Abstract
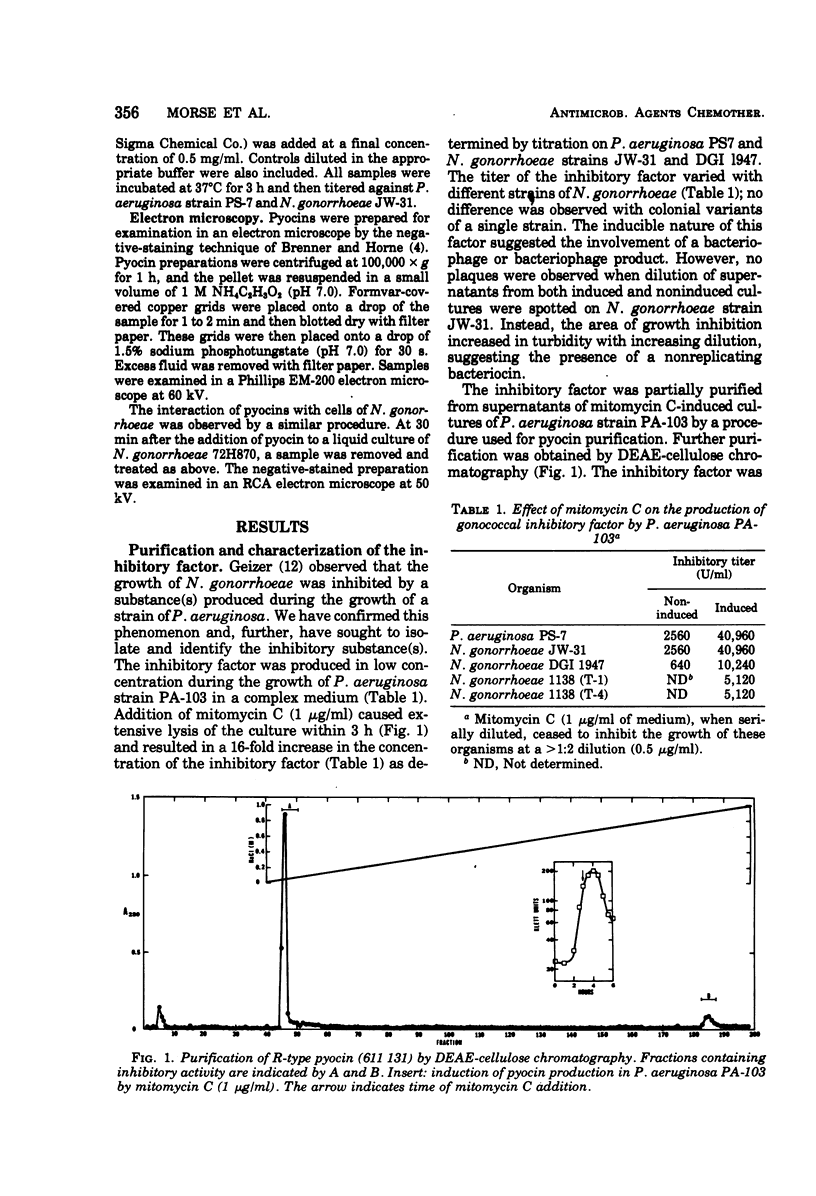
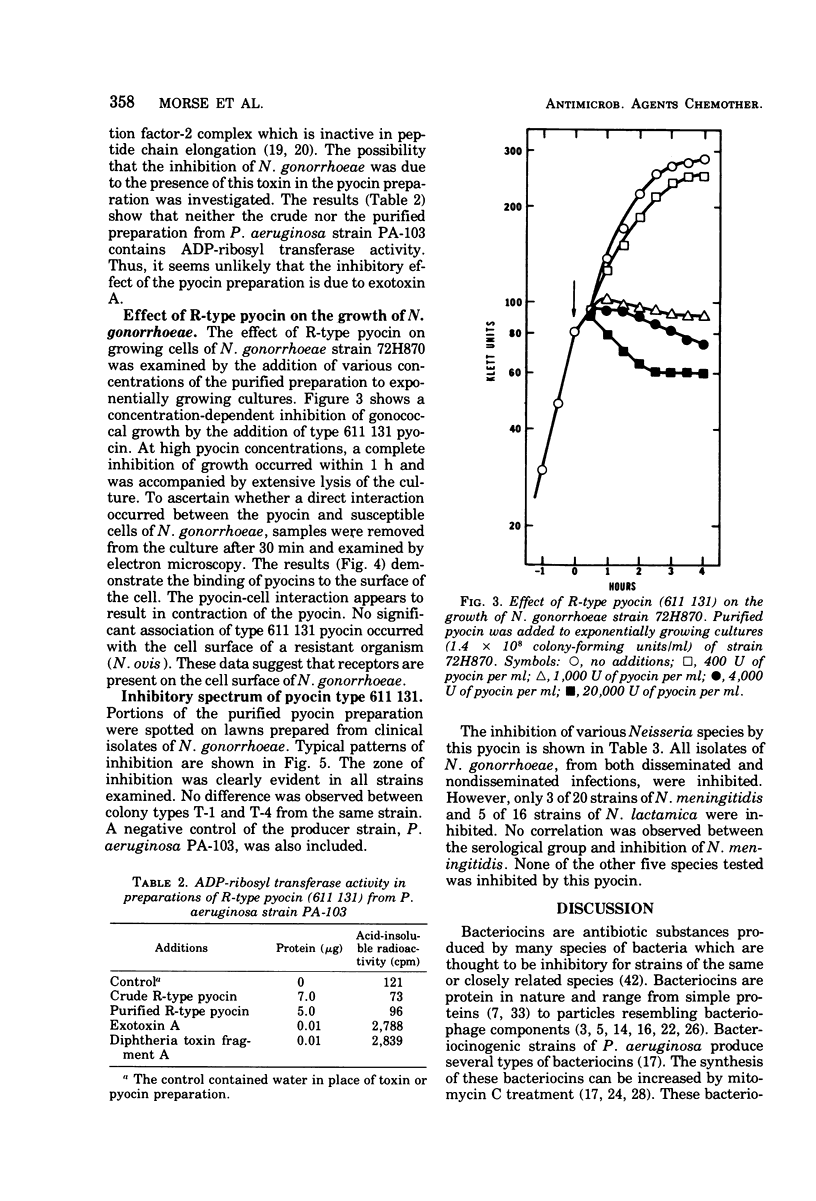
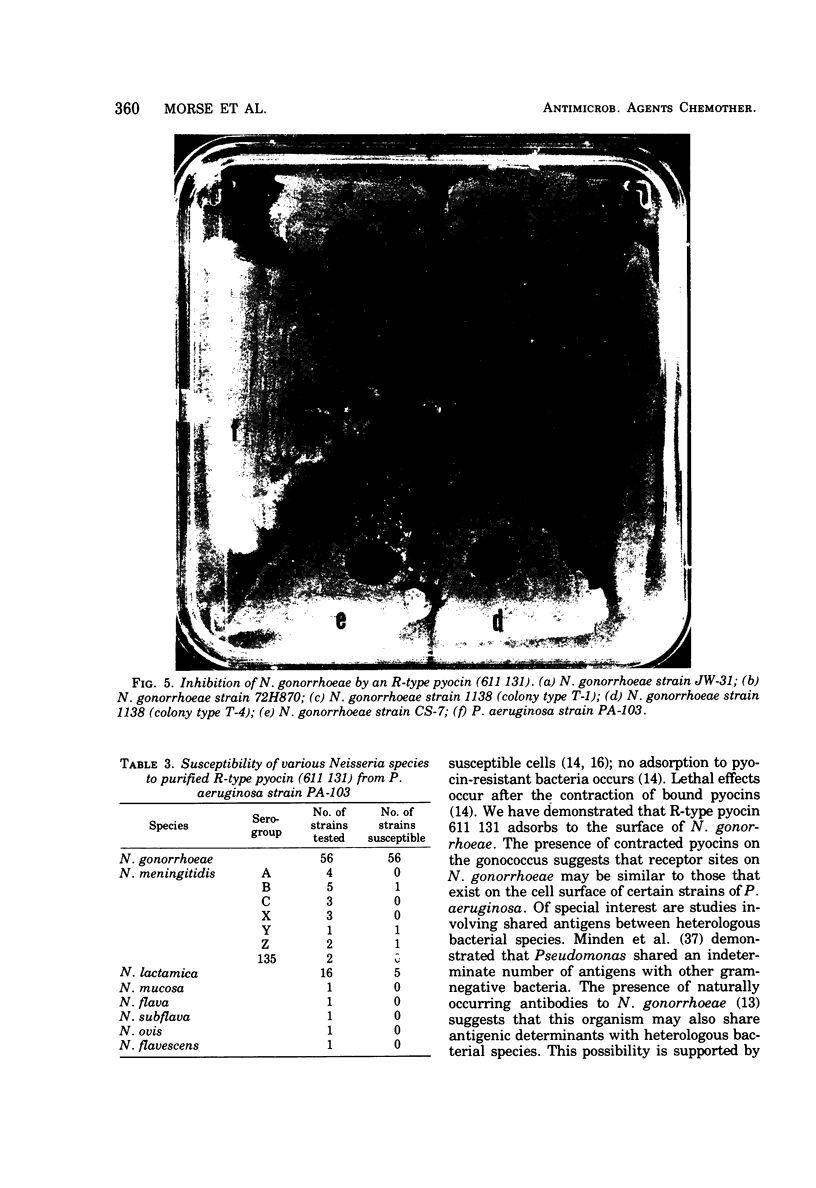
Supernatants from broth-grown cultures of Pseudomonas aeruginosa PA 103 exhibited bactericidal activity against Neisseria gonorrhoeae. The concentration of the bactericidal substance increased significantly after induction by mitomycin C. Purification was effected by salt fractionation, chromatography on diethylaminoethyl-cellulose, and sedimentation by centrifugation at 100,000 × g for 90 min. Electron microscopy of this purified preparation revealed structures resembling R-type pyocins in both the contracted and uncontracted state. Pyocins in the contracted state were observed in association with the gonococcal cell surface. No loss of bactericidal activity was observed after treatment with proteolytic enzymes. Standard pyocin typing procedures identified the pyocin pattern as 611 131. The bactericidal activity of this pyocin was examined on various species of Neisseria. Out of 56 strains of N. gonorrhoeae from disseminated and nondisseminated infections, all were susceptible to pyocin 611 131. However, only 3 of 20 strains of N. meningitidis and 5 of 16 strains of N. lactamica were susceptible. The bactericidal activity that pyocin 611 131 has for N. gonorrhoeae and other species of Neisseria is significant because it departs from the expected specificity that heretofore has distinguished bacteriocins from most “classical” antibiotics.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. H., SCHWEET R. S. Synthesis of hemoglobin in a cell-free system. I. Properties of the complete system. J Biol Chem. 1962 Mar;237:760–767. [PubMed] [Google Scholar]
- Allen J. C., Kelly P. C. Antigenic heterogeneity among pyocins of Pseudomonas aeruginosa. Infect Immun. 1975 Aug;12(2):318–323. doi: 10.1128/iai.12.2.318-323.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee H. L., De Klerk H. C., Coetzee J. N., Smit J. A. Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J Gen Virol. 