Abstract
Background
The T cell suppressive property of bone marrow derived mesenchymal stromal cells (MSCs) has been considered a major mode of action and basis for their utilization in a number of human clinical trials. However, there is no well-established reproducible assay to measure MSC-mediated T cell suppression.
Methods
At the University of Wisconsin-Madison Production Assistance for Cellular Therapy (PACT) Center we developed an in vitro quality control T cell suppression immunopotency assay (IPA) which utilizes anti-CD3 and anti-CD28 antibodies to stimulate T cell proliferation. We measured MSC-induced suppression of CD4+ T cell proliferation at various effector to target cell ratios using defined peripheral blood mononuclear cells and in parallel compared to a reference standard MSC product. We calculated an IPA value for suppression of CD4+ T cells for each MSC product.
Results
Eleven MSC products generated at three independent PACT centers were evaluated for cell surface phenotypic markers and T cell suppressive properties. Flow cytometry results demonstrated typical MSC cell surface marker profiles. There was significant variability in the level of suppression of T cell proliferation with IPA values ranging from 27% to 88%. However, MSC suppression did not correlate with HLA-DR expression.
Discussion
We have developed a reproducible immunopotency assay to measure allogeneic MSC-mediated suppression of CD4+ T cells. Additional studies may be warranted to determine how these in vitro assay results may correlate with other immunomodulatory properties of MSCs, in addition to evaluating the ability of this assay to predict in vivo efficacy.
Keywords: Mesenchymal stromal cells, potency assay, PACT, T cell suppression
Introduction
Mesenchymal stromal cells (MSCs) are fibroblast-like cells that were originally discovered from bone marrow of rodents by Friedenstein and defined based on their osteogenic and hematopoietic supportive potential, but later they were derived from nonhematopoietic cell component of bone marrow from many other species including humans (1-6). These cells are now commonly defined by a certain cell surface expression profile and their multipotency, i.e. their ability to differentiate into bone, fat and cartilage lineages in in vitro assays (7-10). Bone marrow MSCs, or their derivatives, in their in situ niche are believed to be a major component of the microenvironmental stroma supporting hematopoiesis (11). However, it has also been shown that culture expanded MSCs could suppress or modulate immune responses via interaction with many different types of innate and adaptive immune cells. These include the suppression of T cell proliferation and their effector functions, modulation of B cell activity and immunoglobulin production, and changing the immunophenotype of monocytes and macrophages to name a few (12-18). Based on this wide range of immunomodulatory properties and the fact that MSC may be immunoprivileged across HLA barriers (19, 20), they have been investigated as a promising therapy for a wide range of immune mediated disorders. Indeed, initial safety and potential efficacy of MSCs has been demonstrated in graft versus host disease, autoimmune disorders, and solid organ transplantation among others (21-23).
As with many cellular therapies, MSC products are susceptible to inherent genotypic and phenotypic heterogeneity as a result of differences between donors and tissue sources (24-26). Lack of cell product consistency is also further amplified due to the lack of standardization of ex vivo expansion and cell manufacturing methods (27-30). As a result, cellular products generated for each clinical study are likely to have differences in their biological properties with the potential for significant product-to-product variability (31). Furthermore, MSCs are highly reactive cells whose immunodulatory function may depend on the degree of inflammation present in any given in vivo environment (32-34). Thus, it is reasonable to anticipate that different preparations of MSCs exert various degrees of immunomodulation in diverse patient populations and clinical indications (34, 35).
Another major challenge of defining MSC product consistency is the fact that clinical investigators utilize different assays and assay platforms to measure and report immunosuppressive or other properties of their MSC product (36, 37). A popular assay format widely used is based on mixed lymphocyte reaction (MLR) assays that measure specific immunosuppressive effect of MSCs on allogeneic T cell populations that are activated in the MLR reaction (38). Many of these challenges could be minimized or eliminated if an assay was developed to reproducibly measure a relevant immunomodulatory or other functional property of MSCs (39). It should be noted that since MSCs could be used for different clinical indications based on a single or a combination of functions, it may be impractical to anticipate that a single assay would be sufficient to elucidate the plethora of in vivo functions exerted by MSC (16).
Several NHLBI-supported Production Assistance for Cellular Therapy (PACT) centers produce clinical-grade MSC products for a variety of clinical indications (40, 41). These MSC products have been generated using different methodologies adopted and optimized at each PACT Center. We initiated this multicenter project with the goal of developing a standardized immunopotency assay (IPA) to be used to further characterize MSC products and reproducibly measure the ability of MSCs to suppress T cell proliferation, as an intended mechanism of action for treatment of immune disorders such as graft versus host disease (21), Crohn’s disease (42), multiple sclerosis (43) and systemic lupus erythematosus (44).
Materials and Methods
MSC Isolation and Culture Methodology
A summary of the MSC product manufacturing methodologies are briefly described below and a direct side-by-side comparison is provided in Table 1.
Table 1.
Summary of MSC Culture Methodologies
| PACT Center-1 | PACT Center-2 | PACT Center-3 | |
|---|---|---|---|
|
Bone Marrow
Aspirate |
100 mL- fresh | 25 mL- fresh | 25 mL- fresh or frozen |
| MSC Isolation | Ficoll gradient/RBC lysis: Plate MNCs in T-flasks |
Plated aspirate in Quantum |
Ficoll gradient: Plate MNCs in T-flasks |
| Culture Medium | Alpha-MEM 10% FBS 1× GlutaMax |
DMEM 5% human platelet lysate 2mM GlutaMax 10mM N-acetylcysteine 2 IU/mL heparin |
Alpha-MEM 16.5% FBS 1× GlutaMax |
| Culture Conditions | Seed T-225 cm2 grow to 80 to 90% confluence Half-feed day 1, then full feeds every 2-4 days Establish P2 MCB Expand P2 to P5 in Cell Factories (CFs) Seed CFs at 2 to 3 × 103 cells/cm2 |
Seed unfractionated marrow into Quantium Bioreactor (Terumo BCT) Remove unattached cells at 24-48 hours Continuous feed at 0.1 mL/min then when lactate level >4.0 mM; feed at 1.6 mL/min; P1 harvest when lactate >4.0 mM |
Seed T-flasks at 1.0 to 1.5 × 105 cells/cm2 Feed D1 & 2 and every 2-4 days; grow to 70- 80% confluence Seed cell factories at 40- 50 cells/cm2; feed every 2-4 days to 70-80% confluence |
| Cryopreservation | Plasma-Lyte A, 5% HSA, and 2.5% DMSO |
Plasma-Lyte A, 5% HSA, and 10% DMSO |
Plasma-Lyte A, 12.5% HSA, and 10% DMSO |
For method validation only
PACT Center-1
Fresh 100 mL bone marrow-aspirates were collected from healthy donors in accordance with approved IRB protocols. Bone marrow aspirate peripheral blood mononuclear cells (PBMCs) were isolated using standard Ficoll separation (GE healthcare Bio-Sciences, Piscataway, NJ) and red blood cell (RBC) lysis (Sigma-Aldrich, St. Louis, MO) methods before seeding T-225 cm2 culture flasks (Corning, Lowell, MA). PBMCs were plated at 5 × 104 cells/cm2 and grown in alpha-MEM (Mediatech, Manassas, VA) supplemented with 10% FBS (Life Technologies, Grand Island, NY) and 1x GlutaMax (Life Technologies). Approximately half of the culture media was replaced with fresh media on day 1 post-seed, to selectively remove non-adherent cells; cultures were then fed every 2 to 4 days (replaced full media volume) and allowed to grow to 80-90% confluence before each passage. A Master Cell Bank (MCB) was generated at passage 2 (P2) and were frozen using 10% Dimethyl sulfoxide (DMSO, Sigma-Aldrich). The final MSC product was produced by thawing and expanding the P2 MCB to passage 5 (P5) in Cell Factories (Nunc, Thermo Scientific, Waltham, MA). The MSC seeding density into Cell Factories was 2-3 × 103 cells/cm2 and passaged using TrypLE Select (Life Technologies). MSCs were fed every 2-4 days until reaching 80-90% confluence. MSCs were cryopreserved using Plasma-Lyte A (Baxter, Deerfield, IL) containing 2.5% DMSO and 5% Human Serum Albumin (HSA) (Baxter).
