Abstract
Tissues, such as bone, tendon, and ligaments, contain a high fraction of components with “short” and “ultrashort” transverse relaxation times and therefore have short mean transverse relaxation times. With conventional MRI sequences that employ relatively long echo times (TEs), there is no opportunity to encode the decaying signal of short and ultrashort T2/T2* tissues before it has reached zero or near zero. The clinically compatible ultrashort TE (UTE) sequence has been increasingly used to study the musculoskeletal system. This article will review the UTE sequence as well as various modifications that have been implemented since its introduction. These modifications have been used to improve efficiency or contrast as well as provide quantitative analysis. This article also reviews several clinical musculoskeletal applications of UTE.
Keywords: ultrashort TE, musculoskeletal tissues, quantitative MRI, bi-component analysis
Introduction
All biological tissues are heterogeneous and contain a combination of components, each with an individual transverse relaxation time (T2 and T2*). The observed signal intensity of a tissue at the time of magnetic resonance (MR) imaging depends on many variables, including the mean transverse relaxation time of the components within a voxel. One commonly used classification scheme defines transverse relaxation values less than 0.1 ms as “supershort”, 0.1 – 1 ms as “ultrashort”, 1-10 ms as “short” and greater than 10 ms as “long” (1). Some tissues of the musculoskeletal system contain a high fraction of components with long transverse relaxation times, such as cartilage, and therefore have long mean transverse relaxation times. Other tissues, such as bone, tendon, and ligaments, contain a high fraction of components with “short” and “ultrashort” transverse relaxation times and therefore have short mean transverse relaxation times.
With conventional MRI sequences that employ relatively long echo times (TEs), there is limited opportunity to encode the decaying signal of short and ultrashort T2/T2* tissues before it has reached zero or near zero. Although there remains clinical value with seeing the outline of the short T2/T2* tissues, such as hypo-intense tendon outlined by hyper-intense fat, a significant amount of information is lost by not being able to directly view the tissue and its internal structure (Figure 1). Furthermore, there is no possibility of quantification, such as measuring longitudinal (T1) or transverse (T2 or T2*) relaxation times.
Figure 1.
Axial images of an asymptomatic volunteer after Achilles tendon repair. Proton density FSE image using 6.6 ms TE (A) shows no signal within the Achilles tendon (arrow). 3D-UTE-Cones image using 30 μs TE (B) shows internal structure of the repaired Achilles tendon. 30 μs minus 6.6 ms subtraction image (C) highlights short T2* components, which are more abundant in between healed fascicles. Images courtesy of Michael Carl, PhD.
A family of clinically compatible sequences is capable of providing TE values less than 1 ms (1). These can be collectively referred to as the ultrashort TE (UTE) group of sequences, although zero TE (ZTE) is also a member of this family. Since their introduction (2), UTE sequences have been employed on clinical MR systems from every major manufacturer and have been increasingly used to study the musculoskeletal system. With UTE, TEs as short as 8 μs (0.008 ms) have been achieved, although TE values ranging from 20 – 100 μs are more typical.
Images acquired with UTE sequences can be used for qualitative evaluation, such as for alteration of the calcified layer of articular cartilage or tendon ultrastructure. Alternatively UTE sequences can allow for quantitative evaluation, such as evaluation of bi-component fraction of tendon. Furthermore, preparatory pulses can be combined with UTE to sensitize the sequence to particular proton pools, such as for the evaluation of the slow molecular interactions between water and proteoglycans (T1ρ) (3).
This article will review the UTE sequence as well as various modifications that have been implemented since its introduction. These modifications have been used to improve efficiency or contrast as well as provide quantitative analysis. We will also review select clinical musculoskeletal applications of UTE. This review will only focus on proton imaging, although UTE sequences can be useful for imaging of other nuclei including phosphorus or sodium.
UTE Techniques
Conventional echo-forming sequences, such as RARE (rapid acquisition with relaxation enhancement, also known as fast spin echo) and gradient-recalled echo (GRE), cannot be used to generate echo times less than 1 ms on clinical systems. Using these sequences, the majority of signal from short components has decayed prior to echo formation. In fact, significant transverse relaxation can occur even during radiofrequency (RF) pulse excitation.
The UTE sequence uses a short RF pulse and acquires data as soon as possible after excitation finishes. Data acquisition occurs while the readout gradient is being ramped on (Figure 2). The sampling pattern of k-space is radial, filled from the center out, and is nonlinear in time (4). Slice-selection with 2D imaging is achieved with two excitations, each half of a conventional slice selection pulse. The first pulse uses a positive gradient and the second a negative gradient, together resulting in the same scenario as a single complete excitation pulse (2). Half pulses can also be used for volumetric imaging with a variety of methods, each with challenges and proposed solutions (5). Alternatively, 3D imaging can be achieved with a non-selective (hard) excitation pulse followed by a 3D radial acquisition (4). Judicious use and placement of coils allows for signal to originate mainly from the structures of interest. For instance, the use of a surface coil can allow small field-of-view imaging of a superficial anatomic structure without aliasing from deeper structures that are not of interest.
Figure 2.
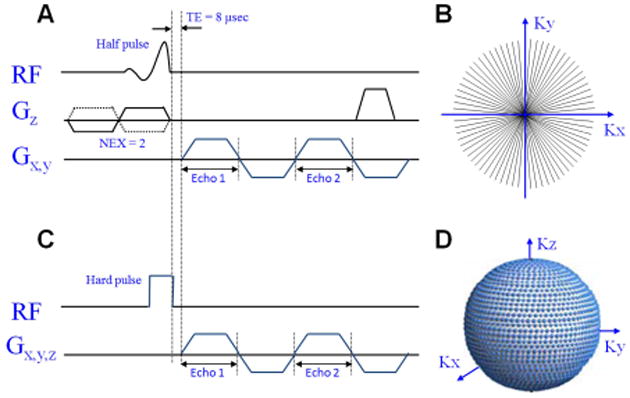
Ultrashort TE (UTE) pulse sequence diagrams and k-space trajectories. 2D-UTE sequence uses a slice-selective half-pulse excitation followed by ramp sampling (A) to fill k-space (B). 3D-UTE sequence uses a short rectangular hard pulse excitation followed by ramp sampling (C) to fill k-space (D).
One of the limitations of radial acquisition is that many more acquisitions are required to satisfy the Nyquist criterion compared with rectangular (Cartesian) sampling. To improve overall efficiency, a number of modifications have been proposed to fill k-space, including twisted, spiral, and cones projection methods (6,7). Other UTE-type sequences that have been used to image species with ultrashort transverse relaxation times include the acquisition-weighted stack of spirals (AWSOS) and variable TE (vTE) sequences. The AWSOS sequence uses selective excitation, variable-duration slice encoding, and mobile spiral readout to sample a cylindrical volume in k-space (8). The vTE sequence uses Cartesian k-space sampling, but TE is shortened with asymmetric RF pulses, partial echoes, and ramp sampling (9). Although all of these methods result in improved overall efficiency compared with radial acquisition, usually longer readout durations are required and blurring may result.
An alternative approach to imaging of tissues with short transverse relaxation is with the application of the readout gradient prior to excitation. The RF pulse that is applied can be a short, hard pulse, referred to as the zero echo time (ZTE) technique (10) or can be a longer, frequency-modulated pulse with interleaved transmit-receive operation, known as sweep imaging with Fourier transformation (SWIFT) (11). The elimination of rapidly switching gradients between TR intervals has many benefits, including decreased acoustic noise and reduced eddy current effects. These sequences are inherently 3D due to the presence of the readout gradient, which prevents simultaneous application of a slice selection gradient in another direction (10). The dead time that is caused by the finite duration of the RF pulse, transmit-receive switching, and digital bandpass filtering results in a gap of data at the center of k-space (10). The missing data challenge can be addressed with acquisition oversampling and algebraic reconstruction in the ZTE technique (10) or filled with a Cartesian single-point imaging technique, known as pointwise encoding time reduction with radial acquisition (PETRA) (12). A limitation of these techniques is less versatile contrast manipulation, although inversion preparation pulses can create T1 contrast (13,14).
Qualitative Imaging for Morphologic or Anatomic Analysis
With the UTE technique, it is possible to obtain signal from tissues with short and ultrashort mean transverse relaxation times. However, images are largely proton-density weighted and contrast between adjacent musculoskeletal structures is often not as dramatic as with conventional images. This highlights the need for manipulation of signal to optimally evaluate tissues and tissue components with rapid transverse relaxation.
Image contrast can be manipulated with appropriate selection of TE, where longer TEs add T2* weighting. Sequences that lack a refocusing pulse are sensitive to both macroscopic and microscopic susceptibility effects (T2*), although use of ultrashort TEs minimizes dephasing. Another basic method is with image subtraction between an UTE image and a second gradient echo image with much longer TE. The UTE image will capture signals from both long and short T2* components whereas the longer TE image will capture signal from the long T2* components. Subtraction of the two images will result in selective display of short T2* components (Figure 1). Rescaling of the UTE image prior to subtraction can be performed to decrease the signal from muscle and fat relative to the unscaled longer TE image, resulting in increased contrast of the short T2* components (15). Subtraction techniques are sensitive to patient in-plane and through-plane motion between source images.
