Abstract
A Salmonella gene encoding a cytolysin has been identified by screening for hemolysis on blood agar. DNA sequence analyses together with genetic mapping in Salmonella suggest that it is unrelated to other toxins or hemolysins. The gene (slyA) is present in every strain of Salmonella examined, in Shigella, and in enteroinvasive Escherichia coli but not in other Enterobacteriaceae. SlyA (salmolysin) purified from a derivative of the original clone has hemolytic and cytolytic activity and has a molecular weight predicted by the DNA sequence. The median lethal dose and infection kinetics in mice suggest that the toxin is required for virulence and facilitates Salmonella survival within mouse peritoneal macrophages.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. W., Abrams C. K., Slatin S. L., Griffiths G. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell. 1990 Jun 29;61(7):1277–1287. doi: 10.1016/0092-8674(90)90692-8. [DOI] [PubMed] [Google Scholar]
- Benson N. R., Goldman B. S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992 Mar;174(5):1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Mackman N., Nicaud J. M., Holland I. B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986 Apr;52(1):63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Muhly M., Korom S., Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J Clin Invest. 1990 Jun;85(6):1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielecki J., Youngman P., Connelly P., Portnoy D. A. Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature. 1990 May 10;345(6271):175–176. doi: 10.1038/345175a0. [DOI] [PubMed] [Google Scholar]
- Braun V., Focareta T. Pore-forming bacterial protein hemolysins (cytolysins). Crit Rev Microbiol. 1991;18(2):115–158. doi: 10.3109/10408419109113511. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Camilli A., Tilney L. G., Portnoy D. A. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol Microbiol. 1993 Apr;8(1):143–157. doi: 10.1111/j.1365-2958.1993.tb01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri S. J., Snyder I. S. Effect of Escherichia coli alpha-hemolysin on human peripheral leukocyte function in vitro. Infect Immun. 1982 Sep;37(3):966–974. doi: 10.1128/iai.37.3.966-974.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. C., Libby S. J., Buchmeier N. A., Loewen P. C., Switala J., Harwood J., Guiney D. G. The alternative sigma factor katF (rpoS) regulates Salmonella virulence. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. M., Long S. R., Brown S. E., Buikema W. J., Ausubel F. M. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene. 1982 Jun;18(3):289–296. doi: 10.1016/0378-1119(82)90167-6. [DOI] [PubMed] [Google Scholar]
- Geoffroy C., Gaillard J. L., Alouf J. E., Berche P. Purification, characterization, and toxicity of the sulfhydryl-activated hemolysin listeriolysin O from Listeria monocytogenes. Infect Immun. 1987 Jul;55(7):1641–1646. doi: 10.1128/iai.55.7.1641-1646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