PACT Center-2
Fresh 25 mL bone marrow-aspirates were collected from healthy donors in accordance with approved IRB protocols. All 25 mL of unfractionated bone marrow-aspirate containing PBMCs was seeded into the Quantum bioreactor (Terumo BCT, Lakewood, CO). Unattached cells were removed after 24 hours and DMEM (Lonza, Walkersville, MD), 5% human platelet lysate was prepared from expired platelet packs (Gulf Coast Regional Blood Center), 2mM GlutaMax (Life Technologies, Grand Island, NY), 10mM N-acetylcysteine (Roxane, Columbus, OH), 2 IU/mL heparin (APP-Fresenius, Schaumburg, IL) medium was perfused through the system. Lactate measurements were obtained daily and when the level reached 4mM the perfusion rate was doubled until flow rate reached 0.4mL/min and the lactate was at 4mM, at which point the cells were harvested using TrypLE Select (Life Technologies). MSCs were then cryopreserved in Plasma-Lyte A (Baxter, Deerfield, IL) containing 10% DMSO (Bioniche Pharma, Lake Forest, IL) and 5% HSA (Baxter).
PACT Center-3
Fresh 100 mL bone marrow aspirates were collected from healthy donors in accordance with approved IRB protocols. Twenty-five (25 mL) aliquots of bone marrow-aspirate, either fresh or frozen, containing PBMCs was seeded at 1.0-1.5 × 105 cells/cm2 in an appropriately sized T-flask (Thermo Scientific, Waltham, MA) after density gradient separation of MNC. Media consisted of alpha-MEM (Life Technologies, Grand Island, NY) containing 16.5% FBS (Thermo Scientific) and 1× Glutamax (Life Technologies). On days one and two, non-adherent cells were removed as spent media was replaced with fresh media. Media was exchanged every 2-4 days until 70-80% confluence was reached between 7 and 10 days. Cells were detached and used to seed a cell factory at 40-50 cells/cm2. Media in the cell factory was replenished every 2 to 4 days over the next 7 to 12 days. On day 18 to 21 when the cultures reached 70-80% confluence, cells were harvested and cryopreserved in Plasma-Lyte A (Baxter, Deerfield, IL) containing 12.5% HSA (Baxter) and 10% DMSO (Bioniche Pharma, Casla Co., Galway, Ireland).
PBMC Isolation and Cryopreservation
Leukapheresis products collected from different normal healthy donors were purchased from SeraCare Life Sciences (Milford, MA). After Ficoll separation, between 4 and 5 × 109 PBMCs were recovered from each of the two leukapheresis products designated as leukopack 4 (LPK4) and leukopack 6 (LPK6). PBMCs from each leukopack were then cryopreserved at 1 × 107 cells/vial using 90% FBS (Atlanta Biologics, Atlanta, GA) and 10% DMSO (Sigma-Aldrich, St. Louis, MO) and stored in vapor phase LN2 before use in this assay.
Immunopotency Assay
The immunopotency assay (IPA) was established using a 48 well tissue culture plate with a total IPA medium volume of 400 ul per well. MSCs and PBMCs, as well as anti-CD3 and anti-CD28 antibodies (clones UCHT1 and 37407, respectively) (R&D Systems, Inc., Minneapolis, MN), were all prepared in IPA medium consisting of RPMI-1640 containing 10% heat inactivated FBS, 1× non-essential amino acids (NEAA) (Mediatech, Inc., Manassas, VA), 1× Glutamine (Mediatech, Inc.), 1X Na Pyruvate (Sigma-Aldrich), and 1× HEPES buffer (Sigma-Aldrich,, St. Louis, MO). Preparation of MSCs for this assay included a thaw, wash, and re-suspension at 4 × 106/mL. To eliminate the confounding factor of their proliferative potential in this evaluation, MSCs were gamma irradiated using 21 Gy before being plated. For a 1:1 (PBMC:MSC) ratio, 4 × 105 MSCs (100ul) were plated and then titrated further to 2 ×104 to achieve a 1:0.05 (PBMC:MSC) ratio. To establish a reliable gating strategy, we used a non-stimulated control consisting of a 1:0.05 PBMC:MSC cell ratio without the addition of anti-CD3 and anti-CD28. T cells in these non-stimulated control wells did not proliferate yet their survival was maintained by the presence of MSCs. In no instance did MSCs induce T cell proliferation without the addition of anti-CD3 and anti-CD28 antibodies which demonstrates the robustness of this as a negative control. The stimulated PBMC to MSC ratios that were evaluated in this assay include 1:0, 1:1, 1:0.5, 1:0.2, 1:0.1, and 1:0.05. The volume of MSCs was held constant at 200 ul/well using IPA medium. After plating, the cells were allowed to settle and adhere to the plastic for 2 hours at 37 °C. To measure proliferation, PBMCs from a single LPK were labeled with carboxyfluoroscein succinate-ester (CFSE) at a final concentration of 1uM for 10 minutes, at 37 °C in the dark, mixing at the 5 minute time point to ensure homogeneous labeling. An equal volume of cold FBS was added for 1 minute to stop the CFSE labeling reaction. PBMCs were then washed twice with IPA medium as defined above before reconstitution at 4 × 106/mL. One hundred microliters of CFSE-labeled PBMCs was added to each well containing MSCs. Every assay included a MSC only control. A 100 ul mixture of 4× concentrated anti-huCD3 and anti-huCD28 antibodies (10 ug/mL and 2 ug/mL, respectively) was added to each well except for the 1:0.05 (PBMC:MSC) non-stimulated control which received 100ul of IPA media instead. Cells were cultured for 4 days at 37 °C before the CD4+ T cells were analyzed for proliferation using standard flow cytometry methodology. Anti-huCD4 APC (R&D Systems, Inc.) was used to gate the CD4+ T cells. All IPA analyses were performed using an Accuri C6 flow cytometer (BD Biosciences, Inc., San Jose, CA) and the associated C6 Plus software was used for the CFSE analysis. Flow cytometry gating strategies are shown in Figure 1 (Results Section).
