Abstract
Background
Injection drug use, infectious disease, and incarceration are inextricably linked in Russia. We aimed to identify factors associated with time to relapse (first opioid injection after release from prison) and using a non-sterile, previously used syringe at relapse in a sample of people who inject drugs in St. Petersburg.
Methods
We collected data on time from release to relapse among individuals with a history of incarceration, a subsample of a larger study among people who inject drugs. Proportional hazards and logistic regression were used to identify factors associated with time to relapse and injection with a non-sterile previously used syringe at relapse, respectively.
Results
The median time to relapse after release was 30 days. Factors that were independently associated with relapsing sooner were being a native of St. Petersburg compared to not being native (AHR: 1.64; 95% CI 1.15 – 2.33), unemployed at relapse compared to employed (AHR: 4.49; 95% CI 2.96 – 6.82) and receiving a previous diagnosis of HBV and HCV compared to no previous diagnosis (AHR: 1.49; 95% CI 1.03 – 2.14). Unemployment at relapse was also significant in modeling injection with a non-sterile, previously used syringe at relapse compared to those who were employed (AOR: 6.80; 95% CI 1.96 – 23.59).
Conclusions
Unemployment was an important correlate for both resuming opioid injection after release and using a non-sterile previously used syringe at relapse. Linkage to medical, harm reduction, and employment services should be developed for incarcerated Russian people who inject drugs prior to release.
Keywords: Russia, incarceration, inject, drugs, relapse
1. INTRODUCTION
Relapse to opioid use is a significant public health problem among people who inject drugs, and the problem can be heightened when returning to the community following incarceration. Upon release, people who use opioids have an increased risk of death (Binswanger et al., 2007; Christensen et al., 2000; Farrell and Marsden, 2008; Kariminia et al., 2007; Merrall et al., 2010) and experiencing a non-fatal overdose (Kinner et al., 2012). Specifically, prior studies have consistently shown a marked increased risk of death due to drug overdose within two to three weeks of release from incarceration in the US and the UK (Binswanger et al., 2007; Bird and Hutchinson, 2003; Seaman et al., 1998).
In Russia, non-violent drug users are disproportionately affected by the criminal justice system, as evidenced by the fact that an estimated 50% of the inmate population in St. Petersburg is incarcerated due to drug offenses (Csete, 2004). Furthermore, Russia has one of the highest incarceration rates in the world (Walmsley, 2011) fueled in great part by the post-Soviet epidemic of heroin injection and making it the country with the largest heroin consumption globally (UNODC, 2010). Previous studies have documented that over 40% of people who inject drugs in Russia have been previously incarcerated (Dolan et al., 2007) and despite an incarceration rate of over 500 per 100,000 (Walmsley, 2011), the Russian prison system suffers from a lack of effective linkage to care services for prisoners being released. This is especially evident regarding opioid substitution therapy, which remains illegal in Russia despite compelling international evidence that it can reduce the incidence of reincarceration (Larney et al., 2012), death (Dolan et al., 2005; Huang et al., 2011; Kinlock et al., 2009) and delay relapse (Gonzalez et al., 2004).
Russia has also experienced an epidemic of HIV that is concentrated among people who inject drugs. With an estimated 83,000 individuals who inject drugs in St. Petersburg (Heimer and White, 2010), or about 1.8% of the population, HIV prevalence exceeds 50% (Eritsyan et al., 2013; Niccolai et al., 2010) and more than 90% are infected with HCV (Heimer et al., 2014; Paintsil et al., 2009). Mandatory HIV testing occurs in Russian prisons and it is where many individuals first learn of their HIV infection (Niccolai et al., 2010). In sum, the high rates of incarceration, injection drug use, and bloodborne pathogens may constitute a syndemic in Russia.
Despite the high prevalence of bloodborne diseases, people who inject drugs in the Russian prison system, and heroin use in the general population, no studies have yet to examine the time to relapse to injection opioids and correlates of high risk injection practices, such as syringe sharing, immediately following release from prison. Syringe sharing is a risk factor for bloodborne disease transmission and has been documented to be more elevated in a cohort of people who inject drugs who reported recent incarceration in Vancouver (Milloy et al., 2009; Wood et al., 2005). High frequency of syringe sharing in Russian prisons has been reported and was responsible for at least one HIV outbreak that occurred in a Russian prison (Bobrik et al., 2005), however the frequency of syringe sharing after release has not been determined. Additionally, this outcome could serve as a marker of high-risk injection behavior directly following release from prison, and then be used to better identify inmates who would most benefit from referral to harm reduction services prior to release.
The overall purpose of this study was to understand to what extent sociodemographic and pre-relapse factors have on time to resumption of injecting opioids post release and injection with a non-sterile previously used syringe at the time of relapse to opioid use. Additionally we were interested in receipt of a positive diagnosis for infectious diseases associated with unsafe injection. Inclusion of this was based on previous studies that reported on the association between awareness of serostatus and injection risk behaviors (Hagan et al., 2006; Metsch et al., 1998; Ompad et al., 2002; Vidal-Trecan et al., 2000). The specific aims of this study were to characterize and identify correlates among a sample of previously incarcerated people who inject drugs on two outcomes of interest: (1) elapsed time from release from prison to first injection and, (2) injection with a non-sterile previously used syringe at the moment of relapse.
2. METHODS
2.1. Recruitment of participants
Recruitment for the parent study occurred in St. Petersburg via respondent driven sampling (RDS), a modified form of peer referral commonly used to recruit individuals from hidden populations (Heckathorn, 1997, 2002). Briefly, our RDS used dual incentives through a structured coupon disbursement procedure where respondents receive an incentive for both participating and recruiting peers. Recruitment began with initial respondents, known as “seeds” who were known to outreach workers and given coupons to distribute to peers (of the same target population). Peers scheduled an appointment to determine eligibility by study staff. The eligibility criteria for the parent study and this analysis were the same: at least 21 years of age, injected drugs in the past 30 days, and ability to provide informed consent. However this analysis was limited to only those who reported ever being incarcerated. Individuals who began injecting drugs after their most recent release from prison were excluded in this analysis (n=5).