Preparation pulses prior to UTE acquisition can introduce additional forms of contrast. T1-weighting can be performed with saturation recovery, where a short rectangular, nonselective 90° pulse (e.g., 256 μs in duration) is delivered followed by a larger crusher gradient (16). Longitudinal magnetizations of both short and long T2* species are saturated and more rapid longitudinal recovery of short T1 components allows for T1-weighting (Figure 3).
Figure 3.
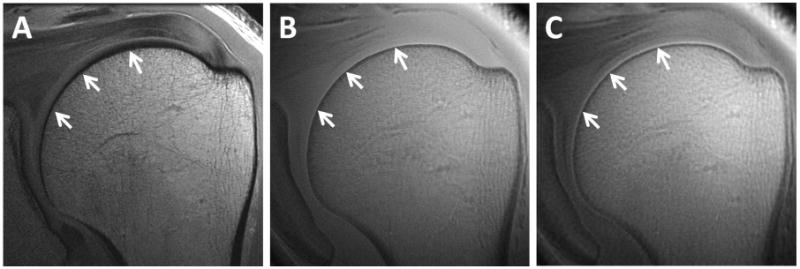
Coronal oblique images of the left shoulder. High-resolution conventional FSE sequence with 23 ms TE (A) shows no signal from the calcified cartilage (arrows). 2D-UTE image with 8 μs TE (B) shows signal but poor contrast from the calcified cartilage (arrows). T1-weighted saturation recovery image with 2D-UTE acquisition (C) highlights the calcified cartilage (arrows).
Preparation pulses can also be used to suppress long T2* species, such as with long T2* saturation or inversion and signal nulling (17). Long T2* saturation techniques use a long 90-110° RF pulse followed by a large crusher gradient (17,18). The short T2* components are then selectively detected with the UTE sequence since they experienced significant transverse relaxation during the long RF preparation pulse and were able to significantly recover their longitudinal magnetization. Two consecutive saturation pulses and subsequent spoiling gradients can be used for combined water- and fat-suppression, known as water- and fat-suppressed projection imaging (WASPI) (17). A limitation of saturation techniques that use hard RF pulses is that they are more sensitive to B0 and B1 inhomogeneities compared with the adiabatic inversion and signal nulling techniques. Long T2* inversion with adiabatic 180° pulses have been used to overcome this limitation (16). Similar to the long T2* saturation technique, the short T2* components are not inverted, but partially saturated during the long T2* inversion process. After an appropriate inversion time, the long T2* signals are nulled and only the short T2* signals are detected with the UTE sequence. A single inversion pulse can be used to reduce approximately 80% of the signal of both fat and long-T2* components (such as muscle) (16) or dual inversion pulses can be used for complete nulling of both (19,20).
Off-resonance saturation with subtraction can also provide contrast for the short T2* components (21). This technique relies on the fact that short T2* components have broad spectral linewidths compared with long T2* components such as fat and water. A high power saturation pulse placed +1 to +2 kHz away from the water peak results in suppression of the short T2* components. Subtraction of two UTE images acquired with and without the off-resonance preparation pulse will accentuate the short T2* components.
Phase differences between tissues and tissue components can also be used to generate contrast with UTE imaging. Phase evolution has been shown to occur during the RF pulse and readout periods of the UTE sequence (22). Phase images may generate more contrast than magnitude images (18) and susceptibility weighted images can also be performed when both care combined.
Fat suppression can be important to increase conspicuity of musculoskeletal tissues. Unfortunately a chemically selective technique, which is the most commonly used in clinical practice, is problematic when the objective is to image short T2* tissues. The broad spectral linewidths of the short T2* components overlap with the main fat peak and the fat-saturation pulse may inadvertently cause direct saturation of the components of interest. The combination of UTE with a chemical shift based water-fat separation method (Iterative Decomposition of Water and Fat With Echo Asymmetry and Least-Squares Estimation, IDEAL) has been successfully implemented to overcome this challenge (23). The UTE-IDEAL sequence utilizes multiple echoes which not only allow for multi-frequency fat spectral modeling, but also provides quantitative T2* information.
Quantitative Imaging
T2* relaxation time is dependent on interactions between spins, tissue hydration, and susceptibility effects (24). T2*quantification is made possible with the acquisition of a series of images with constant repetition time (TR) and variable TE. Tissues with short T2* can be quantified with the UTE sequence. However, increases in TE result in different patterns of eddy currents, leading to variations in slice profile and different degrees of long T2* contamination (25). A number of approaches have been used to reduce errors (18,26). Additionally, quantification of short T2* signal has been performed following long T2* suppression (16).
T2* can be calculated with a mono-exponential decay model or with a multi-exponential decay model. While multi-exponential T2* analysis may provide unique and useful information, a number of challenges exist. These include sensitivity to spatial resolution, image signal-to-noise ratio, the number and spacing of echoes, the number of fitting components, and the differences between component T2* values (27). Knowledge of the total number of components and transverse relaxation time of each individual component in a particular tissue is based on data acquired with high-performance spectroscopic systems using Carr–Purcell–Meiboom–Gill (CPMG) sequences and nonnegative least squares (NNLS) approaches. Although this allows for no a priori assumptions about the total number and T2 relaxation of components, it cannot be used to characterize short T2 tissues on clinical MR systems due to minimal TE limitations. It is assumed that the number and relationship between components made with CPMG and UTE sequences at various field strengths is similar, but UTE sequences will incorporate local susceptibility effects resulting in a shortening of T2* relative to T2. Multi-component analysis in humans is typically performed with the assumption of two components. Use of UTE sequences allows detection of a “short” T2* component (containing short and ultrashort components) and a “long” T2* component (28). In vivo bi-component analysis has been successfully employed with multiple tissues of the musculoskeletal system (25) (Table 1).
Table 1. Approximate T2* Analysis and Composition of Select Musculoskeletal Tissues.
| Mono- exponential UTE T2* |
Bi-Exponential T2* Analysis | Composition | Refs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Short Fraction |
Short T2* |
Long Fraction |
Long T2* |
Water Fraction |
Collagen Fraction (dry weight) |
Proteoglycan Fraction (dry weight) |
Other (dry weight) |
|||
| Cartilage | 10–28 ms | 18% | 0.5 ms | 82% | 35 ms | 65-80% | 60% | 12% | (28,38-40,53) | |
| TMJ disc | 8–19 ms | 80% | 62% | 3% | (77,80) | |||||
| Knee meniscus | 5–8 ms | 49% | 2 ms | 51% | 19 ms | 70% | 60-70% | 2–8% | (67,98) | |
| Tendon | 1–2 ms | 70-90% | < 1 ms | 10-30% | 8-20 ms | 66-75% | 65-87% | 0.2–5% | (28,81-84,99,100) | |
| Bone (cortical) | 0.4–0.8 ms | 65-95% | 0.3 ms | 5-35% | 2 ms | 20% | 30-35% (collagen and protein) | 65-70% calcium phosphate | (16,54,61) | |
The magic angle effect causes variations in transverse relaxation values dependent on collagen fiber orientation relative to B0 (29). Transverse relaxation is shortest when fibers are oriented at 0° and longest when they are oriented at 54.7°. While this phenomenon can be used to improve contrast, it confounds the quantitative evaluation of highly oriented collagen-rich tissues in the clinical setting. The magnitude of the effect on transverse relaxation depends on sequence type and parameters, but intensity variations greater than a factor of 10 have been demonstrated (29). Furthermore, results from spectroscopic systems have shown that both the total number and individual component T2 relaxation times vary depending on orientation in the magnet (30). Despite this, studies performed on clinical MR systems with bi-component UTE T2* analysis suggest that fractional analysis is much less sensitive to magic angle effects compared with single component analysis (31). For instance, Pauli et al showed that one histologically normal patellar cartilage specimen demonstrated CPMG-measured T2 values varying from 30.7 to 79.3 ms, dependent on fiber orientation with the main magnetic field. For the same full-thickness ROIs, short T2* water fractions ranged from 18.8 to 20.7%, appearing much less sensitive to the magic angle effect (31).
T1 relaxation time is dependent on tissue water content and the macromolecular environment of the tissue (24). Rapid T1 quantification of short T2* tissues is possible with the UTE technique combined with a saturation recovery technique for 2D imaging (16,18) or with a variable flip angle (VFA) method for 3D imaging (32). The VFA method relies on the acquisition of 3D UTE datasets with two through six optimized flip angles. Both the saturation recovery and VFA methods demonstrate good agreement with each other (33). Specifically, Wright et al measured T1 values in volunteer Achilles tendons and mean T1 for the regions of interest were 725 ± 42 ms and 698 ± 54 ms for the saturation recovery and VFA UTE techniques, respectively, without significant difference between the two measurements (33).
T1ρ quantification allows for the assessment of extremely slow molecular motions (34). T1ρ has been used to investigate large macromolecules such as proteoglycans and their characteristic polysaccharide chains known as glycosaminoglycans (GAGs). In T1ρ imaging, longitudinal magnetization is brought into the transverse plane with a 90° pulse and subsequently locked with a spin-lock pulse. During the locking pulse, magnetization relaxes by the time constant T1ρ. A -90° pulse is then delivered to flip the magnetization back into the longitudinal plane with subsequent delivery of a crusher gradient to spoil residual transverse magnetization. Magnetization stored in the longitudinal axis is then read out by the UTE sequence, which allows for measurements to be made in short T2* tissues. T1ρ is quantified by repeating the experiment with various spin lock times and fitting the data to an exponential equation (3).
