Abstract
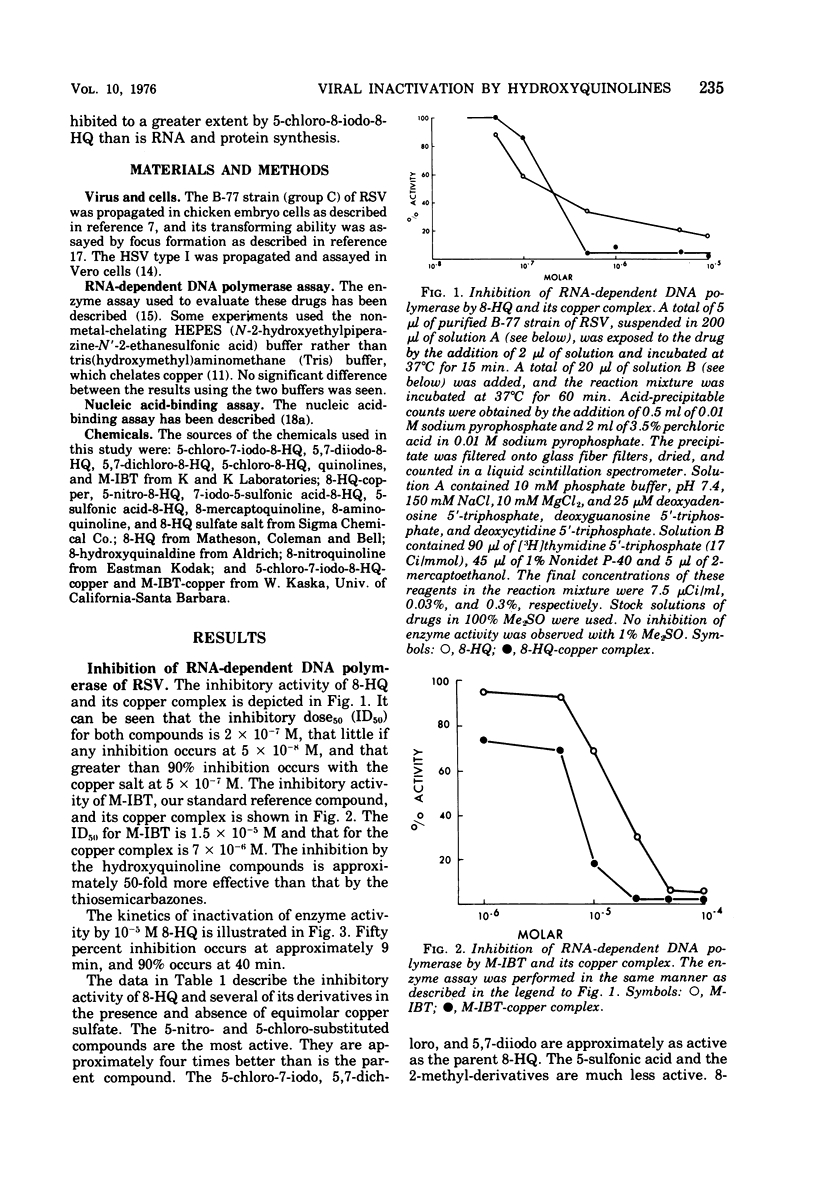
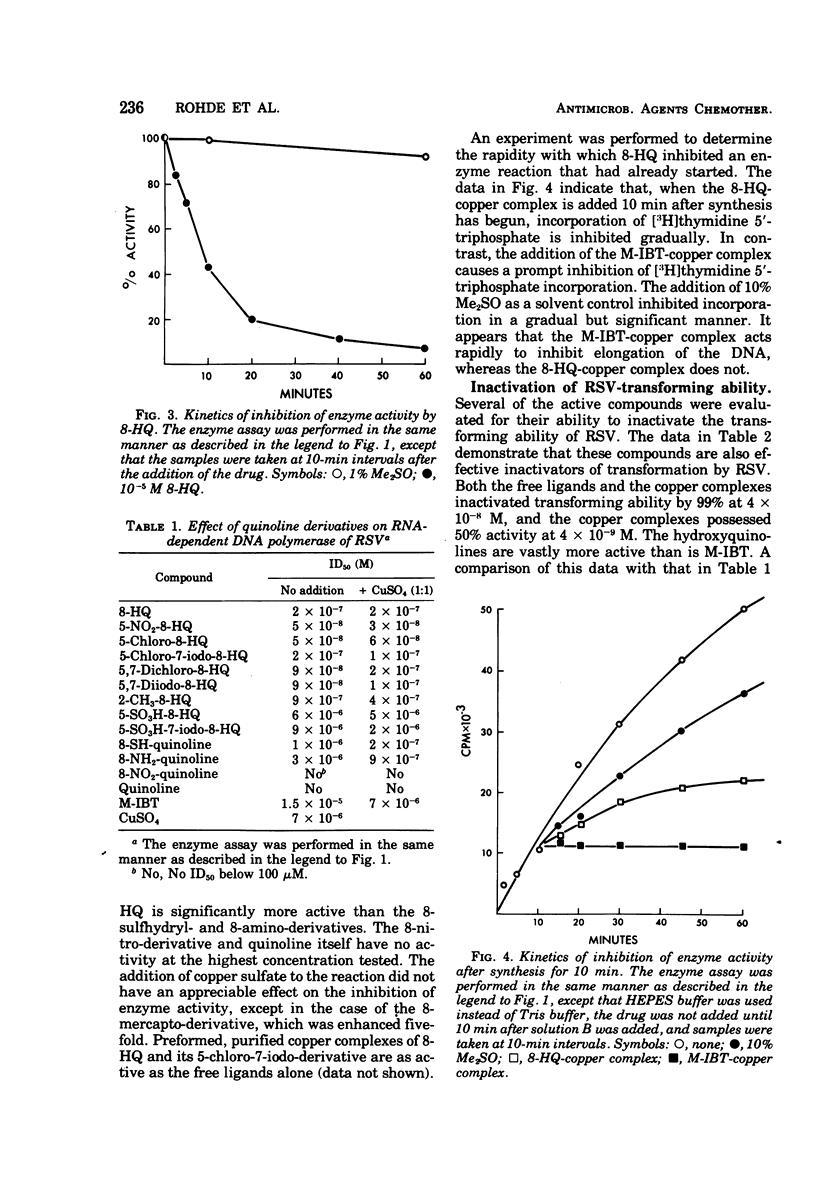
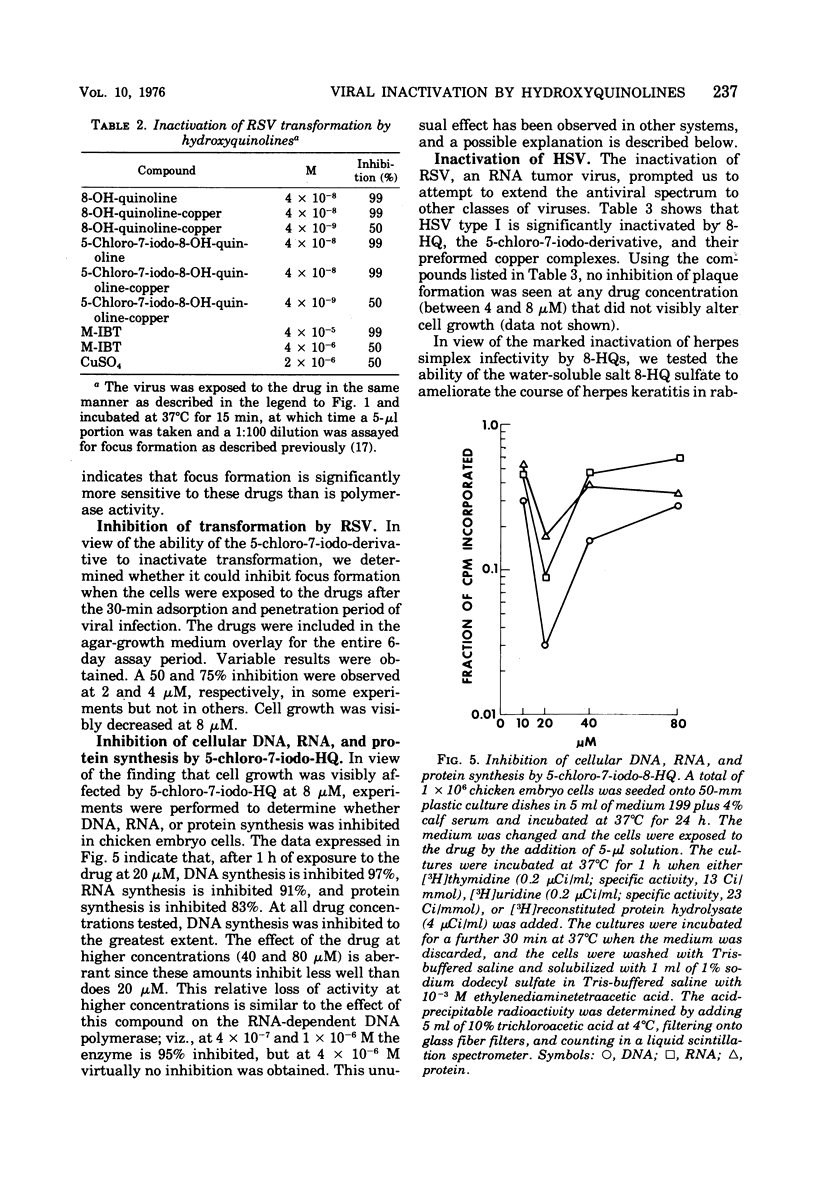
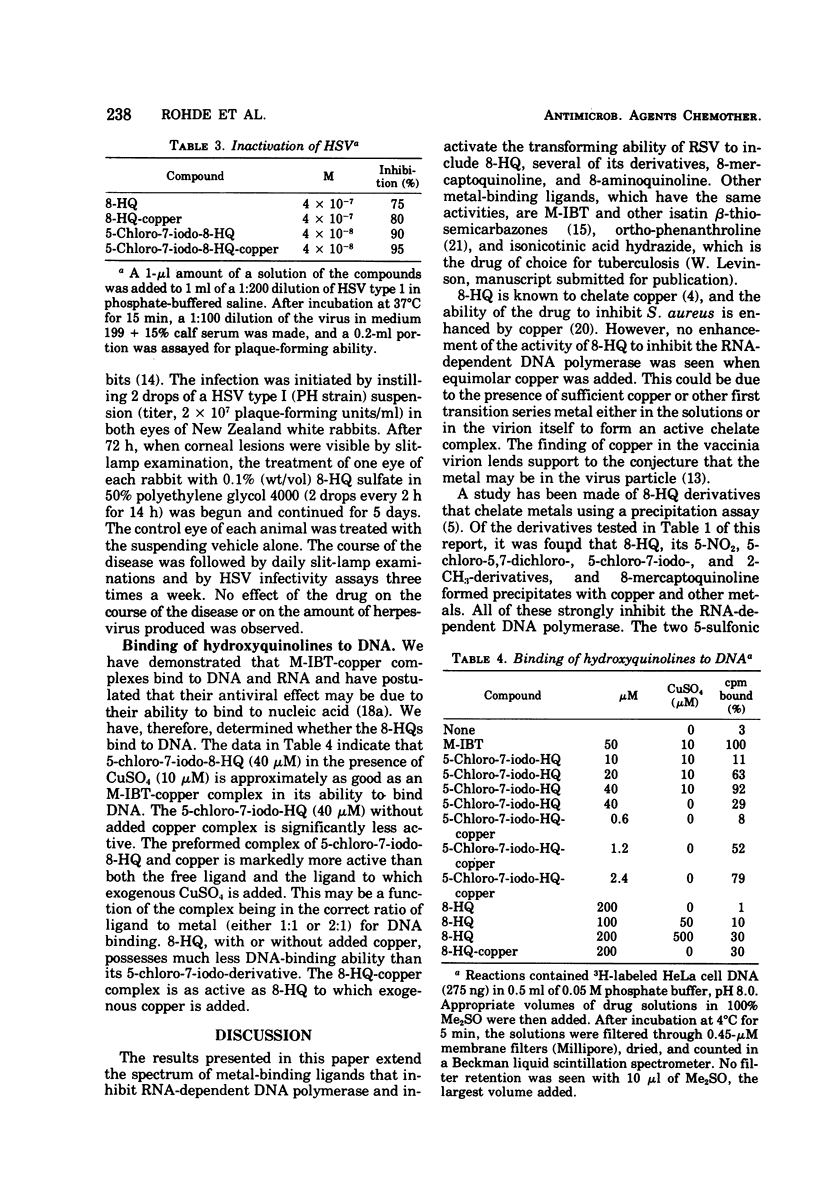
8-Hydroxyquinoline and several of its derivatives inactivate the transforming ability of Rous sarcoma virus and inhibit its ribonucleic acid-dependent deoxyribonucleic acid polymerase activity. The copper complex of these metal-binding ligands is as active as the free ligand. The activity of the 8-hydroxyquinolines is approximately 50-fold more effective than another group of metal-binding compounds that we have tested, the thiosemicarbazones. In contrast to the potency of the 8-hydroxyquinolines to inactivate Rous sarcoma virus, no intracellular inhibition of transformation could be demonstrated at a concentration that did not affect the growth and appearance of the cells. Cellular deoxyribonucleic acid synthesis was inhibited to a greater extent than was ribonucleic acid or protein synthesis. The phenomenon of “concentration quenching” was observed with high concentrations of drug, causing less inhibition of deoxyribonucleic acid synthesis than was observed with lower concentrations. Herpes simplex virus type 1 was inactivated