Trained interviewers administered a questionnaire to eligible participants to collect information on access and use of drug treatment and medical services, incarceration, alcohol, tobacco, and drug use, HIV risk practices associated with injecting drugs, sexual behaviors, HIV, TB, and hepatitis knowledge, overdose risk, physical and mental health, HIV disclosure and stigma. Data collection occurred from September 2012 to June 2013. Participation was voluntary and anonymous. IRB approval was granted by the Yale University Human Investigation Committee and ethical committee of Stellit, a non-governmental organization in St. Petersburg that specializes in HIV services among marginalized populations. After completing the study, participants were reimbursed with a gift worth approximately $15 consisting of personal hygiene products, mobile phone and gift cards, and provided HIV prevention information.
2.2. Outcomes
Our primary outcome was the elapsed number of days from the participants’ most recent release from incarceration to resumption of injecting opioids. We defined relapse as the first moment when the respondent began injecting opioids after release into the community. Since all participants were actively injecting drugs at the time of the interview, no participants were censored: all reported a time to event. Our secondary outcome, injection with a non-sterile previously used syringe at relapse, was dichotomized (yes/no). We piloted the questions that specifically related to our analysis to ensure fidelity and no loss of meaning after translating from English into Russian. None of the study staff reported that the respondents had difficulty in understanding these items and at an interim analysis after 2 months from when data collection began, we verified that all respondents were providing valid, non-missing responses.
2.3. Independent variables
To avoid any issues with temporality between our independent variables and outcomes, variables that could only have occurred before relapse were included in this analysis. Therefore, we included the following sociodemographic and bio-behavioral characteristics: age (at relapse), sex, ethnicity, age at first drink of alcohol, education, how long they had been injecting drugs at the time of their most recent relapse, number of times incarcerated, and whether any drugs were injected during their most recent incarceration. We also created variables that captured receipt of a positive HIV, HCV, and HBV diagnosis prior to the moment of relapse. We included these variables to be markers of prior interaction with the medical system or drug treatment clinics where HIV, HCV and HBV testing is conducted routinely (personal communication with addiction psychiatrist in St. Petersburg). Receipt of positive disease diagnosis was ascertained by the following items: “Has a doctor/medical personnel ever told you that you were infected with HIV/HCV/HBV?” Among those who answered “Yes” to a particular disease, participants were asked when (month/year) the doctor/medical personnel informed them. We added the number of infectious diseases known at the moment of relapse in order to examine the possible effect of multiple comorbid conditions. Previous research has shown important differences in levels of risk by number of comorbid infections in similar populations (Pallas et al., 1999; Ramezani et al., 2014; Saiz de la Hoya et al., 2011)
2.4. Statistical Analysis
Univariate statistics were generated to describe the sample in terms of sociodemographics, incarceration related characteristics, and knowledge of HIV, HCV, and HBV status at relapse after release. We also tested for a potential interaction between previous diagnosis of HIV status and any viral hepatitis diagnosis (either HBV or HCV or both). Finally, we examined the effect of previous diagnosis of viral hepatitis serostatus only (none, either HBV or HCV, or both HBV and HCV).
2.4.1 Time to first opioid injection after incarceration
We used a cross-sectional cohort design to assess our outcomes of interest (Hudson et al., 2005). Cox proportional hazards was used to model time to relapse after release. All bivariate associations significant at P<0.2 were entered into a multivariate model. We used a backward stepwise elimination to obtain the most parsimonious model with only covariates significant at p<0.05. All models were adjusted for potential confounders, including sex, injecting while incarcerated, duration of injection (at moment of relapse), and years since release. We tested for violation of proportional hazards by creating time-dependent covariates and including them in the model and testing them for significance. Kaplan-Meier curves and log-rank tests were generated to examine differences among those who were previously diagnosed with 0, 1, 2, or all 3 diseases of interest.
2.4.2 Modeling utilization of a non-sterile previously used syringe at relapse
For our second outcome, Chi-square, Wilcoxon-Mann-Whitney-U, and t-tests were used to measure the association between modeling injection with a non-sterile, previously used syringe at relapse and the independent variables. All bivariate associations significant at the P< 0.2 level were entered into a multivariate logistic regression model and non-significant covariates were removed by backwards stepwise elimination in order to minimize the Akaike Information Criterion value. All statistical analyses were performed using SAS version 9.3.
3. RESULTS
3.1. Sociodemographics
Sociodemographic characteristics of the sample are found in Table 1. The mean age when participants relapsed after the most recent release from prison was approximately 30 years (range: 16–48). The vast majority of participants was male (86%), of Russian ethnicity (96%), and had obtained at least a secondary school education (89%). The participants, on average, began injecting drugs nearly 12 years prior to when they relapsed after their most recent release.
Table 1.