Magnetization transfer (MT) contrast is based on interactions between the free and restricted proton pools. MT may provide unique information about the microscopic and chemical environment. The MT sequence employs an off-resonance RF saturation pulse, which is typically far from the narrow peak of free water. The RF pulse affects the short T2* components, which have broad spectral linewidths. Exchange with the free water pool leads to a loss of longitudinal magnetization. Combination with the UTE sequence allows for the assessment of the MT effect in short T2* tissues. The MT ratio (MTR) is a useful measure to quantify the MT effects and is derived from one image without the MT preparation pulse (M0) and one image with the MT pulse (MSAT).
| [1] |
Measurement of MT is particularly challenging with short T2* tissues since the RF pulse may directly saturate the free proton pool (35). Furthermore, MT measurements are sensitive to many technical variables and are highly dependent on the specific imaging parameters of the applied pulse sequence. When comparing longitudinal measurements of MTR, one should use the same frequency and power values for the off-resonance pulse. Additionally, the same field strength should be used since MTR is affected by T1 relaxation time and therefore measurements at 3T are expected to yield slightly lower values compared with 1.5T (36). MTR is much less sensitive to magic angle effects compared with transverse relaxation times (37).
Applications
To date, UTE sequences have been used to study many tissues of the musculoskeletal system. This section will focus on anatomical considerations unique to individual tissues as well as review qualitative and quantitative clinical applications.
Articular cartilage
Anatomical Considerations
Articular cartilage refers to hyaline cartilage which lines the ends of the bones in diarthrodial joints. Chondrocytes are scarce in cartilage, but are responsible for producing and maintaining the extracellular matrix (ECM). Cartilage is composed of approximately 65 – 80% water (38) and of the dry weight, approximately 60% is collagen and 12% is sulfated proteoglycan (39,40). Collagen fibrils are predominantly composed of type II collagen. Type X collagen is found in calcified cartilage (41). Aggrecan is the major proteoglycan in articular cartilage, containing chondroitin sulfate and keratin sulfate (GAG) chains.
Articular cartilage can roughly be separated into four zones: the most superficial zone, tangential zone, middle zone, and deep zone (42,43). In general, collagen fibrils vary from being fine and parallel at the articular surface to thicker and perpendicular at the deep zone (44). Furthermore, the collagen network has a preferred orientation known as the split-line direction. Proteoglycan content also shows depth variation, increasing with depth from the surface (45).
The deep zone of cartilage is attached to subchondral bone through a metabolically active region of calcified cartilage, ranging from 79 – 243 μm in thickness (46). With advancing age, the calcified cartilage decreases in thickness. In addition, the number of tidemarks, which represents the junction between the calcified and uncalcified cartilage, also increases with advancing age such that after age 70 nearly all tidemarks will be duplicated with up to four being present (46).
Imaging
Cartilage has a high fraction of long T2* components and therefore cartilage demonstrates high signal with both conventional and UTE sequences. However, with the UTE sequence, signal is also obtained from the short T2* components. Of particular interest is the calcified layer of cartilage, which may be involved in the pathogenesis of chondral degeneration (47). The UTE sequence has been shown to be sensitive to the presence of the calcified cartilage layer (48), largely due to short T1 relaxation. With subtraction or application of preparation pulses such as single or double inversion recovery, selective visualization of the calcified layer can be achieved (19) (Figure 4).
Figure 4.
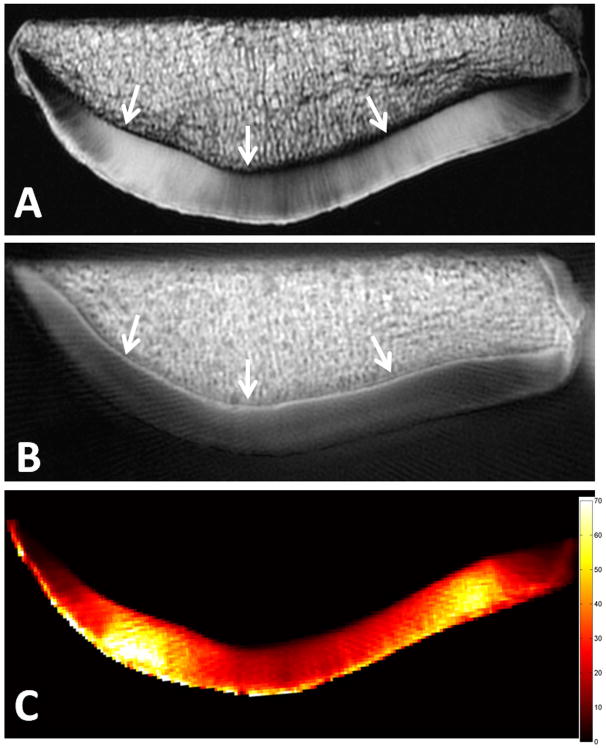
Axial MR images of a patella. High resolution spin-echo sequence with 38 ms TE (A) shows no signal from the deep radial and calcified cartilage (arrows). 8 μs minus 5 ms subtraction image (B) highlights the calcified layer of cartilage (arrows). Pixel map of mono-exponential T2* values generated from UTE images (C) with bi-exponential analysis yielding short T2* of 0.7 ms, short fraction 23%, long T2* 37.7 ms, and long fraction 77% (plot not shown).
Studies performed with articular cartilage on spectroscopic systems suggest that there are up to four pools of protons, each with distinct relaxation times, including bulk water, water trapped between collagen fibers, water associated with proteoglycans, and water associated with collagen (49). The UTE sequence allows for detection of all components except for water associated with collagen, which has supershort transverse relaxation (T2* less than 20 μs) (50). Compared with conventional sequences, detection of these additional components may be useful in determining early stages of disease. Using a mono-exponential decay model, Williams et al found that UTE-T2* values were more sensitive to matrix degeneration compared with conventional T2 values based on histological standards (51). However, in contrast to conventional techniques, the UTE-T2* values decreased with increasing degeneration.
It has been shown that four types of T2* decay patterns can be visualized on clinical MR systems (52), but a bi-component model is the best that can be achieved within clinically allowable imaging times and may be sufficient to describe the decay pattern in cartilage (50). Pauli et al found a significant correlation between increasing short T2* fractions and worsening degrees of degeneration (31).
Evaluation of reproducibility is an integral part of the validation of new imaging techniques. There are limited studies to date presenting reproducibility data for quantitative measures with the UTE techniques. However, one study performed by Williams et al imaged 11 asymptomatic subjects on three consecutive days using UTE-T2* mapping and found intersession precision error of 8% which corresponded to a mean T2* of 1.2 ms (53). Intra-observer reliability was also assessed in the same study and was found to be excellent.
Bone
Anatomical Considerations
Bone is composed of approximately 15% water by volume, either bound to collagen or residing within spaces, and 85% type I collagen and calcium hydroxyapatite (54). From a structural perspective, the periosteum, cortical bone, trabecular bone, and marrow elements are distinct.
Periosteum is a membrane that covers the outer surface of nearly all bones except at the joints. It is composed of a superficial fibrous layer and an inner, thinner osteogenic layer (55). Total periosteal thickness in human mid-diaphyseal regions is about 100 μm for both tibia and femora, with mean fibrous layer thicknesses of 72-77 μm and mean inner layer thickness of 29-23 μm (56). Cortical bone is the dense outer layer, composing approximately 80% of the total mass of the adult skeleton, with approximately 5-10% porosity (57). Cortical bone pores consist of Haversian canals, Volkmann's canals, resorption cavities, lacuna, and canaliculi. These are occupied by blood vessels, nerves, or osteocytes. Trabecular bone is predominantly found in the axial skeleton as well as near the joints of long bones and demonstrates 75-95% porosity (57). It consists of a three-dimensional structure of interconnected plates and rods, filled with bone marrow. Bone marrow may be red or yellow, each with a different chemical composition. Red marrow contains approximately 40% fat, 40% water, and 20% protein and yellow marrow contains approximately 80% fat, 15% water, and 5% protein (58). Precise composition of marrow varies by location and is dependent on age, gender, individual health, and other variables.
Imaging
Normal periosteum as well as alterations due to age, acute fracture or chronic injury can be readily visualized with UTE imaging (55). Although reported mono-exponential T2* values for adult periosteum range from 5 – 11 ms, there is a sufficiently high proportion of short T2* components that signal is readily seen on subtraction between UTE and longer TE images (55).
Studies performed with cortical bone on spectroscopic systems have found three pools of protons, including collagen methylene protons, collagen-bound water, and a broad peak consisting of pore water and lipid (59). UTE sequences can detect all but collagen methylene protons whose T2* values are supershort (less than 50 μs) (60). Mono-exponential T2* values obtained with the UTE sequence have been reported to range from 0.39 – 0.5 ms (16,61). Separation of collagen-bound water from pore water is important since the two are associated with different contributions to bone quality and strength. Expansion of pore volume due to aging or osteoporosis weakens bone (59).