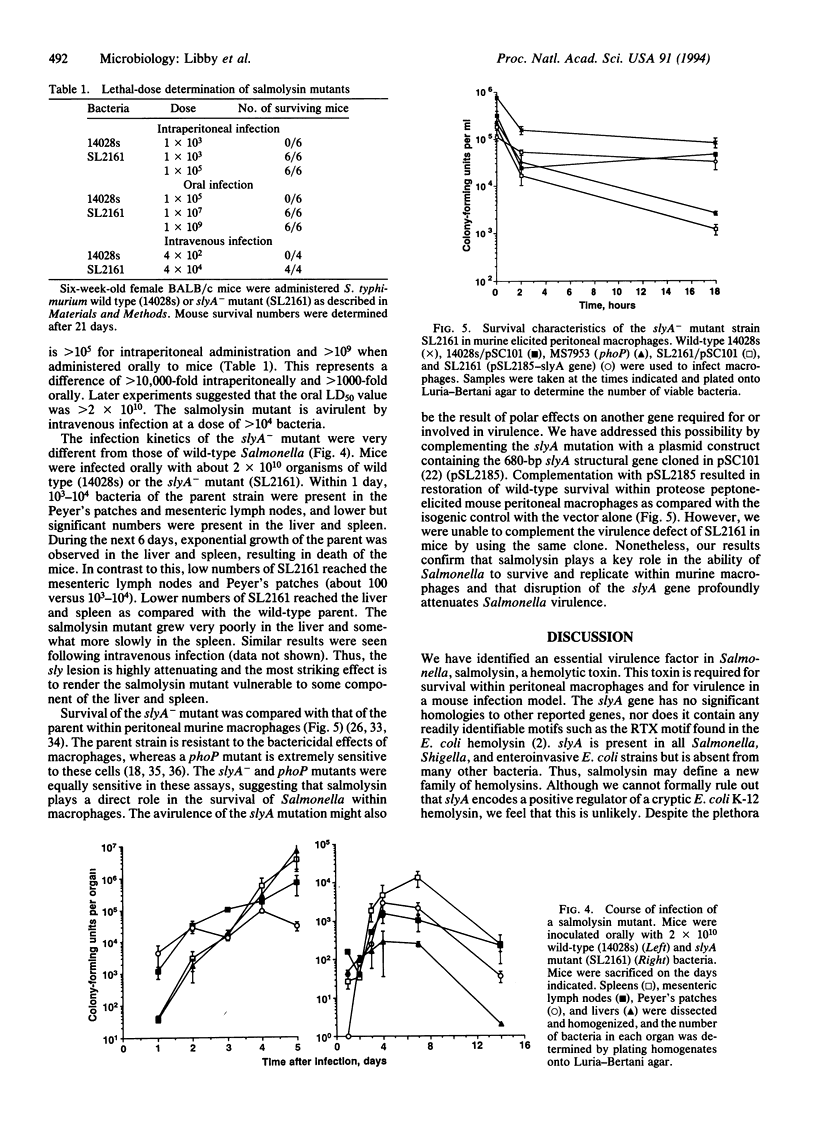
- Groisman E. A., Chiao E., Lipps C. J., Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage-Chahine C. M., Del Giudice G., Lambert P. H., Pechere J. C. Hemolysin-producing Listeria monocytogenes affects the immune response to T-cell-dependent and T-cell-independent antigens. Infect Immun. 1992 Apr;60(4):1415–1421. doi: 10.1128/iai.60.4.1415-1421.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathariou S., Metz P., Hof H., Goebel W. Tn916-induced mutations in the hemolysin determinant affecting virulence of Listeria monocytogenes. J Bacteriol. 1987 Mar;169(3):1291–1297. doi: 10.1128/jb.169.3.1291-1297.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König B., König W., Scheffer J., Hacker J., Goebel W. Role of Escherichia coli alpha-hemolysin and bacterial adherence in infection: requirement for release of inflammatory mediators from granulocytes and mast cells. Infect Immun. 1986 Dec;54(3):886–892. doi: 10.1128/iai.54.3.886-892.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby S. J., Goebel W., Muir S., Songer G., Heffron F. Cloning and characterization of a cytotoxin gene from Salmonella typhimurium. Res Microbiol. 1990 Sep-Oct;141(7-8):775–783. doi: 10.1016/0923-2508(90)90110-c. [DOI] [PubMed] [Google Scholar]
- Mulvey M. R., Switala J., Borys A., Loewen P. C. Regulation of transcription of katE and katF in Escherichia coli. J Bacteriol. 1990 Dec;172(12):6713–6720. doi: 10.1128/jb.172.12.6713-6720.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Rennie R. P., Freer J. H., Arbuthnott J. P. The kinetics of erythrocyte lysis by Escherichia coli haemolysin. J Med Microbiol. 1974 May;7(2):189–195. doi: 10.1099/00222615-7-2-189. [DOI] [PubMed] [Google Scholar]
- Roberts R. C., Burioni R., Helinski D. R. Genetic characterization of the stabilizing functions of a region of broad-host-range plasmid RK2. J Bacteriol. 1990 Nov;172(11):6204–6216. doi: 10.1128/jb.172.11.6204-6216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Songer J. G., Libby S. J., Iandolo J. J., Cuevas W. A. Cloning and expression of the phospholipase D gene from Corynebacterium pseudotuberculosis in Escherichia coli. Infect Immun. 1990 Jan;58(1):131–136. doi: 10.1128/iai.58.1.131-136.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco J., Winkler H. H. Comparison of properties of virulent, avirulent, and interferon-resistant Rickettsia prowazekii strains. Infect Immun. 1991 May;59(5):1647–1655. doi: 10.1128/iai.59.5.1647-1655.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991 Mar;5(3):521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Youderian P., Sugiono P., Brewer K. L., Higgins N. P., Elliott T. Packaging specific segments of the Salmonella chromosome with locked-in Mud-P22 prophages. Genetics. 1988 Apr;118(4):581–592. doi: 10.1093/genetics/118.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]