Figure 1. Gating Strategy.
For the IPA, CFSE-labeled PBMCs are co-cultured with titrated numbers of MSCs for 4 days. Upon cell harvest, co-cultures are incubated with an anti-CD4 APC-labeled antibody and analyzed by flow cytometry. CD4+ cells (Y-axis) are shown in conjunction with CFSE (X-axis) in order to analyze the extent of CD4+ proliferation as measured by the dilution of CFSE intensity. Shown in the top row are representative dot plots with corresponding histograms in the second row. Illustrated in the third row are plots for each PBMC:MSC ratio representing distinct populations generated from the parental population which is highlighted in blue. A CFSE-lo gate was set with the non-stimulated PBMC control (1:0.05 non-stim) in order to assess proliferation in stimulated samples. Lack of MSC in 1:0 (PBMC:MSC) ratio allows stimulated CD4+ cells to proliferate without inhibition. Proliferation is significantly inhibited at a 1:1 ratio which is then regained as MSC numbers are titrated down. The values of the CFSE-lo gate are used for IPAv analysis.
sIL-2R ELISA
The IPA assay was set up as described above. After 4 days, culture supernatants were carefully removed from each well and frozen at −20 °C for future soluble interleukin-2 receptor (sIL-2R) analysis. On the day of analysis, supernatants were thawed on ice for use in a human sIL-2R enzyme-linked immunosorbent assay (ELISA). A Platinum sandwich ELISA kit (eBioscience, Inc., San Diego, CA) was used to quantify sIL-2R levels. Absorbance was measured at 450 nm on an Epoch plate reader (BioTek, Inc., Winooski, VT) and analyzed using Gen5 software (Biotek, Inc.).
Flow Cytometry for MSC Markers
An eight-color flow cytometry panel for cell surface markers of MSC products was performed using a FACS Aria (BD Biosciences, Inc., San Jose, CA) flow cytometer. Antibodies specific for human CD14, CD19, CD34, CD45, CD73, CD90, CD105, HLA-Class I and HLA-DR were all purchased from BD Biosciences. To better assess assay variability, all assays included the use of a UW MSC reference standard (RSP5). All flow analysis was evaluated using the FlowJo software (Tree Star Inc., Ashland, OR).
Determination of the IPA Suppression Value
The IPA value (IPAv) is calculated as the mean ‘white space’ of the titration curve. Each MSC product was tested at five different titrations/ratios to render a titration curve which was used to calculate a single value for each curve (see formula below). This was achieved by determining the suppression value (Sv) for each ratio. To calculate the Sv, the percent proliferation of stimulated T cells (PT) in the presence of MSCs (M) is divided by the percent proliferation of stimulated T cells without MSCs which is then multiplied by 100. This number is then subtracted from 100. The Sv is determined for all titrations. The sum of all suppression values is then divided by the total number of titrations (nt). The resulting ‘mean suppression value’ is a number designated as the IPAv.
Results
MSC Potency Assay for Suppression of CD4+ Proliferation
The IPA measures the suppression of CD4+ T cell proliferation via flow cytometry, using the tracking dye CFSE in conjunction with an anti-CD4 APC-labeled antibody. The gating strategy for CD4+ T cell proliferation of a representative IPA, using the reference standard BM-MSC product RSP5, is depicted in Figure 1. Assay co-cultures were allowed to incubate for four days before harvesting. Cells were then labeled with an anti-huCD4 APC antibody in order to assess proliferation of the CD4+ T cell subset by loss of CFSE intensity (45). At the 1:0 (PBMC:MSC) ratio (no MSCs to suppress proliferation) proliferation was measured to be 84%, whereas at the 1:1 ratio proliferation was suppressed to 2%. At the same time, CD4+ T cell proliferation was recovered as the numbers of MSCs were titrated down. For example, at the ratio of 1:0.1 (PBMC:MSC) CD4+ T cell proliferation was 34% or 60% T cell suppression (relative to the stimulated T cell proliferation of 84% at the 1:0 ratio).
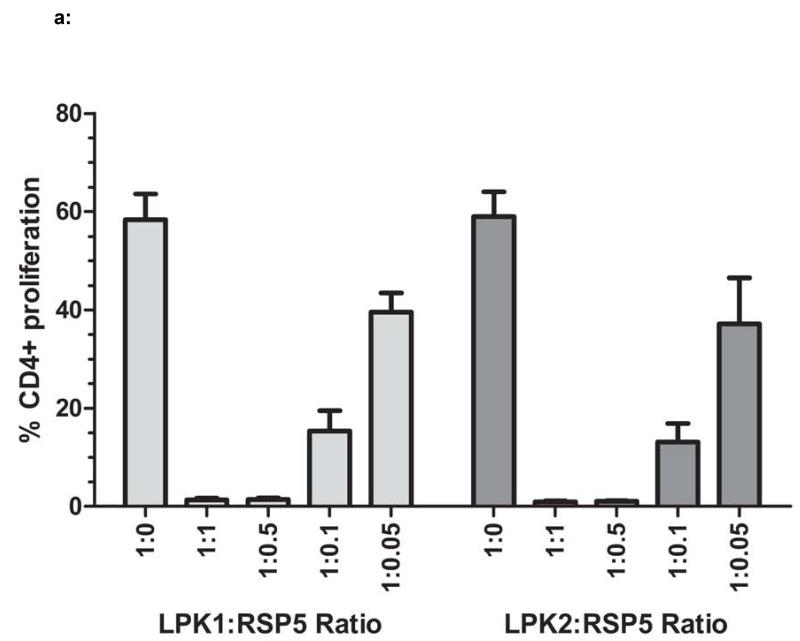
The IPA is highly reproducible, both within a single assay as well as between assay runs. Figure 2a shows the results of MSC reference standard RSP5 used to suppress the proliferation of T cells from two different apheresis donors, LPK1 and LPK2. All titrations were performed in duplicate and replicate assays were performed on three different days. At the 1:0 (PBMC:MSC) ratio, total CD4+ proliferation was approximately 60% with minimal standard error. For the reference standard, suppression was >95% for 1:1 and 1:0.5 ratios, again with a standard error of <1%. As RSP5 MSCs are titrated, proliferation capabilities are regained but usually do not exceed the level of the 1:0 ratio. Occasionally, however, proliferation at the lower 1:0.05 ratio can exceed the proliferation of the 1:0 ratio (no MSC) depending on the MSC product. Such stimulatory properties of MSC at low concentrations have been described by others (38).
Figure 2. Reproducibility of IPA/Titration Curve.