also by the 8-hydroxyquinolines and their copper complexes. No intracellular inhibition of plaque formation was observed. Treatment with 8-hydroxyquinoline sulfate had no effect on the resolution of herpetic keratitis in rabbits. Some 8-hydroxyquinolines bind to deoxyribonucleic acid in the presence of copper, a phenomenon that may be important in their antiviral activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A., GIBSON M. I., RUBBO S. D. The influence of chemical constitution on antibacterial activity. VI. The bactericidal action of 8-hydroxyquinoline (oxine). Br J Exp Pathol. 1953 Apr;34(2):119–130. [PMC free article] [PubMed] [Google Scholar]
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals. III. Pteridines, riboflavin and purines. Biochem J. 1953 Jul;54(4):646–654. doi: 10.1042/bj0540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert A., Gledhill W. S. The choice of a chelating agent for inactivating trace metals: I. A survey of commercially available chelating agents. Biochem J. 1947;41(4):529–533. [PMC free article] [PubMed] [Google Scholar]
- Albert A., Magrath D. The choice of a chelating agent for inactivating trace metals: II. Derivatives of oxine (8-hydroxyquinoline). Biochem J. 1947;41(4):534–545. [PMC free article] [PubMed] [Google Scholar]
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. RNA-dependent DNA polymerase (reverse transcriptase) from avian myeloblastosis virus: a zinc metalloenzyme. Proc Natl Acad Sci U S A. 1974 May;71(5):2091–2095. doi: 10.1073/pnas.71.5.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M., Levinson W. E., Quintrell N., Sullivan D., Fanshier L., Jackson J. The low molecular weight RNAs of Rous sarcoma virus. I. The 4 S RNA. Virology. 1970 Sep;42(1):182–195. doi: 10.1016/0042-6822(70)90251-5. [DOI] [PubMed] [Google Scholar]
- Fraser R. S., Creanor J. Rapid and selective inhibition of RNA synthesis in yeast by 8-hydroxyquinoline. Eur J Biochem. 1974 Jul 1;46(1):67–73. doi: 10.1111/j.1432-1033.1974.tb03597.x. [DOI] [PubMed] [Google Scholar]
- Gershon H. Antifungal activity of bischelates of 5-,7-, and 5,7-halogenated 8-quinolinols with copper(II). Determination of approximate dimensions of the long and short axes of the pores in the fungal spore wall. J Med Chem. 1974 Aug;17(8):824–827. doi: 10.1021/jm00254a009. [DOI] [PubMed] [Google Scholar]