A wide variety of UTE-based techniques have been used to qualitatively and quantitatively evaluate the components of cortical bone water (18). Based on relaxation differences, clinically compatible techniques that can distinguish between bound and pore water include use of bi-component analysis, single adiabatic inversion, or double adiabatic inversion (62) (Figure 5). More recently, two UTE-derived indices that have been significantly correlated with micro-CT porosity are the porosity index (63) and suppression ratio (64), both which have the potential to be readily implemented into clinical practice. The porosity index is calculated from two acquisitions, one with an 50 μs TE, capturing signal from pore water and collagen-bound water, and a second with a 1.2 ms TE, capturing only signal from pore water. Porosity index is defined as the ratio of mean intensity in the 1.2 ms TE image to that of the 50 μs TE image. The suppression ratio is also calculated from two acquisitions, one image with long T2* suppression and an unsuppressed image. The suppression ratio is defined as the ratio of mean intensity between the unsuppressed to suppressed images. The concept is that suppression will increase with increasing pore sizes, which are associated with longer T2* values.
Figure 5.
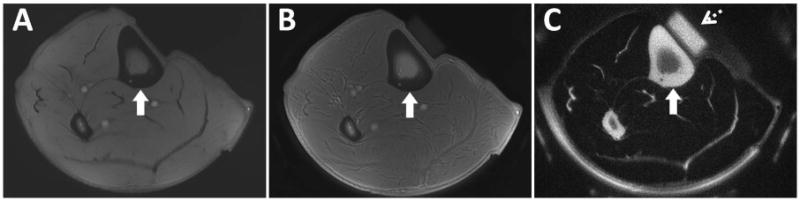
Axial MR images through the leg of a healthy volunteer. Clinical gradient echo sequence (A) shows a signal void in the region of the tibial cortex (arrow). The UTE sequence (B) shows slightly higher signal from the tibial cortex (arrow), but poor contrast due to the high signal from the surrounding muscle and fat which limit the dynamic range for cortical bone. The IR-UTE sequence (C) selectively suppresses signal from fat and muscle creating high contrast for the short T2* components of cortical bone (arrow). An eraser (dashed arrows) with known T1 and T2* values (approximately 200 ms and 300 μs, respectively) was placed for reference.
UTE techniques have not been as widely explored for trabecular bone and bone marrow as they have for cortical bone. Direct imaging of ex vivo trabecular bone architecture has been performed using a ZTE sequence (65). In the presence of trabeculae, bone marrow T2* relaxation time shortens due to magnetic field inhomogeneities. In this setting, UTE techniques may be useful to measure the effective T2* as a surrogate for trabecular density. UTE-IDEAL could potentially be used for the evaluation of bone marrow composition.
Surrogate measures for bone porosity have shown promising reproducibility data. Rad et al measured bone water concentration in 10 subjects twice within two months and found intraclass correlation coefficient of 0.95 (66). Studies using the suppression ratio have also found high reproducibility with intraclass correlation coefficient of 0.99 (64).
Menisci of the Knee
Anatomical Considerations
The menisci of the knee are semilunar shaped fibrocartilaginous structures. They are composed of approximately 70% water and of the dry weight, approximately 60-70% is collagen and 2-8% is proteoglycan (67). There are at least six well-defined fiber groups within the meniscus, including the surface meshwork, lamella, circumferential, radial, vertical, and meshwork fibers around the circumferential fibers (68,69). The circumferential bundles are the largest fiber group, mainly composed of collagen type I, and are continuous into the root ligaments. The radial fibers are another prominent fiber group, which may appear as larger collagen bundles (radial ties) or sheets (70). Radial fibers mainly consist of type I collagen and extend from the peripheral margin of the meniscus towards the free edge, and are generally horizontal in direction. Proteoglycan content also vary by region, with a higher content at the central portion compared with the peripheral portion (71). The adult meniscus is vascularized at the peripheral 10-25%, but avascular at the more central portions (72).
Imaging
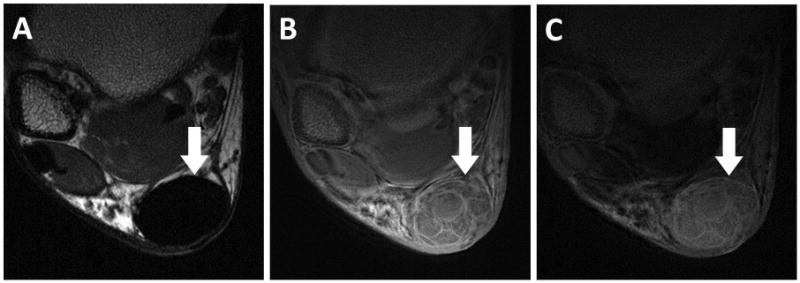
UTE can be used to demonstrate individual fiber groups. Additionally, meniscal calcifications can be imaged and quantified (73). Increased mono-exponential T2* values may indicate sub-clinical meniscus degeneration (74). UTE with bi-component analysis has also been successfully applied to the meniscus (28) and can be useful in quantifying normal versus abnormal meniscal regions (Figure 6). T1ρ relaxation may be sensitive to changes in proteoglycan and GAG content and UTE- T1ρ has been applied to the meniscus (3). The vascularized portions of the meniscus and meniscal root ligaments can been visualized in vivo with UTE imaging after intravenous contrast administration (Figure 7).
Figure 6.
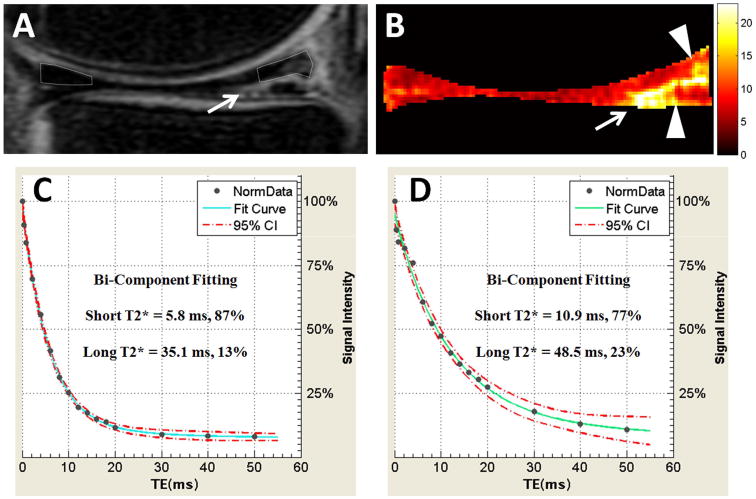
40-year-old patient with longitudinal-horizontal tear of the posterior horn of the medial meniscus. Conventional sagittal-oblique MR image with 38 ms TE (A) shows meniscus tear (arrow). Mono-exponential T2* map generated from UTE images (B) highlights regions of degeneration (arrowheads) adjacent to the tear (arrow). Quantitative bi-exponential fitting through the superior halves of the normal anterior horn (C) and degenerated posterior horn (D) shows notable differences in the degenerated portion with longer short T2* time and smaller short T2* fraction confirming degenerative tear.
Figure 7.
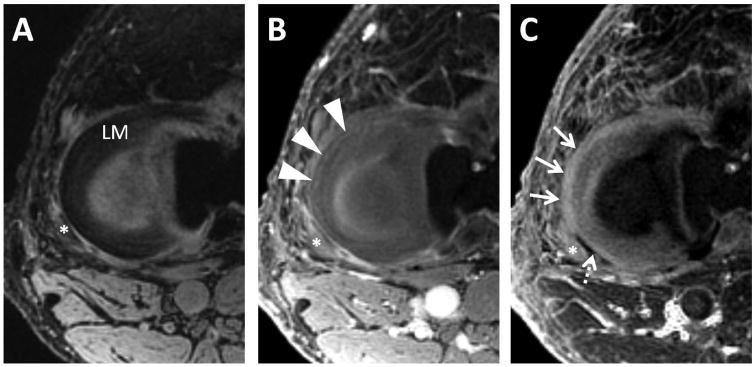
Axial MR images through the lateral meniscus of a healthy volunteer. Clinical gradient echo sequence with 12 ms TE (A) shows the C-shaped lateral meniscus (LM). 3D UTE-Cones image with 30 μs TE (B) was obtained 1 minute after intravenous gadolinium contrast administration, showing early perfusion to the peripheral meniscus (arrowheads). A 30 μs minus 12 ms subtraction image generated from dual-echo 3D UTE-Cones source images (C) 46 minutes after contrast administration shows diffusion of gadolinium into the peripheral meniscus (thin arrows). Note relatively avascular region at the popliteal hiatus (dashed arrow). Popliteus tendon is marked with an asterick.
Temporomandibular Joint
Anatomical Considerations
The temporomandibular joint (TMJ) is one of the most complex and essential joints in the body. The osseous components include the mandibular condyle and the mandibular fossa, which is a concave depression in the squamous portion of the temporal bone. The articular disc typically separates the two bones from direct contact. The articular surfaces of the bones are covered with fibrocartilage which, when compared with hyaline articular cartilage, is less susceptible to the effects of aging and allow for improved healing capacity (75). The TMJ is capable of active remodeling throughout life, including the articular fibrocartilage which maintains the balance between physiologic and pathologic responses (76).
The fibrocartilaginous surfaces of the mandibular condyle and fossa contain water, collagen and proteoglycan. Although the exact percentages have not been well studied, collagen is the most abundant extracellular matrix constituent. TMJ discs are composed of approximately 80% water and the dry weight is composed of approximately 62% collagen and 3% GAG (77).