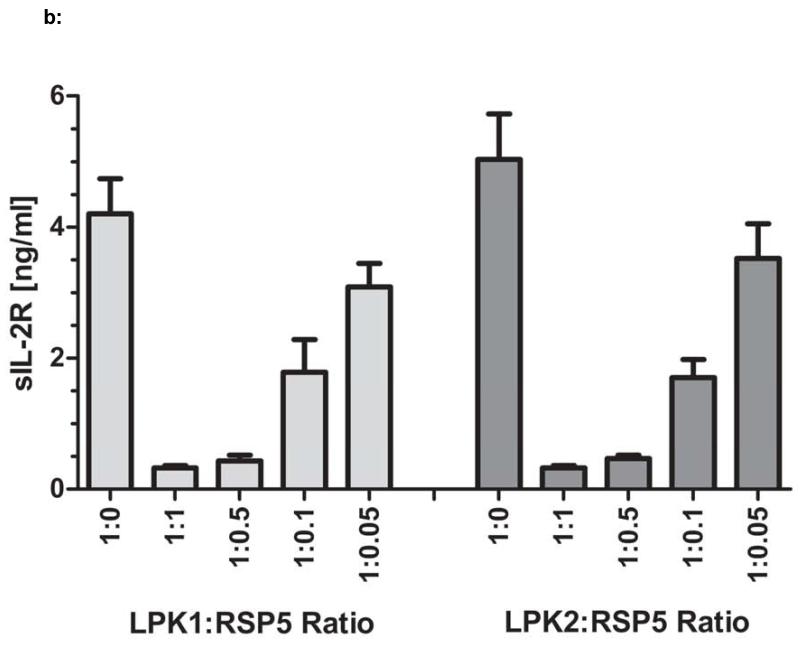
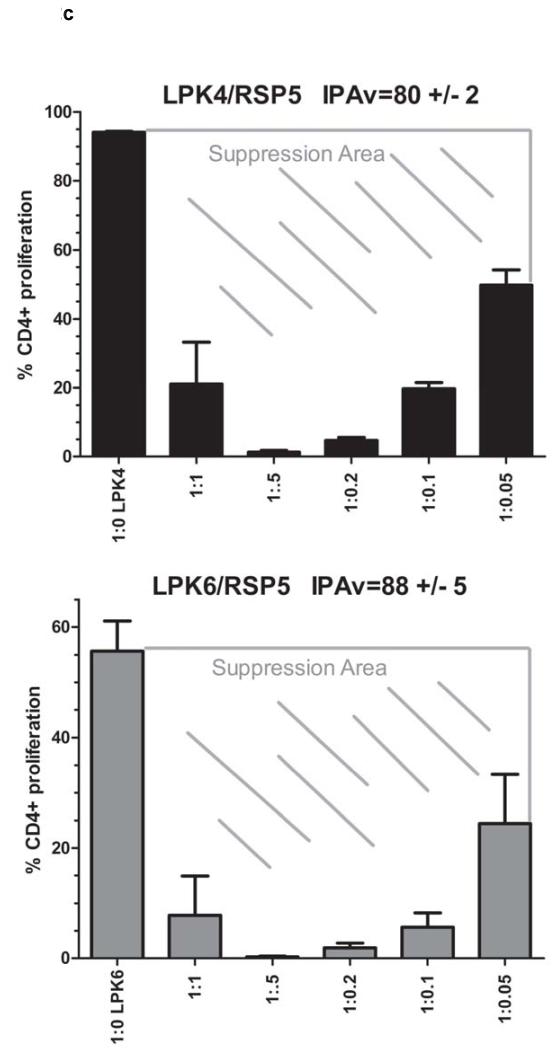
Figure 2a: The reference standard MSC line RSP5 was used in IPAs in which RSP5 was co-cultured with T cell stimulated PBMCs from two different healthy donors, LPK1 or LPK2. These IPAs were repeated on three different days to assess reproducibility of this assay. Standard error of suppression at each PBMC:MSC ratio is shown for LPK1 and LPK2. A suppression value, termed IPAv, was calculated for this assay (see Material and Methods). Figure 2b: The supernatants from the IPA cultures of all assays shown in Figure 2a were assessed for levels of sIL-2R using ELISA. Figure 2c: The IPAv is essentially the ‘suppression area’ or grey cross hatched area represented as a single value used to evaluate each MSC line’s PBMC inhibitory capabilities or potency.
In an effort to confirm the functional utility and correlation of this newly developed IPA with currently established potency assays we compared the IPA to sIL-2R levels using a commercially available quantitative ELISA assay. The detection of sIL-2R in the supernatants of T cell: MSC co-cultures is used as an indirect measurement for T cell activation and proliferation (46). As such, we analyzed the supernatants taken from the IPA co-cultures described above (Figure 2a) for sIL-2R. Using the sIL-2R ELISA, we demonstrated that sIL-2R levels in the IPA supernatants correlated very closely with CD4+ T cell proliferation (Figure 2b). For all assays performed, which included RSP5 co-cultured with LPK1 and LPK2 on three different days, the average correlation coefficient for CFSE-based proliferation versus sIL-2R was R2=0.98 +/−0.01. After verification of the significant correlation between IPA value and sIL-2R levels, we chose the IPA assay for its ease and practicality of use in quality control applications as well as its cost effectiveness.
Importantly, the degree of CD4+ T cell proliferation for the 1:0 PBMC:MSC ratio was dependent on the leukopack used. T cell proliferation ranged from 40 to 95% when evaluating several different leukopacks from a number of different healthy donors (data not shown). Use of PBMCs from donors that demonstrated a wide range of proliferation at the 1:0 ratio (LPK4 and LPK6) allowed the determination of MSC suppression within the context of variable T cell proliferation. We compiled the reference standard IPAs that were performed with each of the MSC products tested to verify assay consistency. For the three assays using RSP5 with LPK4 and LPK6, standard error ranged from 0.3-10% depending on the PBMC:MSC ratio. IPA values, based on the white spaces or grey cross hatched areas of the titration curves as described in Materials and Methods, averaged 80 (+/− 2)% for LPK4-screened RSP5 and 88 (+/− 5)% for LPK6 (Figure 2c).
IPA and Cell Surface Marker Analysis of MSC Products Generated at Three PACT Centers
We determined a single suppression value (IPAv) for eleven MSC products provided by three PACT Centers. All MSC products were evaluated in parallel with the RSP5 reference standard to assess and verify assay-to-assay reproducibility. PBMCs from LPK4 and LPK6 that were co-cultured separately with each titrated MSC product. Flow cytometric analysis for characteristic cell surface MSC, cell recovery, viability, suppression curves and IPAv was measured for each MSC product.
PACT Center-1
Three independent BM-MSC P5 products were generated from three different healthy donors designated as products 1A, 1B, and 1C. Cells were cryopreserved at 1-2 × 106/mL using cryovials to accommodate future evaluation in this IPA. PACT Center-1 products 1B and 1C and the reference standard RSP5 were analyzed. The viability of PACT Center-1 MSC products used in the IPA were all ≥ 90% (Table 2), and they were >95% positive for CD73, CD90, CD105 and HLA Class I, and <3% positive for CD14, CD19, CD34, and CD45 (Figure S1). HLA-DR expression was noted to be between 0.2 to 2.7% for PACT Center-1 products with the reference standard RSP5 measured to be 14% HLA-DR positive. The degree of T cell suppression for products 1A, 1B, and 1C was examined using the IPA. Shown in Figure 3 are the suppression curves using LPK4 and LPK6. The 1C MSC product routinely suppressed T cell proliferation to a greater extent than the other two products when co-cultured with LPK4 and LPK6 as determined by the IPAv. For example, the IPAv of LPK4 screened products was 92% for 1C versus 70% and 69% for 1A and 1B, respectively. The IPAv of LPK6 screened products was 88% for 1C versus 65% and 62% for 1A and 1B, respectively. Therefore, the same IPAv trend was seen for both LPK4 and LPK6-screened products, with product 1C having the highest value of the 3 different donor MSC products evaluated.