- Hanlon D. P., Watt D. S., Westhead E. W. The interaction of divalent metal ions with tris buffer in dilute solution. Anal Biochem. 1966 Aug;16(2):225–233. doi: 10.1016/0003-2697(66)90150-3. [DOI] [PubMed] [Google Scholar]
- Hirai K., Defendi V., Diamond L. Enhancement of simian virus 40 transformation and integration by 4-nitroquinoline 1-oxide. Cancer Res. 1974 Dec;34(12):3497–3500. [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Levinson W., Coleman V., Woodson B., Rabson A., Lanier J., Whitcher J., Dawson C. Inactivation of herpes simplex virus by thiosemicarbazones and certain cations. Antimicrob Agents Chemother. 1974 Apr;5(4):398–402. doi: 10.1128/aac.5.4.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Faras A., Woodson B., Jackson J., Bishop J. M. Inhibition of RNA-dependent DNA polymerase of Rous sarcoma virus by thiosemicarbazones and several cations. Proc Natl Acad Sci U S A. 1973 Jan;70(1):164–168. doi: 10.1073/pnas.70.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Helling R. Inactivation of lambda phage infectivity and lambda deoxyribonucleic acid transfection by N-methyl-isatin beta-thiosemicarbazone-copper complexes. Antimicrob Agents Chemother. 1976 Jan;9(1):160–163. doi: 10.1128/aac.9.