of human joints. Ann Rheum Dis. 1969;28:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hegewald AA, Ringe J, Bartel J, Kruger I, Notter M, Barnewitz D, et al. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 2004; 36:431-8. [DOI] [PubMed] [Google Scholar]
- 31. Kikuchi T, Yamada H, Fujikawa K. Effects of high molecular weight hyaluronan on the distribution and movement of proteoglycan around chondrocytes cultured in alginate beads. Osteoarthritis Cartilage. 2001;9:351-6. [DOI] [PubMed] [Google Scholar]
- 32. Ghosh P, Guidolin D. Potential mechanism of action of intra-articular hyaluronan therapy in osteoarthritis: are the effects molecular weight dependent? Semin Arthritis Rheum. 2002;32:10-37. [DOI] [PubMed] [Google Scholar]
- 33. Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;61:125-35. [DOI] [PubMed] [Google Scholar]