Table 2. MSC Product Characteristics.
| MSC | Recovery (%)* | Viability (%) | LPK4 (%) / LPK6 (%) = IPAv |
HLA-DR (%) |
|---|---|---|---|---|
| RSP5 (Vial) | 90 | 95 | 80/88 | 13.3 |
| Product 1 A (Vial) | 50 | 98 | 70/65 | 2.7 |
| Product 1 B (Vial) | 50 | 96 | 69/62 | 0.2 |
| Product 1 C (Vial) | 50 | 90 | 92/88 | 0.3 |
| Product 2 A (Vial) | 89 | 97 | 64/80 | 3.3 |
| Product 3 D.1 (Vial) | 50 | 90 | 55/58 | 2.2 |
| Product 3 D.2 (Vial) | 45 | 94 | 53/65 | 1.4 |
| Product 3 E (Vial) | 46 | 90 | 48/61 | 1.7 |
| Product 3 A. 1 (Bag) | 49 | 86 | 34/49 | 0.2 |
| Product 3 A.2 (Bag) | 55 | 87 | 27/42 | 0.3 |
| Product 3 B (Bag) | 49 | 74 | 47/59 | 0.6 |
| Product 3 C (Bag) | 55 | 85 | 62/77 | 0.3 |
Recovery (%) = Represents the number of live cells recovered after thaw divided by the number of total cells assessed at the time of cryopreservation.
Figure 3. IPA of PACT Center-1 MSC Products.
Three MSC lines from PACT Center-1, products 1A, 1B, and 1C, were analyzed for their ability to suppress CD4+ T cell proliferation using the IPA assay. PBMCs from two different healthy donors, LPK4 and LPK6, were utilized in these assays. The PBMC:MSC ratios (LPK:MSC ratios) ranged from 1:1 to 1:0.05. The data in top panel are IPA’s performed with LPK4 and the bottom panel represent the same samples performed using LPK6. IPA values (IPAv) were calculated for each IPA. The IPAv’s were highest (i.e., strongest suppression) for MSC Product 1C, using either LPK4 or LPK6.
PACT Center-2
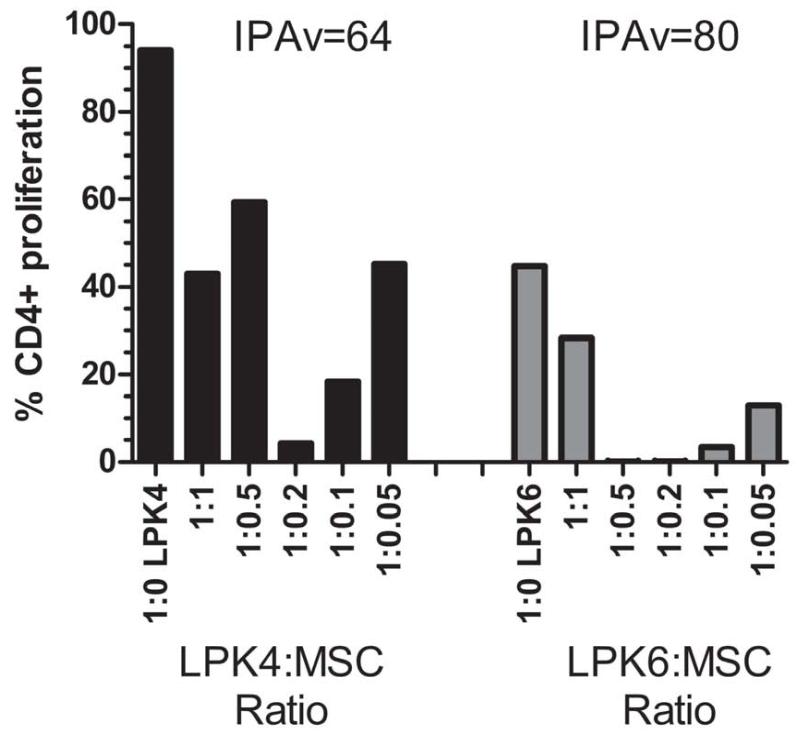
The MSC product from PACT Center-2 was cultured in media supplemented with 5% human platelet lysate. This MSC product was cryopreserved in 1.5 mL cryovials at 1 × 107cells/mL. Viability of this PACT Center product was 97% (Table 2). Flow cytometry analysis for MSC marker expression on the PACT Center-2 MSC product 2A demonstrated >99% positivity for CD73, CD90, CD105 and HLA Class I, and <2% for CD14, CD19, CD34, and CD45. HLA-DR expression was detected to be 2% (Figure S2). The MSC product 2A suppressed T cell proliferation at all ratios tested with an IPAv of 64% for the LPK4 PBMC and 80% for LPK6 (Figure 4). Of note is the higher degree of CD4+ T cell proliferation at the 1:1 (PBMC:MSC) and 1:0.5 ratios for LPK4 screened products compared to the 1:0.2 ratio. Such a titration curve is also not uncommon and highlights the importance of analyzing a range of MSC titrations in order to gain a more complete picture of the suppression capability of specific MSC products proliferation at certain ratios (38).
Figure 4. IPA for PACT Center-2 MSC Product.
The MSC product from PACT Center-2 was analyzed for its ability to suppress the proliferation of CD4+ T cells using the IPA assay. The PBMCs from two different healthy donors, LPK4 and LPK6, were utilized in these assays. The 1:0 (PBMC:MSC) ratio is shown on the left of each graph. The PBMC:MSC ratios (LPK:MSC ratios) ranged from 1:1 to 1:0.05. The plot on the left represents the IPA performed with LPK4 and the plot on the right shows the IPA performed with LPK6. IPA values (IPAv) were calculated to be 64 and 80 for IPAs using LPK4 and LPK6, respectively.
PACT Center-3
MSC products were derived from a bone marrow-aspirate using 16.5% FBS. This methodology includes the use of fresh 100 mL of bone marrow which is typically divided into 25 mL aliquots and one fresh 25 mL aliquot would be plated while the other three aliquots are frozen for later use. Passage 1 (P1) cells are used to inoculate a cell factory allowed to expand until passage 2 (P2) then harvested and cryopreserved (Table 1). We analyzed four MSC products which were cryopreserved in bags (3A.1, 3A.2, 3B, and 3C) and an additional three products that were cryopreserved in vials (3D.1, 3D.2, and 3E) for a total of seven MSC products. Products 3A.1 and 3A.2 represent duplicates of the same MSC product derived from the same donor, as do products 3D.1 and 3D.2. By analyzing the same cell products in duplicate we were able to examine the reproducibility and variability of our assay as well as the cell viability and recovery. Flow cytometry demonstrated typical MSC marker characteristics with CD73, CD90, CD105, and HLA Class I all >98% and CD14, CD19, and CD45 all <2% (Figures S3a and S3b). HLA-DR expression was <2.5% for all PACT Center-3 MSC products tested and CD34 expression ranged from 0.4-8.4%.