Characteristics of Study Participants (N=269)
| Characteristic | N (SD) or (%) |
|---|---|
| SOCIODEMOGRAPHICS AND INCARCERATION INFO | |
| Age at relapse (mean, sd) | 30.0 (5.1) |
| Sex Men Women |
231 (85.9) 38 (14.1) |
| Race/Ethnicity Russian Other |
257 (95.5) 12 (4.4) |
| St. Petersburg native Yes No |
229 (85.1) 40 (14.9) |
| Highest education level attained Less than secondary education Secondary education or higher |
32 (11.9) 237 (88.1) |
| Age of first drink of alcohol (mean, sd) | 14.0 (2.5) |
| Duration of injection drug use at relapse (mean, sd, median) | 11.8 (5.1) |
| # of times incarcerated 1 >1 |
149 (56.0) 117 (44.0) |
| Years since release (mean, sd, median) | 4.6, 3.3, 4.0 |
| Inject drugs last time in prison No Yes |
201 (74.7) 68 (25.3) |
| Location and company at relapse Home alone Home with friends Not at home with friends Other |
48 (17.9) 40 (14.9) 172 (64. 2) 8 (3.0) |
| Employed at relapse No Yes |
168 (63.9) 95 (36.1) |
| Elapsed days from release to relapse median, range | 30 (0–1826) |
| Sterile syringe used at relapse No Yes |
38 (13.9) 225 (85.6) |
| KNOWLEDGE OF SEROSTATUS (awareness prior to relapse) | |
| HIV positive | 107 (40.7) |
| Number of diseases known 0 1 2 3 |
51 (19.0) 79 (29.4) 77 (28.6) 62 (23.1) |
| Viral hepatitis serostatus Negative for both HBV and HCV Positive for either HBV or HCV Positive for HBV and HCV |
64 (24.2) 88 (33.3) 112 (42.4) |
Slightly more than half had been incarcerated only once and a quarter had injected drugs during their most recent incarceration period. More than two-thirds of participants were released from prison within the last 5 years. Most participants had resumed injecting drugs after incarceration in the company of a friend and away from home while around 18% had relapsed at home by themselves. Approximately one-third of the sample was employed at the time they relapsed. The median time to relapse after release was 30 days, ranging from 0 days (injected the same day as release) to 1826 days (5 years). Nearly 15% had injected with a non-sterile, previously used syringe at relapse after release from prison. At the time of relapse, 41% had been told by a clinician that they were infected with HIV and 42% had received a positive diagnosis for HBV and HCV before they relapsed. Approximately half of the participants were previously diagnosed with at least two infections prior to relapse.
3.2. Survival analysis – time from release to relapse
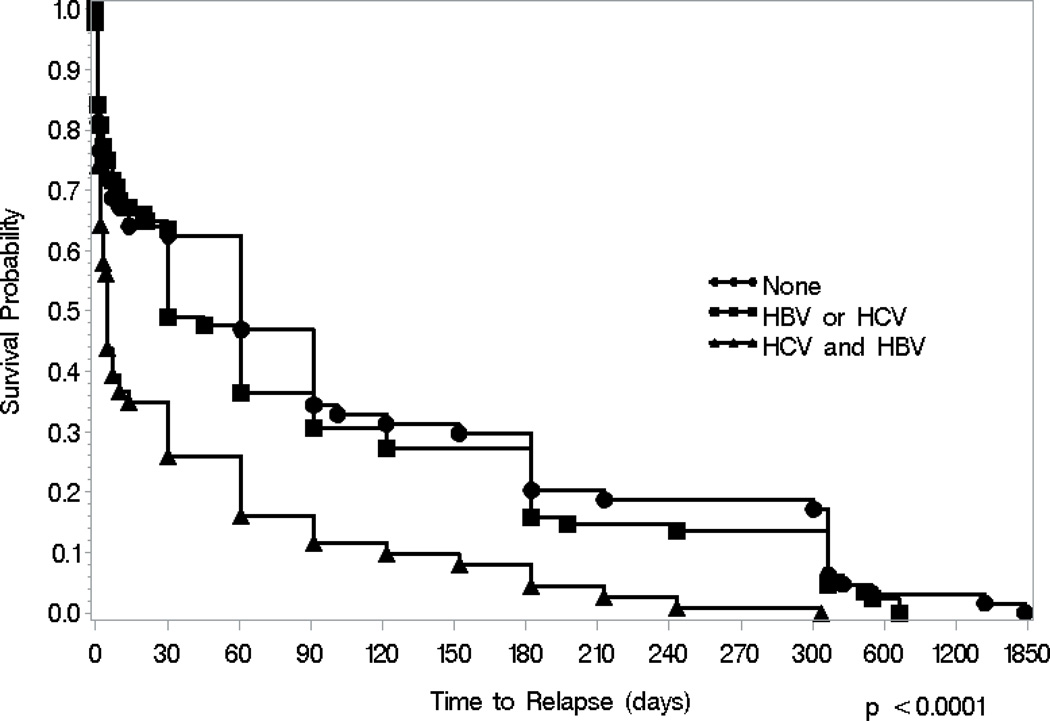
Results from the Cox proportional hazards regression are found in Table 2. In the unadjusted analyses, a moderate protective effect was found among those who had at least a high school education compared to those who did not finish high school (HR: 0.59; 95% CI: 0.40, 0.85). A strong independent risk factor for relapse was not being employed at the moment of relapse (HR: 4.84; 95% CI: 3.25, 7.20). We did not find a significant interaction with time to relapse between having been diagnosed with HIV and having been diagnosed with HBV or HCV. However, compared to those who were not previously diagnosed with viral hepatitis infection, there was a significantly increased hazard of relapsing among those who were previously diagnosed with both infections (HBV and HCV) (HR: 2.16; 95% CI: 1.57, 2.99). Figure 1 displays the Kaplan-Meier curves for those who were previously diagnosed with both HBV and HCV, previously diagnosed with either HBV or HCV, or never diagnosed with these diseases at relapse (log-rank test: p-value <0.0001). The median time to relapse for those who were previously diagnosed for both HBV and HCV was 5 days, while those had been previously diagnosed with either HBV or HCV infection had a median time to relapse of 30 days and those who were never diagnosed with HBV or HCV had a median time to relapse of 61 days. After adjusting for potential confounders, several correlates remained significant in the multivariate model. Notably, those who were not employed were at a significantly increased risk of relapsing (AHR: 4.49; 95% CI: 2.96, 6.82) compared to those who were employed. We conducted sensitivity analyses by limiting the sample to those released within the past 2, 3, and 5 years. We found similar magnitudes of effect and direction for the covariates that remained significant in the proportional hazards. However, some covariates became less significant as power was reduced (data not shown).