Imaging
3D UTE images have been used to evaluate condylar shape and can readily visualize the fibrocartilaginous surface (78). The TMJ disc contains a sufficiently large long T2* component that it can be readily detected with conventional spin-echo imaging. However, with UTE imaging, T2* contrast can be optimized between the disc and lamina (79). Quantitative T2* values have been generated for the TMJ discs and condylar fibrocartilage (80) (Figure 8). Increasing UTE T2* values of TMJ disc have correlated with lower indentation stiffness (softer disc) and less collagen orientation as indicated by polarized light microscopy (80). Quantitative UTE T2* maps can facilitate characterization of the individual portions of the TMJ disc (Figure 9).
Figure 8.
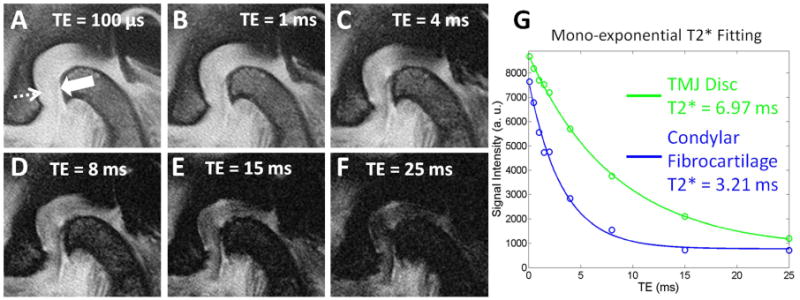
Sagittal MR images through the TMJ. Select multi-echo images ranging from 100 μs to 25 ms (A-F) show condylar fibrocartilage (solid arrow) and TMJ disc (dashed arrow). Mono-exponential T2* fitting curve (G) shows excellent curve fitting with T2* values of 6.97 ms and 3.21 ms for the disc and fibrocartilage, respectively.
Figure 9.
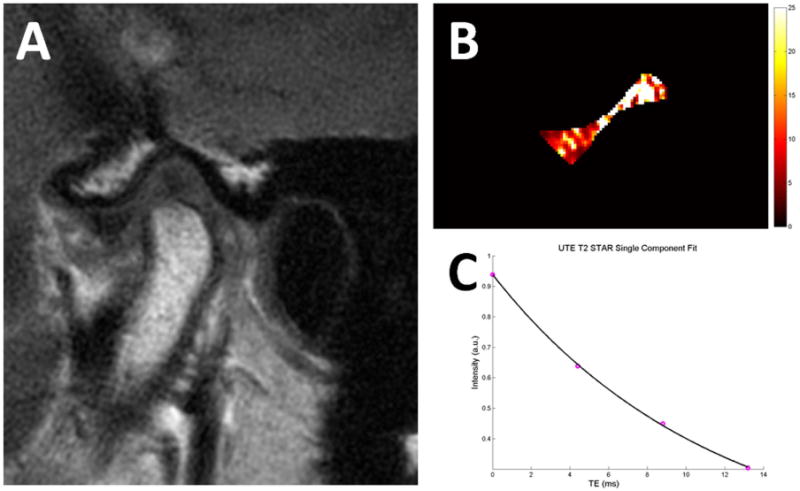
Temporomandibular joint disc of a 35-year old asymptomatic volunteer. (A) Conventional T1-weighted fast-spin echo image with mouth closed (A) shows the normal position of the TMJ disc. Quantitative pixel map generated from UTE images (B) show increased T2* values at the intermediate zone and posterior band, suggestive of early-stage degeneration. Mono-exponential T2* analysis of the entire disc (C) shows excellent curve fitting with T2* value of 11.4 ms.
Tendons, Ligaments, and Entheses
Anatomical Considerations
Tendon composition varies by anatomic location in addition to health. Total water content of healthy Achilles tendons is approximately 66% (81) whereas normal rotator cuff tendons contain about 75% water (82). Of the dry weight of tendon, approximately 65-87% is collagen (mainly type I) and 0.2-5% is GAG (83,84). In regions subject primarily to tension, such as the tensile zone of the Achilles, collagen content is higher and GAG content is lower. In regions also subject to pressure, such as the rotator cuff or near the entheses (where soft tissue connects to bone), collagen content is lower and GAG content is higher. Ligaments are grossly similar to tendons, but contain slightly lower amounts of collagen and more GAGs (84).
Tendon and ligaments are highly ordered due to the assembly of type I collagen into fibrils that aggregate to form larger fiber bundle units. Endotenon and endoligament invest and bind the collagen fibers together, and also serve as a conduit for neurovascular structures (83,85). Regions of fibrocartilage or fat can be found within tendons, ligaments, and entheses at sites subject to compression, such as within the distal Achilles, rotator cuff, or tibialis posterior (86).
Tendon degeneration is a necessary precursor to tendon tearing. Degenerated tendons demonstrate higher total water content, decrease in collagen content, collagen fiber disruption, and GAG accumulation (81,82,87). Fibrocartilaginous metaplasia and an accumulation of other substances such as mucous, fat, and eosinophils can also be seen in degeneration (87). Entheses are a characteristic site of involvement with spondyloarthropathies (88).
Imaging
Tendons, ligaments, entheses, and their individual components can be readily visualized with the UTE sequence (89). Differences in relaxation between collagen fascicles (shorter T2*) and endotenon, endoligament, and fibrocartilage (longer T2*) can be used to create internal contrast. Tendon alterations which are subtle or not visible at higher TEs are readily visible on UTE images with or without exogenous contrast enhancement (89).
Studies performed with tendon on spectroscopic systems suggest that there are up to four pools of protons (90), although many other studies have found three, likely corresponding to tightly bound water, weakly bound water, and free water (30). The number of components and T2* values vary depending tendon orientation and spatial resolution (30). Bi-exponential T2* analysis has been successfully performed in vivo using both UTE and vTE sequences, and fractions and T2* values vary depending on tendon location, consistent with different tendon compositions. More recently, Juras et al have found bi-exponential T2* values to be more useful for distinguishing between healthy volunteers and patients with Achilles tendinopathy compared with mono-exponential values (91). Bicomponent analysis can also be useful in quantifying the injured or postoperative tendon (Figure 10).
Figure 10.
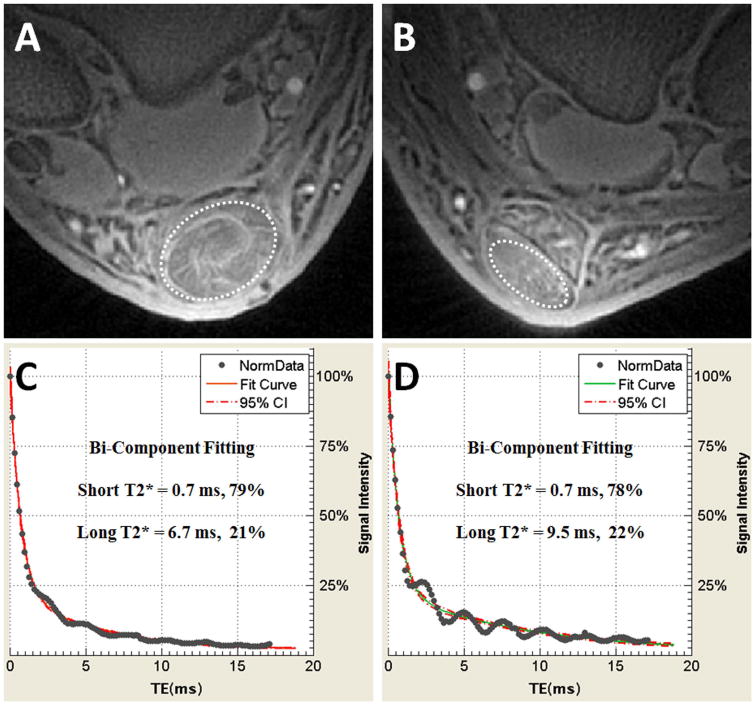
60-year old man several years after successful right Achilles tendon repair. Axial UTE MR images of the repaired right Achilles tendon (A) compared with the same patient's asymptomatic left Achilles tendon (B). Quantitative bi-component analysis performed in the tendons (dashed ovals) show that the short T2* value and fraction of the repaired right tendon (C) has approached the asymptomatic left side (D), confirming adequate collagen remodeling.
Intervertebral Disc
Anatomical Considerations
The intervertebral discs of the spine contain a central nucleus pulposus surrounded by a peripheral annulus fibrosus. Healthy nucleus pulposi contain about 70-90% water and of the dry weight, 65% is proteoglycan and 15-20% is type II collagen (92). The annulus fibrosus contains about 60-70% water and of the dry weight, 20% is proteoglycan, and 50-60% is collagen (92). The collagen of the annulus is also highly ordered, forming more than 20 concentric lamellar sheets. The individual lamellae within a disc alternate in orientation, approximately ±25° relative to the endplates (93).
The cartilaginous endplate (CEP) is an approximately 0.6 – 1 mm thick layer of hyaline-like cartilage that forms an interface between the intervertebral disc and the vertebral body. The CEP is strongly attached to the annulus fibrosus, but only weakly attached to the vertebral body, which is why it is predominantly considered a component of the disc (94). Similar to hyaline cartilage at other sites, the deep zone of the CEP attaches to bone through a thin layer of calcified cartilage. The intervertebral disc is avascular and blood vessels in the bone and longitudinal ligaments serve as routes for nutrients and waste. Solutes move by diffusion through the CEP and annulus fibrosus. Calcification of the CEP with advancing age or degeneration may hinder nutrition of the intervertebral disc and result in deterioration of biomechanical integrity (95).