Overall, all seven MSC products (3A.1, 3A.2, 3B, 3C, 3D.1, 3D.2, and 3E) from PACT Center-3 demonstrated some level of CD4+ T cell proliferation suppression (Table 2). For LPK4 screened MSC products 3A (3A.1 and 3A.2), 3B, and 3C IPA suppression values ranged from 27 to 62% as shown in Figure 5a, while for LPK6 screened MSC products, IPA values ranged from 42% to 77%. The IPA values correlated well between LPK4 and LPK6 screens, since the lower suppression values were generated from MSC products 3A.1 and 3A.2, and the highest values were generated for product 3C.
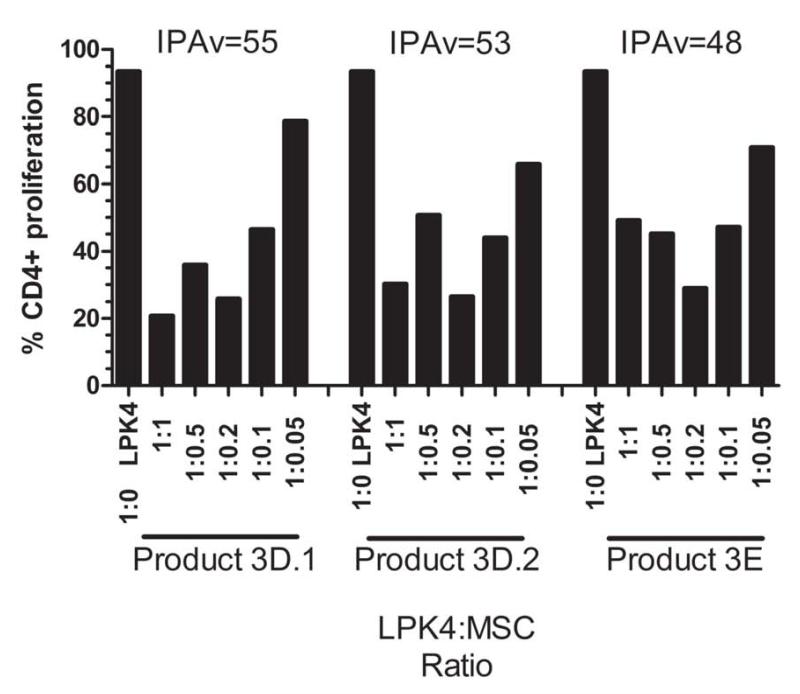
Figure 5. IPA for PACT Center-3 Products.
The MSC products from PACT Center-3 were assessed for their ability to suppress the proliferation of CD4+ T cells using the IPA assay. PBMCs from two different healthy donors, LPK4 and LPK6, were used in these assays. Five different PBMC:MSC ratios (LPK:MSC ratios) were used and ranged from 1:1 to 1:0.05. The 1:0 (PBMC:MSC) ratio is shown on the left. The suppression values, or IPA values (IPAv), were calculated for each IPA assay and are shown directly above each plot. Figure 5a: The IPAs of products 3A.1, 3A.2, 3B, 3C, 3D.1, 3D.2, and 3E cultured with PBMCs from healthy donor LPK4. Figure 5b: The IPAs of products 3A.1, 3A.2, 3B, 3C, 3D.1, 3D.2, and 3E cultured with PBMCs from healthy donor LPK6.
The IPA values for MSC products 3D.1, 3D.2, and 3E that were screened with LPK4 and LPK6 are provided in Figure 5b. The IPA values for products 3D.1 and 3D.2 are 55% and 53%, respectively, for LPK4 co-cultures. For LPK6 screened MSC products, IPA values for products 3D.1 and 3D.2 are 58 and 65% respectively. Viability upon thaw ranged from 74 to 94% for the Center-3 MSC products (Table 2).
Viability and DR levels do not correlate with In Vitro Suppression Values
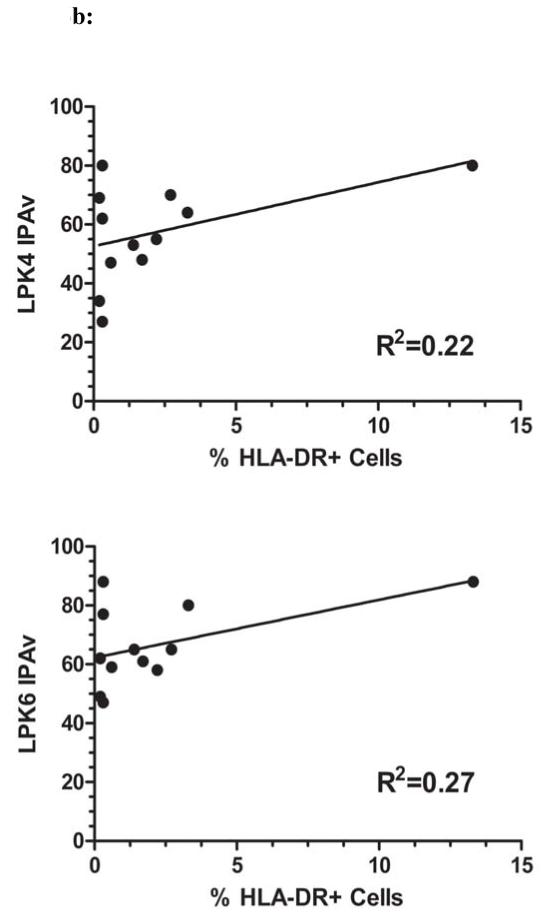
We examined whether the differences in IPA values could be explained by differences in cell viability at the time of assay setup. Thus, we compared the IPA values with viability of all MSC products from the three PACT centers and the reference standard. As shown in Figure 6a, there was little correlation between IPA and viability results. Correlation coefficients were R2=0.21 for LPK4 screened products and R2=0.12 for LPK6 screened MSC products. In addition, suppression did not correlate strongly with HLA-DR expression as correlation coefficients were R2=0.22 and R2=0.27 for LPK4 and LPK6 screened MSC products, respectively (Figure 6b).
Figure 6. IPAv Correlation with Viability and MHC Class II.
Figure 6a: The IPA values for each product were assessed for their degree of correlation with the cell viability of that product. IPAv’s from assays using either LPK4 or LPK6 were used. The correlation coefficients were R2=0.21 and R2=0.12 for LPK4 and LPK6, respectively. Figure 6b: IPA values for products were evaluated for correlation with cell surface HLA-DR (MHC Class II) expression which was measured using flow cytometry analysis. The correlation coefficients were R2=0.22 and R2=0.27 for LPK4 and LPK6, respectively.