Table 2.
Unadjusted and Adjusted Hazard Ratios- Time to Relapse
| Characteristic | Unadjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|
| SOCIODEMOGRAPHICS AND INCARCERATION INFO | ||
| Age at relapse | 0.98 (0.96, 1.01) | |
| Sex Men Women |
1.24 (0.88, 1.77) 1.00 |
1.02 (0.71, 1.47) 1.00 |
| Native of St. Petersburg Yes No |
1.39 (0.99, 1.95) 1.00 |
1.64 (1.15, 2.33)** 1.00 |
| Race/Ethnicity Non-Russian Russian |
0.78 (0.68, 1.04) 1.00 |
|
| Highest education level attained Secondary school or higher Less than secondary school |
0.59 (0.40, 0.85)*** 1.00 |
|
| Age of first alcohol drink | 0.91 (0.86, 0.96)** | |
| Duration of injection drug use at relapse | 1.00 (0.98, 1.03) | 0.99 (0.96, 1.02) |
| # of times incarcerated >1 1 |
1.35 (1.05, 1.72)* 1.00 |
|
| Years since release | 0.91 (0.88, 0.95)*** | 0.93 (0.89, 0.97)** |
| Inject drugs last time in prison Yes No |
1.59 (1.20, 2.10)*** 1.00 |
1.89 (1.41, 2.54)*** 1.00 |
| Location and company Not at home with friends Other Home with friends Home alone |
0.87 (0.63, 1.19) 1.09 (0.52, 2.31) 1.14 (0.74, 1.73) 1.00 |
|
| Employed at relapse No Yes |
4.84 (3.25, 7.20)*** 1.00 |
4.49 (2.96, 6.82)*** 1.00 |
| KNOWLEDGE OF SEROSTATUS (prior to relapse) | ||
| HIV positive | 1.25 (0.98, 1.60) | |
| Diseases known | 1.32 (1.16, 1.49)*** | |
| Both HBV and HCV Either HBV or HCV None |
2.16 (1.57, 2.99)*** 1.15 (0.83, 1.59) 1.00 |
1.49 (1.03, 2.14)* 1.02 (0.72, 1.46) 1.00 |
p<0.05
p<0.01
p<0.001
Figure 1.
Time-to-relapse to first opioid injection after release from incarceration stratified by receipt of positive diagnosis of viral hepatitis
3.3. Correlates of non-sterile, previously used syringe at relapse
Results from the unadjusted and adjusted logistic regression analyses modeling injection with a non-sterile previously used syringe at relapse are found in Table 3. Similar to our findings from the proportional hazards modeling, in the unadjusted logistic regression we found that those who were not employed at relapse were at a significantly greater odds of using a nonsterile previously used syringe at relapse (OR: 8.23 95% CI: 2.46, 27.57). Additionally, those who had ever received a positive HBV and HCV diagnosis before relapse were also significantly more likely to use a non-sterile previously used syringe compared to those who had never received a diagnosis for viral hepatitis (OR: 4.33 95% CI: 1.58, 11.84). Furthermore, for every year increase in age from when the participant had their first alcoholic drink, there was 28% decreased odds in injecting with a non-sterile previously used syringe at relapse. In our multivariate model, we found a protective effect for older age at first alcoholic drink (AOR 0.76; 95% CI: 0.64, 0.90)), after adjusting for potential confounders. Not being employed at relapse (AOR 6.80; 95% CI: 1.96, 23.59) remained a strong risk factor in the multivariate model. Sensitivity analyses also showed a similar magnitude of effect for unemployment status when we limited the sample to those who were released more recently (data not shown).
Table 3.
Unadjusted and Adjusted Odds Ratios- Utilization of non-sterile previously used syringe at relapse
| Characteristic | Unadjusted OR (95% CI) |
Adjusted OR (95% CI) |
|---|---|---|
| SOCIODEMOGRAPHICS AND INCARCERATION INFO | ||
| Age, years total | 0.95 (0.89, 1.02) | |
| Sex Men Women |
1.17 (0.45, 3.06) 1.00 |
1.33 (0.47, 3.77) 1.00 |
| Race/Ethnicity Non-Russian Russian |
0.76 (0.27, 2.16) 1.00 |
|
| Native of St. Petersburg Yes No |
0.71 (0.29, 1.75) 1.00 |
|
| Highest education level attained Secondary school or higher Less than secondary school |
0.43 (0.18, 1.04) 1.00 |
|
| Age of first alcohol drink | 0.72 (0.61, 0.84)*** | 0.76 (0.64, 0.90)** |
| Duration of injection drug use at relapse | 0.99 (0.92, 1.06) | 0.98 (0.90, 1.06) |
| # of times incarcerated >1 1 |
1.01 (0.50, 2.03) 1.00 |
|
| Years since release | 1.00 (0.90, 1.10) | 1.03 (0.91, 1.17) |
| Inject drugs last time in prison Yes No |
1.46 (0.69, 3.09) 1.00 |
1.06 (0.47, 2.40) 1.00 |
| Company and location at relapse Not at home with friends Other Home with friends Home alone |
5.14 (1.18, 22.31)* 3.14 (0.25, 39.43) 1.19 (0.16, 8.86) 1.00 |
|
| Employed at relapse No Yes |
8.23 (2.46, 27.57)*** 1.00 |
6.80 (1.96, 23.59)** 1.00 |
| KNOWLEDGE OF SEROSTATUS (prior to relapse) | ||
| HIV positive | 1.58 (0.79, 3.15) | |
| Diseases known | 2.06 (1.41, 3.01)** | |
| Both HBV and HCV Either HBV or HCV None |
4.33 (1.58, 11.84)** 0.41 (0.09, 1.77) 1.00 |
|
p<0.05
p<0.01
p<0.001
4. DISCUSSION
Understanding the risk profile of individuals who are more likely to engage in these high-risk behaviors after release from prison is important in order to improve the referral process of linking them to drug treatment, HIV care, and harm reduction services. By identifying such factors associated with earlier relapse and high-risk behaviors, we can better design and tailor interventions to former inmates with a history of injection drug use in resource-limited settings. Since health care in Russia’s criminal justice system is severely underfunded (Parfitt, 2010), implementing cost-effective interventions to delay resumption of drug abuse as a means to reduce recidivism and further spread of infectious diseases within the population of people who inject drugs who enter and are released from the prison system should be highly prioritized.