1968 Jan;2(1):29–36. doi: 10.1099/0022-1317-2-1-29. [DOI] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Dandeu J. P. Chemical and immunological study of colicins e(1), k, a, and q. Infect Immun. 1971 Jan;3(1):1–9. doi: 10.1128/iai.3.1.1-9.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke J., Berk R. S. Growth inhibition and pyocin receptor properties of endotoxin from Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1974 Apr;145(4):1405–1408. doi: 10.3181/00379727-145-38023. [DOI] [PubMed] [Google Scholar]
- FEARY T. W., FISHER E., Jr, FISHER T. N. Lysogeny and phage resistance in Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1963 Jun;113:426–430. doi: 10.3181/00379727-113-28386. [DOI] [PubMed] [Google Scholar]
- Flynn J., McEntegart M. G. Bacteriocins from Neisseria gonorrhoeae and their possible role in epidemiological studies. J Clin Pathol. 1972 Jan;25(1):60–61. doi: 10.1136/jcp.25.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geizer E. Antibacterial substances produced by different bacteria inhibiting the growth of Neisseria gonorrhoea (preliminary report). J Hyg Epidemiol Microbiol Immunol. 1968;12(2):241–243. [PubMed] [Google Scholar]
- Glynn A. A., Ward M. E. Nature and Heterogeneity of the Antigens of Neisseria gonorrhoeae Involved in the Serum Bactericidal Reaction. Infect Immun. 1970 Aug;2(2):162–168. doi: 10.1128/iai.2.2.162-168.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: morphology and mode of action of contractile pyocins. J Gen Microbiol. 1974 Jan;80(1):1–15. doi: 10.1099/00221287-80-1-1. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Studies on the pyocins of Pseudomonas aeruginosa: production of contractile and flexuous pyocins in Pseudomonas aeruginosa. J Gen Microbiol. 1974 Jan;80(1):17–30. doi: 10.1099/00221287-80-1-17. [DOI] [PubMed] [Google Scholar]