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson W., Rubin H. Radiation studies of avian tumor viruses and of Newcastle disease virus. Virology. 1966 Apr;28(4):533–542. doi: 10.1016/0042-6822(66)90238-8. [DOI] [PubMed] [Google Scholar]
- Mikelens P. E., Woodson B. A., Levinson W. E. Association of nucleic acids with complexes of N-methyl isatin-beta-thiosemicarbazone and copper. Biochem Pharmacol. 1976 Apr 1;25(7):821–827. doi: 10.1016/0006-2952(76)90153-2. [DOI] [PubMed] [Google Scholar]
- Ogilvie A., Wiebauer K., Spitzbarth P., Kersten W. Inhibition of leucyl-tRNA synthetase in Escherichia coli by the cytostatic 5,8-dioxo-6-amino-7-chloroquinoline. Biochim Biophys Acta. 1975 Oct 15;407(3):357–364. doi: 10.1016/0005-2787(75)90103-3. [DOI] [PubMed] [Google Scholar]
- RUBBO S. D., ALBERT A., GIBSON M. I. The influence of chemical constitution on antibacterial activity. V. The antibacterial action of 8-hydroxyquinoline (oxine). Br J Exp Pathol. 1950 Jun;31(3):425–441. [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Morris R. W., Faras A., Levinson W., Rutter W. J. Are all nucleotidyl transferases metalloenzymes? Biochem Biophys Res Commun. 1973 Aug 6;53(3):1036–1041. doi: 10.1016/0006-291x(73)90196-4. [DOI] [PubMed] [Google Scholar]