A strong and consistent finding was the large magnitude of effect between employment status and relapsing sooner to opioid injection and using a non-sterile previously used syringe at relapse. This may suggest that employment provides some stability after release. Linking inmates to assistance with employment prior to or at release could be a critical component in reducing the likelihood of relapse, criminal behavior, and re-incarceration. Indeed, previous studies, including one intervention, have demonstrated that employment reduced the likelihood of relapse to heroin (Hser et al., 2013; Shah et al., 2006; Stenbacka et al., 2007; Strathdee et al., 2010). Moreover, difficulties in obtaining employment have been associated with more substance abuse problems (Henkel, 2011). It is possible that individuals with the most severe substance abuse problems are at the highest risk of relapse, which would create challenges in securing employment after their release. Russian officials have recognized the discrimination and difficulties faced with obtaining employment for ex-convicts and have begun undertaking reforms as a means to reduce recidivism (Salnik, 2013).
While incarceration is often a setting where inmates can learn of their disease status, previous studies in the US, Canada, and Scandinavia suggest that risky injection behaviors may not change after a positive diagnosis for viral hepatitis (Hagan et al., 2006; Kwiatkowski et al., 2002; Norden et al., 2009; Ompad et al., 2002) or increase after becoming aware of one’s disease status (Vidal-Trecan et al., 2000). The findings on injection behaviors following HIV diagnosis are less clear, with conflicting reports of no change or risk reductions (Brogly et al., 2002) and persistence of injection-related risk behaviors after receipt of a positive HIV diagnosis (Metsch et al., 1998). In this study we found a significant association between being previously diagnosed with both HBV and HCV and an increased hazard of relapse compared to those who had never received a diagnosis of viral hepatitis. Despite HBV and HCV being different diseases, we created a 3-level variable for 0, 1, and 2 viral hepatitis diagnoses due to the confusion that often arises between a positive HBV or HCV diagnosis alone (Best et al., 1999; Kuo et al., 2004). Given this confusion between the two diseases, we found that there was not a significant difference in time to relapse after receiving a positive diagnosis for either disease compared to those who had not received a positive diagnosis. However, our results do suggest that those who had received a positive diagnosis for HBV and HCV were more likely to relapse sooner, irrespective of previous diagnosis of HIV infection, than those who had never received a positive diagnosis for either HBV or HCV. In other words, receiving a positive diagnosis might be more than not protective; it might be associated with negative health behaviors. Although we do not know the mechanism by which previous diagnoses might increase the risk of relapsing sooner after release from prison in our study population, the public health implications of these findings could eventually be used to strengthen linkage to services that can slow relapse to injection and promote safer injection among recently released prisoners. Further, those who had been previously diagnosed with HCV and HBV infections prior to release could be prioritized and referred to harm reduction and medical services before discharge as a means to reduce the likelihood of an earlier relapse.
Finally, we found that a later onset of alcohol use was associated with a decreased odds in syringe sharing at relapse. While this relationship may be distal, it could be an important marker to consider for referral to harm reduction services since it has been shown that earlier onset of alcohol use is associated with more severe substance use and dependence disorders (Dawson et al., 2008; McGue et al., 2001; von Diemen et al., 2008).
4.1. Limitations
Given the challenges of conducting longitudinal studies among people who inject drugs, in Russia, we were limited to cross-sectional data. As a result, we included only those variables that we knew explicitly occurred before relapse. It is possible that some other risk factors not measured and accounted for in this analysis could influence the associations we found. Additionally, we cannot say anything about causality between previous disease diagnosis and relapse because we do not know whether those who relapsed sooner did so because they had been previously diagnosed and thus relapsed as a means to cope with the trauma of being infected or whether they relapsed because they had more severe addiction and were more likely to contract diseases due to their riskier behaviors.
Recall is also a concern given that some participants were asked about events that occurred in the distant past. However retrospective studies that involve describing the salient events related to incarceration have been used previously (Buavirat et al., 2003; van Haastrecht et al., 1998) and a high consistency of responses related to self-reported drug use has been documented (Darke, 1998; Shillington et al., 1995). Further, we asked numerous questions related to the context of the relapse episode, which may have helped with recall (Means et al., 1991).
Thus far, we have been unable to follow a sample of prisoners being released prospectively to determine the timing of and influences prior to relapse. Given what is known about the risk of overdose death shortly after release, if there is a high rate of death due to overdose after release from prison, these relapse events would not be captured in our study and would overestimate the time to relapse. Also, since this study only included people who were actively injecting drugs, it is possible that some individuals were released from prison and never relapsed. However, there is little reason to believe that incarceration provides long-term effective rehabilitation. We expect the number of people to never resume injecting drugs once released into the community to be low due to the lack of effective drug treatment options. According to providers of drug treatment in Russia, 95% of patients fail their treatment regimen in a clinical setting (Torban et al., 2011).
Lastly, there is a potential for selection bias given that RDS was used to recruit participants and consequently the potential direction of this bias is unclear. While it has been suggested that RDS could produce population-based estimates for a hidden population, the mathematical theory and assumptions supporting the RDS estimates as reliable estimators of the underlying population have been questioned (Gile and Handcock, 2010; Goel and Salganik, 2010; Heimer, 2005).