- Higerd T. B., Baechler C. A., Berk R. S. Morphological studies on relaxed and contracted forms of purified pyocin particles. J Bacteriol. 1969 Jun;98(3):1378–1389. doi: 10.1128/jb.98.3.1378-1389.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Goto S., Shionoya H. Relationship between pyocine and temperate phage of Pseudomonas aeruginosa. II. Isolation of pyocines from strain P 1-III and their characteristics. Jpn J Exp Med. 1967 Oct;37(5):373–393. [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K., Egami F. Receptor substance for pyocin R. I. Partial purification and chemical properties. J Biochem. 1969 Apr;65(4):603–609. doi: 10.1093/oxfordjournals.jbchem.a129053. [DOI] [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- JACOB F. Biosynthèse induite et mode d'action d'une pyocine, antibiotique de Pseudomonas pyocyanea. Ann Inst Pasteur (Paris) 1954 Feb;86(2):149–160. [PubMed] [Google Scholar]
- Jones L. F., Zakanycz J. P., Thomas E. T., Farmer J. J., 3rd Pyocin typing of Pseudomonas aeruginosa: a simplified method. Appl Microbiol. 1974 Feb;27(2):400–406. doi: 10.1128/am.27.2.400-406.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAGEYAMA M., IKEDA K., EGAMI F. STUDIES OF A PYOCIN. III. BIOLOGICAL PROPERTIES OF THE PYOCIN. J Biochem. 1964 Jan;55:59–64. doi: 10.1093/oxfordjournals.jbchem.a127841. [DOI] [PubMed] [Google Scholar]
- KAGEYAMA M. STUDIES OF A PYOCIN. I. PHYSICAL AND CHEMICAL PROPERTIES. J Biochem. 1964 Jan;55:49–53. doi: 10.1093/oxfordjournals.jbchem.a127839. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Falkow S., Holmes K. K. Reevaluation of bacteriocinogeny in Neisseria gonorrhoeae. J Clin Pathol. 1975 Apr;28(4):274–278. doi: 10.1136/jcp.28.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krămer J., Brandis H. Purification and Characterization of two bacteriocins from Streptococcus faecium. J Gen Microbiol. 1975 May;88(1):93–100. doi: 10.1099/00221287-88-1-93. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966 Feb;116(1):112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- Minden P., McClatchy J. K., Farr R. S. Shared antigens between heterologous bacterial species. Infect Immun. 1972 Oct;6(4):574–582. doi: 10.1128/iai.6.