Imaging
Studies performed with spectroscopic systems suggest that there are four water components in both the nucleus pulposus and annulus fibrosus (96), likely corresponding to water tightly bound to collagen, water loosely bound to collagen, water associated with residue proteins, and water associated with GAG. Healthy nucleus pulposi were shown to contain a majority of water associated with GAG whereas healthy annuli were shown to contain a majority of water loosely bound to collagen and associated with residue proteins (96).
UTE techniques allow for visualization of components of the intervertebral disc that cannot be directly seen with conventional techniques. These include the longitudinal ligaments, annulus fibrosus, and the CEP (Figure 11). The uncalcified and calcified portions of the CEP can be visible with UTE images, creating a bilaminar appearance (97).
Figure 11.
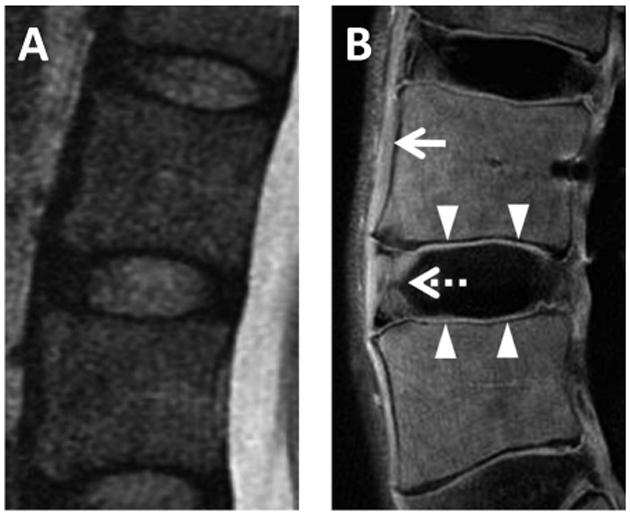
Lumbar spine imaged with conventional T2-weighted FSE sequence (A) and UTE subtraction image (B) generated from 10 μs TE minus 10 ms TE. Short T2* components are directly visible in the anterior longitudinal ligament (arrow), outer annulus fibrosus (dashed arrow), and cartilaginous endplate (arrowheads) on UTE subtraction image, but not on the conventional image. Images courtesy of Won C. Bae, PhD.
Challenges
We have summarized various qualitative and quantitative UTE techniques as well as potential applications in the musculoskeletal system. Notably, there are relatively few clinical studies to date using UTE techniques. This highlights a number of challenges that are continuously being improved upon. Challenges to clinical translation can be broken up into three categories, including hardware limitations, software availability, and validation studies.
Hardware continues to improve and many challenges are relatively solvable, such as sufficiently fast transmit-receive switches and precise RF waveform transmission. One of the most important challenges to UTE imaging is the requirement for high gradient performance. Unfortunately this problem is more difficult to solve. In particular, gradient waveforms are often far from ideal due to current induction from the rapidly switching gradients (eddy currents). Short term eddy currents disturb the UTE measurement in a number of complicated ways (26). These can be improved with gradient calibration, minimized with certain imaging tactics, or overcome with more complex pulse sequences. For instance, positioning at magnet isocenter typically results in improved image quality. This is clearly easier to obtain with peripheral joints such as the ankle compared with more centrally located joints such as the shoulder or hip. Imaging more spherical or cylindrical parts such as the leg will decrease susceptibility and typically result in higher image quality compared with less cylindrical body parts such as the fingers with the many air-tissue interfaces. Removal of the slice-select gradient with the use of 3D imaging together with a short rectangular excitation pulse also decreases eddy currents.
Software availability is another major challenge to widespread use. At the time of this article, there are no product UTE-type sequences available, although nearly all major vendors have a near-product UTE sequence in development. Furthermore, the near-product sequences are typically qualitative and used for morphologic or anatomic analysis. Cross-platform quantitative sequences are scarcer. Finally, there are relatively few validation and reproducibility studies compared with feasibility studies, in part because they are more time consuming and expensive. However, as shown in this article, these types of studies are occurring and the results are quite encouraging.
Conclusion
In summary, UTE sequence and its modifications should be considered as complementary to conventional MR techniques and may allow for improved evaluation of the musculoskeletal system. Tissues with short mean T2* and short T2* components can now be qualitatively highlighted or quantitatively evaluated. Clinical translation of UTE techniques is likely to occur in the near future and ongoing research will provide validation for widespread use.
Acknowledgments
The authors gratefully acknowledge grant support from the VA Clinical Science Research and Development Service (Career Development Grant 1IK2CX000749 and Merit Award I01CX000625) and the NIH (R01DE022068 and R21AR063894).
References
- 1.Bydder GM. Review. The Agfa Mayneord lecture: MRI of short and ultrashort T(2) and T(2)* components of tissues, fluids and materials using clinical systems. Br J Radiol. 2011;84(1008):1067–1082. doi: 10.1259/bjr/74368403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pauly JM, Conolly SI, Nishimura D, Macovski A. Slice-Selective Excitation for Very Short T2 Species. Proceedings of the 8th Annual Meeting of ISMRM; Amsterdamn, Netherlands. 1989; abstract 28. [Google Scholar]
- 3.Du J, Carl M, Diaz E, et al. Ultrashort TE T1rho (UTE T1rho) imaging of the Achilles tendon and meniscus. Magn Reson Med. 2010;64(3):834–842. doi: 10.1002/mrm.22474. [DOI] [PubMed] [Google Scholar]
- 4.Rahmer J, Bornert P, Groen J, Bos C. Three-dimensional radial ultrashort echo-time imaging with T2 adapted sampling. Magn Reson Med. 2006;55(5):1075–1082. doi: 10.1002/mrm.20868. [DOI] [PubMed] [Google Scholar]
- 5.Pauly JM. Selective Excitation for Ultrashort Echo Time Imaging. eMagRes: John Wiley & Sons, Ltd; 2012. [DOI] [Google Scholar]
- 6.Du J, Bydder M, Takahashi AM, Chung CB. Two-dimensional ultrashort echo time imaging using a spiral trajectory. Magnetic resonance imaging. 2008;26(3):304–312. doi: 10.1016/j.mri.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Gurney PT, Hargreaves BA, Nishimura DG. Design and analysis of a practical 3D cones trajectory. Magn Reson Med. 2006;55(3):575–582. doi: 10.1002/mrm.20796. [DOI] [PubMed] [Google Scholar]
- 8.Qian Y, Boada FE. Acquisition-weighted stack of spirals for fast high-resolution three-dimensional ultra-short echo time MR imaging. Magn Reson Med. 2008;60(1):135–145. doi: 10.1002/mrm.21620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song HK, Wehrli FW. Variable TE gradient and spin echo sequences for in vivo MR microscopy of short T2 species. Magn Reson Med. 1998;39(2):251–258. doi: 10.1002/mrm.1910390212. [DOI] [PubMed] [Google Scholar]
- 10.Weiger M, Pruessmann KP. MRI with Zero Echo Time. eMagRes: John Wiley & Sons, Ltd; 2012. [DOI] [Google Scholar]
- 11.Garwood M, Idiyatullin D, Corum CA, et al. Capturing Signals from Fast-relaxing Spins with Frequency-Swept MRI:SWIFT. eMagRes: John Wiley & Sons, Ltd; 2012. [DOI] [Google Scholar]
- 12.Grodzki DM, Jakob PM, Heismann B. Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA) Magn Reson Med. 2012;67(2):510–518. doi: 10.1002/mrm.23017. [DOI] [PubMed] [Google Scholar]
- 13.Weiger M, Brunner DO, Wyss M, Dietrich BE, Wilm BJ, Pruessmann KP. ZTE Imaging with T1 Contrast; Proceedings of the 22nd Annual Meeting of ISMRM; Milan, Italy. 2014; abstract 4262. [Google Scholar]
- 14.Ida M, Wakayama T, Nielsen ML, Abe T, Grodzki DM. Quiet T1-weighted imaging using PETRA: Initial clinical evaluation in intracranial tumor patients. J Magn Reson Imaging. 2014 doi: 10.1002/jmri.24575. [DOI] [PubMed] [Google Scholar]
- 15.Du J, Bydder M, Takahashi AM, Carl M, Chung CB, Bydder GM. Short T2 contrast with three-dimensional ultrashort echo time imaging. Magnetic resonance imaging. 2011;29(4):470–482. doi: 10.1016/j.mri.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010;207(2):304–311. doi: 10.1016/j.jmr.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y, Ackerman JL, Chesler DA, Graham L, Wang Y, Glimcher MJ. Density of organic matrix of native mineralized bone measured by water- and fat-suppressed proton projection MRI. Magn Reson Med. 2003;50(1):59–68. doi: 10.1002/mrm.10512. [DOI] [PubMed] [Google Scholar]
- 18.Du J, Bydder GM. Qualitative and quantitative ultrashort-TE MRI of cortical bone. NMR Biomed. 2013;26(5):489–506. doi: 10.1002/nbm.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J, Carl M, Bae WC, et al. Dual inversion recovery ultrashort echo time (DIR-UTE) imaging and quantification of the zone of calcified cartilage (ZCC) Osteoarthritis Cartilage. 2013;21(1):77–85. doi: 10.1016/j.joca.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du J, Takahashi AM, Bae WC, Chung CB, Bydder GM. Dual inversion recovery, ultrashort echo time (DIR UTE) imaging: creating high contrast for short-T(2) species. Magn Reson Med. 2010;63(2):447–455. doi: 10.1002/mrm.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Du J, Takahashi AM, Bydder M, Chung CB, Bydder GM. Ultrashort TE imaging with off-resonance saturation contrast (UTE-OSC) Magn Reson Med. 2009;62(2):527–531. doi: 10.1002/mrm.22007. [DOI] [PubMed] [Google Scholar]