Discussion
In this report, we describe a well-defined immunopotency assay to measure the relative T cell suppression strength of human bone marrow derived MSC products. This method was developed into a Quality Control Specification (QCS) for evaluation of MSC products to support cellular therapy characterization in a manufacturing environment regulated by current Good Manufacturing Practice (cGMP). This assay is straightforward, reproducible, and comprehensive in design. Currently, there is no standard methodology used to generate bone marrow MSCs for research or clinical applications and no bench mark assay or cell line has been reported to compare cell therapies across different manufacturing practices (39). Although the impact of a number of potential variables on the in vitro and in vivo properties of MSCs is unknown, notable differences in clinical study results have been attributed to differences in culture methodologies (47). However, there are a number of other reasons that could lead to variability in clinical outcomes. For example, differences in clinical study design such as type or severity of the disease in a patient population, dose and frequency of MSC administration, or variability in concomitant medications may all greatly contribute to variability in clinical study outcome (48). Another important variable is the passage number of MSCs as it has shown that biological properties of MSCs evolve over different passages (49). Of importance, the MSCs from each of the PACT Centers are final cellular products of clinical quality which were evaluated using our IPA.
As a first step in evaluating variability in MSC products, we developed a standardized biological assay that reproducibly measures the inhibition of CD4+ T cell proliferation, one of the main proposed mechanisms of action of MSCs. The IPA has been comprehensively evaluated using a series of PBMC:MSC ratios for each MSC product. Additionally, the results of each MSC product were compared to a reference standard to ensure assay integrity and reproducibility. Another controlled aspect of this assay is that human PBMCs from healthy apheresis donors are purified and cryopreserved in large numbers to enable use of the same PBMCs for every experiment, thereby minimizing assay-to-assay variability in these assays. The standardization of PBMC stocks not only allowed for the direct comparison of suppression results, but it also provided a level of consistency between experiments which is one of the basic requirements of a good quality control assay.
This assay takes into account the suppression mediated in tandem by both soluble suppression factors and mechanisms initiated upon PBMC/MSC contact. The assay was designed as a non-exclusive screen for any immune suppression mechanism and as a result avoids assuming that any single immune factor is more important than another. Still, despite the complex nature of the suppression mechanisms, a single suppression value can be generated using a wide range of PBMC:MSC ratios. To our knowledge, this is the first MSC assay to be described in which an actual suppression value (IPAv) is assigned to an MSC product. Analysis of additional effector to target ratios could result in an enhanced evaluation of suppression and confidence in determining significant differences that may exist between MSC products.
This IPA is flexible in that the proliferative suppression of specific leukocyte subsets can be evaluated. For example, this assay could be modified to analyze B cell proliferation with the addition of an anti-IgM/CD40L stimulus, or identify the suppression of a specific clonal population of T or B cells using receptor-specific tetramers. Using this IPA format could also allow for titrating T cell stimulatory antibodies and generate a stimulus curve to better mimic the pro-inflammatory environments seen in any given patient population. Flexibility can also be attributed to the assay in that any number of soluble factors, produced either by the MSC or the PBMC, could be measured in the supernatants of each co-culture. We have shown that soluble IL-2R levels could be used as an indication of T cell activation and proliferation. Additionally, rather than evaluation of a broad function such as proliferation potential, one could measure cytokine levels expressed by a specific T cell subset or specific activities of MSC-derived suppression factors. Ideally, use of this assay format with patient PBMCs could prove helpful in identifying which MSC product may be better suited as a cellular therapeutic for a particular patient.
Although the IPA described here is robust, flexible, and reproducible it is important to consider other factors that have the potential to influence the outcome of this assay. For example, in order to simplify upfront assay preparation and yet develop a dependable quantitative assay, T cells or CD4+ cells were not isolated prior to their use in this MSC co-culture assay. As such, other cell populations such as monocytes, macrophages, dendritic cells, or B cells may influence this IPA by affecting the responsiveness of MSCs and CD4+ T cells (50). Therefore, using PBMCs may not be a direct measurement of CD4+ T cell inhibition by MSCs. However, using T cells within a PBMC milieu, as we have here, may more closely mimic the in vivo inflammatory environment that MSCs are exposed to upon patient administration. This IPA also does not directly address the effect of MSCs on other cellular components of PBMCs such as B cells, T regulatory cells, dendritic cells, and monocytes and macrophages. Thus, unless a series of MSC products are rigorously evaluated and demonstrated to correlate with in vivo outcomes this IPA assay should only be used as a research tool to screen and compare T cell suppressive properties of different MSC products and as a platform for further functional characterization and comparison of these products.
Testing of MSC products generated from different donors with different manufacturing techniques using this QCS assay is an initial step toward much needed efforts to standardize MSC immunopotency assays for clinical trials.
Supplementary Material
Acknowledgments
This work was supported in part by Production Assistance for Cellular Therapies (PACT) program from NIH/NHLBI at Baylor College of Medicine Center for Cell and Gene Therapy (PACT Contract # HHSN268201000007C); University of Minnesota Molecular and Cellular Therapeutics Facility (PACT Contract # HHSN268201000008C); Center for Human Cell Therapy Boston (PACT Contract # HHSN268201000009C); University of Wisconsin–Madison Waisman Biomanufacturing (PACT Contract # HHSN268201000010C); PACT Coordinating Center: The EMMES Corporation (PACT Contract # HHSN268201000006C). Peiman Hematti lab is also supported by the UW Comprehensive Cancer Center Support Grant P30 CA014520, Crystal Carney Fund for Leukemia Research, and University of Wisconsin Graft Versus Host Disease Program.