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Adaptation of the Minitek system for the rapid identification of Neisseria gonorrhoeae. J Clin Microbiol. 1976 Jan;3(1):8–13. doi: 10.1128/jcm.3.1.8-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Stein S., Hines J. Glucose metabolism in Neisseria gonorrhoeae. J Bacteriol. 1974 Nov;120(2):702–714. doi: 10.1128/jb.120.2.702-714.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REEVES P. THE BACTERIOCINS. Bacteriol Rev. 1965 Mar;29:24–45. doi: 10.1128/br.29.1.24-45.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., Myerowitz L., Whisnant J. K., Argaman M., Schneerson R., Handzel Z. T., Gotschlich E. C. Enteric bacteria cross-reactive with Neisseria meningitidis groups A and C and Diplococcus pneumoniae types I and 3. Infect Immun. 1972 Nov;6(5):651–656. doi: 10.1128/iai.6.5.651-656.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead A., Main J. S., Ward M. E., Watt P. J. Studies on lipopolysaccharides isolated from strains of Neisseria gonorrhoeae. J Gen Microbiol. 1975 May;88(1):123–131. doi: 10.1099/00221287-88-1-123. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Studies on group A (phage tail) bacteriocins of Serratia marcescens. IV. Preliminary characterization of receptors of subgroup II bacteriocins. Zentralbl Bakteriol Orig A. 1974 Dec;229(4):490–502. [PubMed] [Google Scholar]
- Volk J., Kraus S. J. Asymptomatic meningococcal urethritis. Possible protective value against gonococcal infection by bacteriocin production. Br J Vener Dis. 1973 Dec;49(6):511–512. doi: 10.1136/sti.49.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON J. F. The extracellualr polysaccharides of bacteria. Bacteriol Rev. 1958 Mar;22(1):46–73. doi: 10.1128/br.22.1.46-73.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad D. L., Reitz R. C., Sparling P. F. Growth inhibition among strains of Neisseria gonorrhoeae due to production of inhibitory free fatty acids and lysophosphatidylethanolamine: absence of bacteriocins. Infect Immun. 1974 Sep;10(3):481–488. doi: 10.1128/iai.10.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]