- 22.Carl M, Chiang JTA. Investigations of the origin of phase differences seen with ultrashort TE imaging of short T2 meniscal tissue. Magn Reson Med. 2012;67(4):991–1003. doi: 10.1002/mrm.23075. [DOI] [PubMed] [Google Scholar]
- 23.Wang K, Yu H, Brittain JH, Reeder SB, Du J. k-space water-fat decomposition with T2* estimation and multifrequency fat spectrum modeling for ultrashort echo time imaging. J Magn Reson Imaging. 2010;31(4):1027–1034. doi: 10.1002/jmri.22121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gore JC, Anderson AW. The Physics of Relaxation. eMagRes: John Wiley & Sons, Ltd; 2013. [DOI] [Google Scholar]
- 25.Du J. Quantitative Ultrashort TE (UTE) Imaging of Short T2 Tissues. eMagRes: John Wiley & Sons, Ltd; 2012. [DOI] [Google Scholar]
- 26.Lu A, Daniel BL, Pauly JM, Pauly KB. Improved slice selection for R2* mapping during cryoablation with eddy current compensation. J Magn Reson Imaging. 2008;28(1):190–198. doi: 10.1002/jmri.21396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia Y. MRI of articular cartilage at microscopic resolution. Bone & joint research. 2013;2(1):9–17. doi: 10.1302/2046-3758.21.2000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du J, Diaz E, Carl M, Bae W, Chung CB, Bydder GM. Ultrashort echo time imaging with bicomponent analysis. Magn Reson Med. 2012;67(3):645–649. doi: 10.1002/mrm.23047. [DOI] [PubMed] [Google Scholar]
- 29.Fullerton GD, Cameron IL, Ord VA. Orientation of tendons in the magnetic field and its effect on T2 relaxation times. Radiology. 1985;155(2):433–435. doi: 10.1148/radiology.155.2.3983395. [DOI] [PubMed] [Google Scholar]
- 30.Wang N, Xia Y. Anisotropic analysis of multi-component T2 and T1rho relaxations in achilles tendon by NMR spectroscopy and microscopic MRI. J Magn Reson Imaging. 2013;38(3):625–633. doi: 10.1002/jmri.24012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pauli C, Bae WC, Lee M, et al. Ultrashort-echo time MR imaging of the patella with bicomponent analysis: correlation with histopathologic and polarized light microscopic findings. Radiology. 2012;264(2):484–493. doi: 10.1148/radiol.12111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Springer F, Steidle G, Martirosian P, Syha R, Claussen CD, Schick F. Rapid assessment of longitudinal relaxation time in materials and tissues with extremely fast signal decay using UTE sequences and the variable flip angle method. Invest Radiol. 2011;46(10):610–617. doi: 10.1097/RLI.0b013e31821c44cd. [DOI] [PubMed] [Google Scholar]
- 33.Wright P, Jellus V, McGonagle D, Robson M, Ridgeway J, Hodgson R. Comparison of two ultrashort echo time sequences for the quantification of T1 within phantom and human Achilles tendon at 3 T. Magn Reson Med. 2012;68(4):1279–1284. doi: 10.1002/mrm.24130. [DOI] [PubMed] [Google Scholar]
- 34.Sepponen RE, Pohjonen JA, Sipponen JT, Tanttu JI. A method for T1 rho imaging. J Comput Assist Tomogr. 1985;9(6):1007–1011. doi: 10.1097/00004728-198511000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Springer F, Martirosian P, Schick F. Magnetization Transfer – Ultrashort Echo Time (MT-UTE) Imaging. eMagRes: John Wiley & Sons, Ltd; 2012. [DOI] [Google Scholar]
- 36.Stanisz GJ, Odrobina EE, Pun J, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54(3):507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 37.Hopfgarten C, Kirsch S, Reisig G, Kreinest M, Schad LR. Identification of in vitro degenerated porcine meniscal tissue: MTR contrast prevents misinterpretation due to the magic angle effect. Proceedings of the 20th Annual Meeting of ISMRM; Sydney, Australia. 2012; abstract 1397. [Google Scholar]
- 38.Maroudas A. Physico-chemical properties of articular cartilage. In: Freeman MAR, editor. Adult articular cartilage. 2d. Tunbridge Wells, Eng.: Pitman Medical; 1979. pp. 215–290. [Google Scholar]
- 39.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports health. 2009;1(6):461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson D, Pedowitz RA. Practical orthopaedic sports medicine and arthroscopy. Philadelphia: Lippincott Williams & Wilkins; 2007. p. 1216. [Google Scholar]
- 41.Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J Cell Biol. 1985;100(2):598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37(4):271–284. doi: 10.1002/(SICI)1097-0029(19970515)37:4<271::AID-JEMT3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 43.Fujioka R, Aoyama T, Takakuwa T. The layered structure of the articular surface. Osteoarthritis Cartilage. 2013;21(8):1092–1098. doi: 10.1016/j.joca.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Aspden RM, Hukins DW. Collagen organization in articular cartilage, determined by X-ray diffraction, and its relationship to tissue function. Proc R Soc Lond B Biol Sci. 1981;212(1188):299–304. doi: 10.1098/rspb.1981.0040. [DOI] [PubMed] [Google Scholar]
- 45.Muir H. Proteoglycans of cartilage. J Clin Pathol Suppl (R Coll Pathol) 1978;12:67–81. [PMC free article] [PubMed] [Google Scholar]
- 46.Lane LB, Bullough PG. Age-related changes in the thickness of the calcified zone and the number of tidemarks in adult human articular cartilage. The Journal of bone and joint surgery British volume. 1980;62(3):372–375. doi: 10.1302/0301-620X.62B3.7410471. [DOI] [PubMed] [Google Scholar]
- 47.Boyde A, Riggs CM, Bushby AJ, McDermott B, Pinchbeck GL, Clegg PD. Cartilage damage involving extrusion of mineralisable matrix from the articular calcified cartilage and subchondral bone. Eur Cell Mater. 2011;21:470–478. doi: 10.22203/ecm.v021a35. [DOI] [PubMed] [Google Scholar]
- 48.Bae WC, Dwek JR, Znamirowski R, et al. Ultrashort echo time MR imaging of osteochondral junction of the knee at 3 T: identification of anatomic structures contributing to signal intensity. Radiology. 2010;254(3):837–845. doi: 10.1148/radiol.09081743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lattanzio PJ, Marshall KW, Damyanovich AZ, Peemoeller H. Macromolecule and water magnetization exchange modeling in articular cartilage. Magn Reson Med. 2000;44(6):840–851. doi: 10.1002/1522-2594(200012)44:6<840::aid-mrm4>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 50.Qian Y, Williams AA, Chu CR, Boada FE. Multi-component T2* relaxation of knee cartilage under UTE acquisitions; Proceedings of the 18th Annual Meeting of ISMRM; Stockholm, Sweden. 2010; abstract 830. [Google Scholar]
- 51.Williams A, Qian Y, Bear D, Chu CR. Assessing degeneration of human articular cartilage with ultra-short echo time (UTE) T2* mapping. Osteoarthritis Cartilage. 2010;18(4):539–546. doi: 10.1016/j.joca.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian Y, Williams AA, Chu CR, Boada FE. Multicomponent T2* mapping of knee cartilage: technical feasibility ex vivo. Magn Reson Med. 2010;64(5):1426–1431. doi: 10.1002/mrm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Williams A, Qian Y, Chu CR. UTE-T2 * mapping of human articular cartilage in vivo: a repeatability assessment. Osteoarthritis Cartilage. 2011;19(1):84–88. doi: 10.1016/j.joca.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wehrli FW, Song HK, Saha PK, Wright AC. Quantitative MRI for the assessment of bone structure and function. NMR Biomed. 2006;19(7):731–764. doi: 10.1002/nbm.1066. [DOI] [PubMed] [Google Scholar]
- 55.Reichert IL, Benjamin M, Gatehouse PD, et al. Magnetic resonance imaging of periosteum with ultrashort TE pulse sequences. J Magn Reson Imaging. 2004;19(1):99–107. doi: 10.1002/jmri.10432. [DOI] [PubMed] [Google Scholar]
- 56.Moore SR, Milz S, Knothe Tate ML. Periosteal thickness and cellularity in mid-diaphyseal cross-sections from human femora and tibiae of aged donors. Journal of anatomy. 2014;224(2):142–149. doi: 10.1111/joa.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin RB, Burr DB, Sharkey NA. Skeletal tissue mechanics. New York: Springer; 1998. p. 392. [Google Scholar]
- 58.Vande Berg BC, Malghem J, Lecouvet FE, Maldague B. Magnetic resonance imaging of normal bone marrow. European radiology. 1998;8(8):1327–1334. doi: 10.1007/s003300050547. [DOI] [PubMed] [Google Scholar]
- 59.Horch RA, Gochberg DF, Nyman JS, Does MD. Non-invasive predictors of human cortical bone mechanical properties: T(2)-discriminated H NMR compared with high resolution X-ray. PLoS One. 2011;6(1):e16359. doi: 10.1371/journal.pone.0016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fernandez-Seara MA, Wehrli SL, Takahashi M, Wehrli FW. Water content measured by proton-deuteron exchange NMR predicts bone mineral density and mechanical properties. J Bone Miner Res. 2004;19(2):289–296. doi: 10.1359/JBMR.0301227. [DOI] [PubMed] [Google Scholar]