Abbreviations
- CFSE
Carboxyfluorescein succinimidyl ester
- cGMP
current Good Manufacturing Practice
- CTL
cytotoxic T lymphocyte
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
Dimethyl sulfoxide
- FBS
Fetal Bovine Serum
- HSA
Human Serum Albumin
- IPA
immunopotency assay
- LPK
leukopack
- MCB
Master Cell Bank
- MLR
mixed lymphocyte reaction
- MSC
Mesenchymal stromal cells
- NHLBI
National Heart, Lung, and Blood Institute
- NIH
National Institutes of Health
- PACT
Production Assistance for Cellular Therapies
- PBMCs
peripheral blood mononuclear cell
- IPAv
IPA value
- IRB
Institutional Review Board
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedenstein AJ, Piatetzky S, II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. Journal of embryology and experimental morphology. 1966 Dec;16(3):381–90. [PubMed] [Google Scholar]
- 2.Keating A. Mesenchymal stromal cells: new directions. Cell stem cell. 2012 Jun 14;10(6):709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz EM, Keating A. Nonhematopoietic mesenchymal stem cells: what are they? Cytotherapy. 2000;2(5):387–8. doi: 10.1080/146532400539305. [DOI] [PubMed] [Google Scholar]
- 4.Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Experimental hematology. 2004 May;32(5):414–25. doi: 10.1016/j.exphem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy. 2012 May;14(5):516–21. doi: 10.3109/14653249.2012.677822. [DOI] [PubMed] [Google Scholar]
- 6.Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica. 2009 Feb;94(2):258–63. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006;8(4):315–7. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 8.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999 Apr 2;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991 Sep;9(5):641–50. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 10.Phinney DG, Sensebe L. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy. 2013 Feb;15(2):140–5. doi: 10.1016/j.jcyt.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Dazzi F, Ramasamy R, Glennie S, Jones SP, Roberts I. The role of mesenchymal stem cells in haemopoiesis. Blood reviews. 2006 May;20(3):161–71. doi: 10.1016/j.blre.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Keating A. How do mesenchymal stromal cells suppress T cells? Cell stem cell. 2008 Feb 7;2(2):106–8. doi: 10.1016/j.stem.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nature reviews Immunology. 2012 May;12(5):383–96. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 14.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007 Jul 30; doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental hematology. 2009 Dec;37(12):1445–53. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz EM, Dominici M. How do mesenchymal stromal cells exert their therapeutic benefit? Cytotherapy. 2008;10(8):771–4. doi: 10.1080/14653240802618085. [DOI] [PubMed] [Google Scholar]
- 17.Stagg J, Galipeau J. Mechanisms of immune modulation by mesenchymal stromal cells and clinical translation. Current molecular medicine. 2013 Jun;13(5):856–67. doi: 10.2174/1566524011313050016. [DOI] [PubMed] [Google Scholar]
- 18.Ghannam S, Pene J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010 Jul 1;185(1):302–12. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 19.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nature biotechnology. 2014 Mar;32(3):252–60. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Experimental hematology. 2003 Oct;31(10):890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 21.Battiwalla M, Hematti P. Mesenchymal stem cells in hematopoietic stem cell transplantation. Cytotherapy. 2009;11(5):503–15. doi: 10.1080/14653240903193806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014 May;54(5):1418–37. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prockop DJ, Brenner M, Fibbe WE, Horwitz E, Le Blanc K, Phinney DG, et al. Defining the risks of mesenchymal stromal cell therapy. Cytotherapy. 2010 Sep;12(5):576–8. doi: 10.3109/14653249.2010.507330. [DOI] [PubMed] [Google Scholar]
- 24.Phinney DG. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. Journal of cellular biochemistry. 2012 Sep;113(9):2806–12. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 25.Phinney DG. Biochemical heterogeneity of mesenchymal stem cell populations: clues to their therapeutic efficacy. Cell Cycle. 2007 Dec 1;6(23):2884–9. doi: 10.4161/cc.6.23.5095. [DOI] [PubMed] [Google Scholar]
- 26.Ho AD, Wagner W, Franke W. Heterogeneity of mesenchymal stromal cell preparations. Cytotherapy. 2008;10(4):320–30. doi: 10.1080/14653240802217011. [DOI] [PubMed] [Google Scholar]
- 27.Goodrich AD, Hematti P. Mesenchymal stem cell therapies: the quest for fine-tuning. Exp Dermatol. 2014 May 7; doi: 10.1111/exd.12432. [DOI] [PubMed] [Google Scholar]
- 28.Ren J, Stroncek DF, Zhao Y, Jin P, Castiello L, Civini S, et al. Intra-subject variability in human bone marrow stromal cell (BMSC) replicative senescence: molecular changes associated with BMSC senescence. Stem Cell Res. 2013 Nov;11(3):1060–73. doi: 10.1016/j.scr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabatino M, Ren J, David-Ocampo V, England L, McGann M, Tran M, et al. The establishment of a bank of stored clinical bone marrow stromal cell products. J Transl Med. 2012;10:23. doi: 10.1186/1479-5876-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner W, Ho AD. Mesenchymal stem cell preparations--comparing apples and oranges. Stem Cell Rev. 2007 Dec;3(4):239–48. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 31.Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-Based Product Characterization for Clinical Trials: An FDA Perspective. Cell Stem Cell. 2014 Feb 6;14(2):141–5. doi: 10.1016/j.stem.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4):e10088. doi: 10.1371/journal.pone.0010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romieu-Mourez R, Francois M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. Journal of immunology. 2009 Jun 15;182(12):7963–73. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 34.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-independent suppression of T cell effector function by IFN-gamma-licensed human mesenchymal stromal cells. Journal of immunology. 2014 Feb 15;192(4):1491–501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 35.Nemeth K. Mesenchymal stem cell therapy for immune-modulation: the donor, the recipient, and the drugs in-between. Exp Dermatol. 2014 May 26; doi: 10.1111/exd.12459. [DOI] [PubMed] [Google Scholar]
- 36.Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L. Immunological characterization of multipotent mesenchymal stromal cells--The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013 Sep;15(9):1054–61. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell stem cell. 2008 Apr 10;2(4):313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003 Jan;57(1):11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, et al. Soliciting Strategies for Developing Cell-Based Reference Materials to Advance MSC Research and Clinical Translation. Stem cells and development. 2014 Mar 10; doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanley PJ, Mei Z, Durett AG, da Graca Cabreira-Harrison M, Klis M, Li W, et al. Efficient manufacturing of therapeutic mesenchymal stromal cells with the use of the Quantum Cell Expansion System. Cytotherapy. 2014 Apr 10; doi: 10.1016/j.jcyt.2014.01.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood D, Wesselschmidt R, Hematti P, Gee AP, Rooney C, Silberstein L, et al. An Update from the United States National Heart, Lung, and Blood Institute-funded Production Assistance for Cellular Therapies (PACT) Program: A Decade of Cell Therapy. Clin Transl Sci. 2014 Apr;7(2):93–9. doi: 10.1111/cts.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forbes GM, Sturm MJ, Leong RW, Sparrow MP, Segarajasingam D, Cummins AG, et al. A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn’s disease refractory to biologic therapy. Clin Gastroenterol Hepatol. 2014 Jan;12(1):64–71. doi: 10.1016/j.cgh.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. 2010 Apr;16(4):503–10. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013;22(12):2267–77. doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trivedi P, Hematti P. Derivation and immunological characterization of mesenchymal stromal cells from human embryonic stem cells. Experimental hematology. 2008 Mar;36(3):350–9. doi: 10.1016/j.exphem.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Blanc K, Rasmusson I, Gotherstrom C, Seidel C, Sundberg B, Sundin M, et al. Mesenchymal stem cells inhibit the expression of CD25 (interleukin-2 receptor) and CD38 on phytohaemagglutinin-activated lymphocytes. Scandinavian journal of immunology. 2004 Sep;60(3):307–15. doi: 10.1111/j.0300-9475.2004.01483.x. [DOI] [PubMed] [Google Scholar]
- 47.Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013 Jan;15(1):2–8. doi: 10.1016/j.jcyt.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Yin F, Battiwalla M, Ito S, Feng X, Chinian F, Melenhorst JJ, et al. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: correlation of biological markers with clinical responses. Stem Cells. 2014 May;32(5):1278–88. doi: 10.1002/stem.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lennon DP, Schluchter MD, Caplan AI. The effect of extended first passage culture on the proliferation and differentiation of human marrow-derived mesenchymal stem cells. Stem Cells Transl Med. 2012 Apr;1(4):279–88. doi: 10.5966/sctm.2011-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.