4.2 Conclusion
Since a high proportion of people who inject drugs infected with bloodborne pathogens circulate through the Russian criminal justice system, incarcerated settings should be viewed as opportunities for public health interventions as a way to test and treat individuals and link them to care before they are released to the community. Our results suggest that many people who inject drugs who have ever received a positive diagnosis for both HCV and HBV resume injecting opioids sooner after release compared to those who have not received a positive disease diagnosis. Additionally, the strong associations between employment status and relapsing sooner after release and using a non-sterile previously used syringe at the moment of relapse warrant further exploration. Interventions in the form of strengthening linkage to harm reduction and social services upon discharge from a criminal justice setting in Russia should be enacted. Such collaborations have been achieved between the AIDS Foundation East-West and the Russian Penitentiary Service in the form of delivering discharge planning and case management services (AIDS Foundation East-West, 2013). Community based programs should strive to financially stabilize people who inject drugs upon release and more structural interventions are needed to reduce the barriers in obtaining employment for recently released people who inject drugs.
Highlights.
Russia has high rates of injection drug use, infectious disease, and incarceration
We modeled time to opioid relapse and unsterile syringe use after release from prison
Unemployment, previous diagnosis of HCV and HBV were associated with relapsing sooner
Being unemployed was also associated with unsterile syringe use at relapse
Linkage from prison to health care and social services must be strengthened in Russia
Acknowledgements
The authors wish to thank all participants in the study.
Role of funding source
This research was supported by National Institutes of Health grants (F31DA03570901, 5R01DA02988804, 5T32MH020031 and 2P30MH06229411) from the National Institute on Drug Abuse (NIDA) and the National Institute of Mental Health (NIMH). The funding agencies had no role in the design, collection, analysis, and interpretation of the data. The authors are solely responsible for the writing of the manuscript and decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication., As a service to our customers we are providing this early version of the manuscript., The manuscript will undergo copyediting, typesetting, and review of the resulting proof, before it is published in its final citable form. Please note that during the production, process errors may be discovered which could affect the content, and all legal disclaimers, that apply to the journal pertain.
Contributors
J.C. and R.H. designed the study. J.C. analyzed the data with assistance from L.N., T.K., and R.H. A.L. and O.L. were responsible for overseeing recruitment of study participants and data collection. J.C. wrote the first draft of the manuscript. All authors provided feedback and approved the final version of the manuscript.
Conflicts of Interest
All other authors declare that they have no conflicts of interest.
REFERENCES
- AIDS Foundation East-West. Improving Access to HIV Prevention and Care Programmes for Injecting Drug Users and in Prison Settings in the Russian Federation. [Retrieved 16 October, 2014];2013 from http://www.afew.org/cetest-firstpage/prisons/project/?tx_afewregions_pi1%5Bproject%5D=18&cHash=11200c17c27eaaff 38b36cdf0231e9f1. [Google Scholar]
- Best D, Noble A, Finch E, Gossop M, Sidwell C, Strang J. Accuracy of perceptions of hepatitis B and C status: cross sectional investigation of opiate addicts in treatment. BMJ. 1999;319:290–291. doi: 10.1136/bmj.319.7205.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binswanger IA, Stern MF, Deyo RA, Heagerty PJ, Cheadle A, Elmore JG, Koepsell TD. Release from prison--a high risk of death for former inmates. N. Engl. J. Med. 2007;356:157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird SM, Hutchinson SJ. Male drugs-related deaths in the fortnight after release from prison: Scotland, 1996–99. Addiction. 2003;98:185–190. doi: 10.1046/j.1360-0443.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- Bobrik A, Danishevski K, Eroshina K, McKee M. Prison health in Russia: the larger picture. J. Public Health Policy. 2005;26:30–59. doi: 10.1057/palgrave.jphp.3200002. [DOI] [PubMed] [Google Scholar]
- Brogly SB, Bruneau J, Lamothe F, Vincelette J, Franco EL. HIV-positive notification and behavior changes in Montreal injection drug users. AIDS Educ. Prev. 2002;14:17–28. doi: 10.1521/aeap.14.1.17.24333. [DOI] [PubMed] [Google Scholar]
- Buavirat A, Page-Shafer K, van Griensven GJ, Mandel JS, Evans J, Chuaratanaphong J, Chiamwongpat S, Sacks R, Moss A. Risk of prevalent HIV infection associated with incarceration among injecting drug users in Bangkok, Thailand: case-control study. BMJ. 2003;326:308. doi: 10.1136/bmj.326.7384.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen PB, Krarup HB, Niesters HG, Norder H, Georgsen J. Prevalence and incidence of bloodborne viral infections among Danish prisoners. Eur. J. Epidemiol. 2000;16:1043–1049. doi: 10.1023/a:1010833917242. [DOI] [PubMed] [Google Scholar]
- Csete J. Lessons not learned: Human rights abuses and HIV/AIDS in the Russian Federation. New York: Human Rights Watch; 2004. [Google Scholar]
- Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51(3):253–263. doi: 10.1016/s0376-8716(98)00028-3. discussion 267–258. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol. Clin. Exp. Res. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K, Kite B, Black E, Aceijas C, Stimson GV Reference Group on HIV/AIDS Prevention and Care Among Injecting Drug Users In Developing and Transitional Countries. HIV in prison in low-income and middle-income countries. Lancet Infect. Dis. 2007;7:32–41. doi: 10.1016/S1473-3099(06)70685-5. [DOI] [PubMed] [Google Scholar]
- Dolan K, Shearer J, White B, Zhou J, Kaldor J, Wodak AD. Four-year follow-up of imprisoned male heroin users and methadone treatment: mortality, re-incarceration and hepatitis C infection. Addiction. 2005;100:820–828. doi: 10.1111/j.1360-0443.2005.01050.x. [DOI] [PubMed] [Google Scholar]
- Eritsyan KU, Levina OS, White E, Smolskaya TT, Heimer R. HIV prevalence and risk behavior among injection drug users and their sex partners in two Russian cities. AIDS Res. Hum. Retroviruses. 2013;29:687–690. doi: 10.1089/aid.2012.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell M, Marsden J. Acute risk of drug-related death among newly released prisoners in England and Wales. Addiction. 2008;103:251–255. doi: 10.1111/j.1360-0443.2007.02081.x. [DOI] [PubMed] [Google Scholar]
- Gile KJ, Handcock MS. Respondent-driven sampling: an assessment of current methodology. Sociol. Methodol. 2010;40:285–327. doi: 10.1111/j.1467-9531.2010.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel S, Salganik MJ. Assessing respondent-driven sampling. Proc. Natl. Acad. Sci. U.S.A. 2010;107:6743–6747. doi: 10.1073/pnas.1000261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Expert Opin. Pharmacother. 2004;5:713–725. doi: 10.1517/14656566.5.4.713. [DOI] [PubMed] [Google Scholar]
- Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, Kapadia F, Hudson S, Garfein RS. Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep. 2006;121:710–719. doi: 10.1177/003335490612100611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc. Probl. 1997;44:174–199. [Google Scholar]
- Heckathorn DD. Respondent-driven sampling II: deriving valid population estimates from chain-referral samples of hidden populations. Soc. Probl. 2002;49:11–134. [Google Scholar]
- Heimer R. Critical issues and further questions about respondent-driven sampling: comment on Ramirez-Valles, et al. (2005) AIDS Behav. 2005;9:403–408. doi: 10.1007/s10461-005-9030-1. discussion 409–413. [DOI] [PubMed] [Google Scholar]
- Heimer R, Eritsyan K, Barbour R, Levina OS. Hepatitis C virus seroprevalence among people who inject drugs and factors associated with infection in eight Russian cities. BMC Infect. Dis. 2014;14(Suppl. 6):S12. doi: 10.1186/1471-2334-14-S6-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer R, White E. Estimation of the number of injection drug users in St. Petersburg, Russia. Drug Alcohol Depend. 2010;109:79–83. doi: 10.1016/j.drugalcdep.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel D. Unemployment and substance use: a review of the literature (1990–2010) Curr. Drug Abuse Rev. 2011;4:4–27. doi: 10.2174/1874473711104010004. [DOI] [PubMed] [Google Scholar]
- Hser YI, Fu L, Wu F, Du J, Zhao M. Pilot trial of a recovery management intervention for heroin addicts released from compulsory rehabilitation in China. J. Subst. Abuse Treat. 2013;44:78–83. doi: 10.1016/j.jsat.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YF, Kuo HS, Lew-Ting CY, Tian F, Yang CH, Tsai TI, Gange SJ, Nelson KE. Mortality among a cohort of drug users after their release from prison: an evaluation of the effectiveness of a harm reduction program in Taiwan. Addiction. 2011;106:1437–1445. doi: 10.1111/j.1360-0443.2011.03443.x. [DOI] [PubMed] [Google Scholar]
- Hudson JI, Pope HG, Jr, Glynn RJ. The cross-sectional cohort study: an underutilized design. Epidemiology. 2005;16:355–359. doi: 10.1097/01.ede.0000158224.50593.e3. [DOI] [PubMed] [Google Scholar]
- Kariminia A, Law MG, Butler TG, Corben SP, Levy MH, Kaldor JM, Grant L. Factors associated with mortality in a cohort of Australian prisoners. Eur. J. Epidemiol. 2007;22:417–428. doi: 10.1007/s10654-007-9134-1. [DOI] [PubMed] [Google Scholar]
- Kinlock TW, Gordon MS, Schwartz RP, Fitzgerald TT, O'Grady KE. A randomized clinical trial of methadone maintenance for prisoners: results at 12 months postrelease. J. Subst. Abuse Treat. 2009;37:277–285. doi: 10.1016/j.jsat.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinner SA, Milloy MJ, Wood E, Qi J, Zhang R, Kerr T. Incidence and risk factors for non-fatal overdose among a cohort of recently incarcerated illicit drug users. Addict. Behav. 2012;37:691–696. doi: 10.1016/j.addbeh.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo I, Mudrick DW, Strathdee SA, Thomas DL, Sherman SG. Poor validity of self-reported hepatitis B virus infection and vaccination status among young drug users. Clin. Infect. Dis. 2004;38:587–590. doi: 10.1086/381440. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski CF, Fortuin Corsi K, Booth RE. The association between knowledge of hepatitis C virus status and risk behaviors in injection drug users. Addiction. 2002;97:1289–1294. doi: 10.1046/j.1360-0443.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- Larney S, Toson B, Burns L, Dolan K. Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction. 2012;107:372–380. doi: 10.1111/j.1360-0443.2011.03618.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol. Clin. Exp. Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Means B, Swan GE, Jobe JB, Esposito JL. An alternative approach to obtaining personal history. In: Biemer PP, Groves RM, Lyberg LE, Mathiowetz NA, Sudman S, editors. Measurement Errors in Surveys. New York: John Wiley and Sons; 1991. pp. 167–183. [Google Scholar]
- Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, Hutchinson SJ, Bird SM. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsch LR, McCoy CB, Lai S, Miles C. Continuing risk behaviors among HIV-seropositive chronic drug users in Miami, Florida. AIDS Behav. 1998;2:161–169. [Google Scholar]