- 61.Techawiboonwong A, Song HK, Leonard MB, Wehrli FW. Cortical bone water: in vivo quantification with ultrashort echo-time MR imaging. Radiology. 2008;248(3):824–833. doi: 10.1148/radiol.2482071995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Horch RA, Gochberg DF, Nyman JS, Does MD. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magn Reson Med. 2012;68(6):1774–1784. doi: 10.1002/mrm.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bashoor-Zadeh M, Li C, Sun W, et al. Simple ultrashort echo time MRI measure associated with cortical bone porosity; Proceedings of the 21st Annual Meeting of ISMRM; Salt Lake City, Utah, USA. 2013; abstract 1599. [Google Scholar]
- 64.Li C, Seifert AC, Rad HS, et al. Cortical Bone Water Concentration: Dependence of MR Imaging Measures on Age and Pore Volume Fraction. Radiology. 2014 doi: 10.1148/radiol.14132585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiger M, Stampanoni M, Pruessmann KP. Direct depiction of bone microstructure using MRI with zero echo time. Bone. 2013;54(1):44–47. doi: 10.1016/j.bone.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Rad HS, Lam SC, Magland JF, et al. Quantifying cortical bone water in vivo by three-dimensional ultra-short echo-time MRI. NMR Biomed. 2011;24(7):855–864. doi: 10.1002/nbm.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blain E, Duance V. Meniscus. In: Hutson MA, Speed C, editors. Sports injuries. Oxford; New York: Oxford University Press; 2011. pp. 45–53. [Google Scholar]
- 68.Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anatomy and embryology. 1998;197(4):317–324. doi: 10.1007/s004290050141. [DOI] [PubMed] [Google Scholar]
- 69.Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. J Orthop Res. 2005;23(1):142–149. doi: 10.1016/j.orthres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 70.Skaggs DL, Warden WH, Mow VC. Radial tie fibers influence the tensile properties of the bovine medial meniscus. J Orthop Res. 1994;12(2):176–185. doi: 10.1002/jor.1100120205. [DOI] [PubMed] [Google Scholar]
- 71.Nakano T, Dodd CM, Scott PG. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J Orthop Res. 1997;15(2):213–220. doi: 10.1002/jor.1100150209. [DOI] [PubMed] [Google Scholar]
- 72.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. The American journal of sports medicine. 1982;10(2):90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 73.Omoumi P, Bae WC, Du J, et al. Meniscal calcifications: morphologic and quantitative evaluation by using 2D inversion-recovery ultrashort echo time and 3D ultrashort echo time 3.0-T MR imaging techniques--feasibility study. Radiology. 2012;264(1):260–268. doi: 10.1148/radiol.12111439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams A, Qian Y, Golla S, Chu CR. UTE-T2 * mapping detects sub-clinical meniscus injury after anterior cruciate ligament tear. Osteoarthritis Cartilage. 2012;20(6):486–494. doi: 10.1016/j.joca.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wadhwa S, Kapila S. TMJ disorders: future innovations in diagnostics and therapeutics. Journal of dental education. 2008;72(8):930–947. [PMC free article] [PubMed] [Google Scholar]
- 76.Kuang B, Dai J, Wang QY, et al. Combined degenerative and regenerative remodeling responses of the mandibular condyle to experimentally induced disordered occlusion. American journal of orthodontics and dentofacial orthopedics: official publication of the American Association of Orthodontists, its constituent societies, and the American Board of Orthodontics. 2013;143(1):69–76. doi: 10.1016/j.ajodo.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 77.Kuo J, Zhang L, Bacro T, Yao H. The region-dependent biphasic viscoelastic properties of human temporomandibular joint discs under confined compression. Journal of biomechanics. 2010;43(7):1316–1321. doi: 10.1016/j.jbiomech.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Geiger D, Bae WC, Statum S, Du J, Chung CB. Quantitative 3D ultrashort time-to-echo (UTE) MRI and micro-CT (muCT) evaluation of the temporomandibular joint (TMJ) condylar morphology. Skeletal radiology. 2014;43(1):19–25. doi: 10.1007/s00256-013-1738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carl M, Sanal HT, Diaz E, et al. Optimizing MR signal contrast of the temporomandibular joint disk. J Magn Reson Imaging. 2011;34(6):1458–1464. doi: 10.1002/jmri.22810. [DOI] [PubMed] [Google Scholar]
- 80.Sanal HT, Bae WC, Pauli C, et al. Magnetic resonance imaging of the temporomandibular joint disc: feasibility of novel quantitative magnetic resonance evaluation using histologic and biomechanical reference standards. J Orofac Pain. 2011;25(4):345–353. [PMC free article] [PubMed] [Google Scholar]
- 81.de Mos M, van El B, DeGroot J, et al. Achilles tendinosis: changes in biochemical composition and collagen turnover rate. The American journal of sports medicine. 2007;35(9):1549–1556. doi: 10.1177/0363546507301885. [DOI] [PubMed] [Google Scholar]
- 82.Riley GP, Harrall RL, Constant CR, Chard MD, Cawston TE, Hazleman BL. Tendon degeneration and chronic shoulder pain: changes in the collagen composition of the human rotator cuff tendons in rotator cuff tendinitis. Annals of the rheumatic diseases. 1994;53(6):359–366. doi: 10.1136/ard.53.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kannus P. Structure of the tendon connective tissue. Scand J Med Sci Sports. 2000;10(6):312–320. doi: 10.1034/j.1600-0838.2000.010006312.x. [DOI] [PubMed] [Google Scholar]
- 84.Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- 85.Villegas DF, Donahue TL. Collagen morphology in human meniscal attachments: a SEM study. Connect Tissue Res. 2010;51(5):327–336. doi: 10.3109/03008200903349639. [DOI] [PubMed] [Google Scholar]
- 86.Benjamin M, Redman S, Milz S, et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Annals of the rheumatic diseases. 2004;63(12):1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scand J Med Sci Sports. 1997;7(2):86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 88.Benjamin M, McGonagle D. The enthesis organ concept and its relevance to the spondyloarthropathies. Advances in experimental medicine and biology. 2009;649:57–70. doi: 10.1007/978-1-4419-0298-6_4. [DOI] [PubMed] [Google Scholar]
- 89.Benjamin M, Milz S, Bydder GM. Magnetic resonance imaging of entheses. Part 2. Clinical radiology. 2008;63(6):704–711. doi: 10.1016/j.crad.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 90.Peto S, Gillis P, Henri VP. Structure and dynamics of water in tendon from NMR relaxation measurements. Biophys J. 1990;57(1):71–84. doi: 10.1016/S0006-3495(90)82508-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Juras V, Apprich S, Szomolanyi P, Bieri O, Deligianni X, Trattnig S. Bi-exponential T2 analysis of healthy and diseased Achilles tendons: an in vivo preliminary magnetic resonance study and correlation with clinical score. European radiology. 2013;23(10):2814–2822. doi: 10.1007/s00330-013-2897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bogduk N, Endres SM. Clinical anatomy of the lumbar spine and sacrum. New York: Elsevier/Churchill Livingstone; 2005. p. 272. [Google Scholar]
- 93.Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23(1):75–88. doi: 10.3109/03008208909103905. [DOI] [PubMed] [Google Scholar]
- 94.Levangie PK, Norkin CC. Joint structure and function : a comprehensive analysis. Philadelphia, PA: F.A. Davis Co.; 2005. p. 640. [Google Scholar]
- 95.Roberts S, Urban JP, Evans H, Eisenstein SM. Transport properties of the human cartilage endplate in relation to its composition and calcification. Spine (Phila Pa 1976) 1996;21(4):415–420. doi: 10.1097/00007632-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 96.Nightingale T, MacKay A, Pearce RH, Whittall KP, Flak B. A model of unloaded human intervertebral disk based on NMR relaxation. Magn Reson Med. 2000;43(1):34–44. doi: 10.1002/(sici)1522-2594(200001)43:1<34::aid-mrm5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 97.Bae WC, Statum S, Zhang Z, et al. Morphology of the cartilaginous endplates in human intervertebral disks with ultrashort echo time MR imaging. Radiology. 2013;266(2):564–574. doi: 10.1148/radiol.12121181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Robson MD, Gatehouse PD, Bydder M, Bydder GM. Magnetic resonance: an introduction to ultrashort TE (UTE) imaging. J Comput Assist Tomogr. 2003;27(6):825–846. doi: 10.1097/00004728-200311000-00001. [DOI] [PubMed] [Google Scholar]
- 99.Gold GE, Pauly JM, Macovski A, Herfkens RJ. MR spectroscopic imaging of collagen: tendons and knee menisci. Magn Reson Med. 1995;34(5):647–654. doi: 10.1002/mrm.1910340502. [DOI] [PubMed] [Google Scholar]
- 100.Chang EY, Bae WC, Statum S, Du J, Chung CB. Effects of repetitive freeze-thawing cycles on T2 and T2 of the Achilles tendon. Eur J Radiol. 2014;83(2):349–353. doi: 10.1016/j.ejrad.2013.10.014. [DOI] [PubMed] [Google Scholar]