- Milloy MJ, Buxton J, Wood E, Li K, Montaner JS, Kerr T. Elevated HIV risk behaviour among recently incarcerated injection drug users in a Canadian setting: a longitudinal analysis. BMC Public Health. 2009;9:156. doi: 10.1186/1471-2458-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccolai LM, Toussova OV, Verevochkin SV, Barbour R, Heimer R, Kozlov AP. High HIV prevalence, suboptimal HIV testing, and low knowledge of HIV-positive serostatus among injection drug users in St. Petersburg, Russia. AIDS Behav. 2010;14:932–941. doi: 10.1007/s10461-008-9469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden L, Saxon L, Kaberg M, Kall K, Franck J, Lidman C. Knowledge of status and assessment of personal health consequences with hepatitis C are not enough to change risk behaviour among injecting drug users in Stockholm County, Sweden. Scand. J. Infect. Dis. 2009;41:727–734. doi: 10.1080/00365540903159279. [DOI] [PubMed] [Google Scholar]
- Ompad DC, Fuller CM, Vlahov D, Thomas D, Strathdee SA. Lack of behavior change after disclosure of hepatitis C virus infection among young injection drug users in Baltimore, Maryland. Clin. Infect. Dis. 2002;35:783–788. doi: 10.1086/342063. [DOI] [PubMed] [Google Scholar]
- Paintsil E, Verevochkin SV, Dukhovlinova E, Niccolai L, Barbour R, White E, Toussova OV, Alexander L, Kozlov AP, Heimer R. Hepatitis C virus infection among drug injectors in St Petersburg, Russia: social and molecular epidemiology of an endemic infection. Addiction. 2009;104:1881–1890. doi: 10.1111/j.1360-0443.2009.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas JR, Farinas-Alvarez C, Prieto D, Delgado-Rodriguez M. Coinfections by HIV, hepatitis B and hepatitis C in imprisoned injecting drug users. Eur. J. Epidemiol. 1999;15:699–704. doi: 10.1023/a:1007619614350. [DOI] [PubMed] [Google Scholar]
- Parfitt T. Crime and unjust punishment in Russia. Lancet. 2010;376:1815–1816. doi: 10.1016/s0140-6736(10)62152-6. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Amirmoezi R, Volk JE, Aghakhani A, Zarinfar N, McFarland W, Banifazl M, Mostafavi E, Eslamifar A, Sofian M. HCV, HBV, HIV seroprevalence, coinfections, and related behaviors among male injection drug users in Arak, Iran. AIDS Care. 2014;26:1122–1126. doi: 10.1080/09540121.2014.882485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz de la Hoya P, Marco A, Garcia-Guerrero J, Rivera A Prevalhep Study Group. Hepatitis C and B prevalence in Spanish prisons. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:857–862. doi: 10.1007/s10096-011-1166-5. [DOI] [PubMed] [Google Scholar]
- Salnik V. Employment assistance for ex-cons - a remedy for recidivism? [Retrieved 20 November, 2013];2013 from http://english.pravda.ru/society/anomal/13-04-2013/124262-employment_assistance-0/ [Google Scholar]
- Seaman SR, Brettle RP, Gore SM. Mortality from overdose among injecting drug users recently released from prison: database linkage study. BMJ. 1998;316:426–428. doi: 10.1136/bmj.316.7129.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NG, Galai N, Celentano DD, Vlahov D, Strathdee SA. Longitudinal predictors of injection cessation and subsequent relapse among a cohort of injection drug users in Baltimore, MD, 1988–2000. Drug Alcohol Depend. 2006;83:147–156. doi: 10.1016/j.drugalcdep.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Shillington AM, Cottler LB, Mager DE, Compton WM. Self-report stability for substance use over 10 years: data from the St. Louis Epidemiologic Catchment Study. Drug Alcohol Depend. 1995;40:103–109. doi: 10.1016/0376-8716(95)01176-5. [DOI] [PubMed] [Google Scholar]
- Stenbacka M, Beck O, Leifman A, Romelsjo A, Helander A. Problem drinking in relation to treatment outcome among opiate addicts in methadone maintenance treatment. Drug Alcohol Rev. 2007;26:55–63. doi: 10.1080/09595230601036994. [DOI] [PubMed] [Google Scholar]
- Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, Hankins CA. HIV and risk environment for injecting drug users: the past, present, and future. Lancet. 2010;376:268–284. doi: 10.1016/S0140-6736(10)60743-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torban MN, Heimer R, Ilyuk RD, Krupitsky EM. Practices and attitudes of addiction treatment providers in the Russian Federation. J. Addict. Res. Ther. 2011;2:104. [Google Scholar]
- UNODC. World Drug Report. Vienna, Austria: 2010. pp. 37–63. [Google Scholar]
- van Haastrecht HJ, Bax JS, van den Hoek AA. High rates of drug use, but low rates of HIV risk behaviours among injecting drug users during incarceration in Dutch prisons. Addiction. 1998;93:1417–1425. doi: 10.1046/j.1360-0443.1998.939141712.x. [DOI] [PubMed] [Google Scholar]
- Vidal-Trecan G, Coste J, Varescon-Pousson I, Christoforov B, Boissonnas A. HCV status knowledge and risk behaviours amongst intravenous drug users. Eur. J. Epidemiol. 2000;16:439–445. doi: 10.1023/a:1007622831518. [DOI] [PubMed] [Google Scholar]
- von Diemen L, Bassani DG, Fuchs SC, Szobot CM, Pechansky F. Impulsivity, age of first alcohol use and substance use disorders among male adolescents: a population based case-control study. Addiction. 2008;103:1198–1205. doi: 10.1111/j.1360-0443.2008.02223.x. [DOI] [PubMed] [Google Scholar]
- Walmsley R. World Prison Population List. 9th ed. London: International Centre for Prison Studies; 2011. [Google Scholar]
- Wood E, Li K, Small W, Montaner JS, Schechter MT, Kerr T. Recent incarceration independently associated with syringe sharing by injection drug users. Public Health Rep. 2005;120:150–156. doi: 10.1177/003335490512000208. [DOI] [PMC free article] [PubMed] [Google Scholar]