Abstract
Rationale
Limited access nicotine self-administration decreases hippocampal neurogenesis, providing a mechanism for the deleterious effects of nicotine on hippocampal neuronal plasticity. However, recent studies have shown that limited access nicotine self-administration does not exhibit key features of nicotine dependence such as motivational withdrawal and increased motivation for nicotine after deprivation.
Objectives
The present study used extended access nicotine self-administration (0.03 mg/kg/infusion, 21h/day (d), 4d) with intermittent periods of deprivation (3d) for 14 weeks, to test the hypothesis that this model enhances nicotine seeking and produces distinct responses in hippocampal neurogenesis when compared with limited access (1h/day, 4d) intake. Animals in the extended access group were either perfused prior to or following their final deprivation period, whereas animals in the limited access group were perfused after their last session.
Results
Limited access nicotine self-administration and extended access nicotine self-administration with periodic deprivation did not affect proliferation and differentiation of oligodendrocyte progenitors in the medial prefrontal cortex (mPFC). Conversely, extended access nicotine self-administration with periodic deprivation enhanced proliferation and differentiation of hippocampal neural progenitors. Furthermore, in the hippocampus, the number of differentiating NeuroD-labeled cells strongly and positively correlated with enhanced nicotine seeking in rats that experienced extended access nicotine self-administration.
Conclusions
These findings demonstrate that extended access versus limited access to nicotine self-administration differentially affects the generation of new oligodendroglia and new neurons during adulthood. The increases in the number of differentiating cells in extended access nicotine self-administering rats may consequently contribute to aberrant hippocampal neurogenesis and may contribute to maladaptive addiction-like behaviors dependent on the hippocampus.
Keywords: addiction, dependence, abstinence, Ki-67, Olig2, NeuroD
Introduction
Despite the well-known health consequences of smoking, approximately a fifth of the adults in the United States were current cigarette smokers in 2009 and among developing nations, tobacco use is rising annually by 3.4% (CDC 2010; WHO 2002). The development of tobacco dependence is considered to be motivated by the reinforcing effects of nicotine, the primary psychoactive and addictive compound found in tobacco (Jaffe and Kanzler 1979; Pich et al. 1997; Pontieri et al. 1996; Stolerman and Jarvis 1995). The positive reinforcing effect of nicotine has been observed in nonhuman primate and rodent models of limited and extended access to nicotine self-administration (Corrigall and Coen 1989; Goldberg et al. 1981; Valentine et al. 1997). Furthermore, rats with extended (but not limited) access to nicotine self-administration exhibit key features of nicotine dependence, particularly when allowed periodic deprivation (George et al. 2007). These features are observed as increased motivation for nicotine and emergence of a negative emotional state during abstinence (Cohen et al. 2012; Cohen et al. 2013; George et al. 2007).
Clinical studies have demonstrated that following abstinence from smoking, nicotine dependent subjects show profound impairments in behaviors dependent on the hippocampus and prefrontal cortex (PFC; e.g. concentration (Hendricks et al. 2006; Hughes et al. 1991; Hughes et al. 1994), attention (Hughes et al. 1989; Jacobsen et al. 2005), learning and memory (Jacobsen et al. 2007; Mendrek et al. 2006; Merritt et al. 2010; Snyder et al. 1989), and maladaptive cortical plasticity (Grundey et al. 2012). Remarkably, few studies have used the extended access self-administration model in rodents to investigate the effects of nicotine self-administration with periodic deprivation on neuroplasticity in the hippocampus and PFC. Such studies may help elucidate the neurobiological mechanisms contributing to the pathology of tobacco addiction in humans (Abrous et al. 2002; Cohen et al. 2012; Cohen et al. 2013; George et al. 2007; Wei et al. 2012).
In the context of the above studies, nicotinic cholinergic input in the hippocampus influences adult hippocampal neurogenesis. Newly born neurons during their differentiation and maturation stages express functional ionotropic nicotinic acetylcholine receptors (nAChRs), whose expression is critical for normal survival and integration of newly born neurons into the hippocampal circuitry (Cooper-Kuhn et al. 2004; Ide et al. 2008; Kaneko et al. 2006; Mohapel et al. 2005). Additionally, recent evidence supports a causal role for nAChRs in the differentiation, maturation, survival and integration of newly born neurons, suggesting that nAChR signaling is vital for proper functioning and networking of newly born granule cells in the dentate gyrus (Campbell et al. 2010; Harrist et al. 2004; Mechawar et al. 2004). In contrast, chronic nicotine exposure affects various developmental stages of newly born neurons. For example, nicotine self-administration or experimenter delivered nicotine at high doses reduces proliferation, differentiation and survival of newly born neurons (Abrous et al. 2002; Scerri et al. 2006; Shingo and Kito 2005; Wei et al. 2012), indicating a dissociation between nicotine and endogenous acetylcholine, and their contribution to regulation of adult hippocampal neurogenesis (Nakauchi and Sumikawa 2012). However, it has yet to be determined whether nicotine exposure alters proliferation and differentiation of progenitors in the PFC, a brain region that generates glial progenitors and newly born oligodendrocytes (Mandyam and Koob 2012). As a result, the present study explores whether limited access versus extended access nicotine self-administration differentially alters proliferation and differentiation of newly born hippocampal progenitors and cortical progenitors.
A periodic deprivation model of nicotine self-administration was used, and in this model 4 days of extended access (21 hours (h)) or limited access (1 h) nicotine self-administration was followed by 3 days of abstinence. As previously demonstrated in the extended access conditions with periodic deprivation model, an increase in nicotine intake is observed on the first post-deprivation day and this effect is not evident on the subsequent 3 days of self-administration (Cohen et al. 2013; George et al. 2007). The enhanced nicotine seeking resulting from deprivation is assumed to represent the consequence of withdrawal symptoms on the motivation to consume nicotine (Cohen et al., 2013). We therefore used the deprivation model to examine the motivational effects of nicotine withdrawal that accompany its effects on developmental stages of neurogenesis in the hippocampus and gliogenesis in the medial prefrontal cortex (mPFC). The hypothesis of the current study is that withdrawal from extended access nicotine self-administration via deprivation of nicotine produces aberrant responses in adult hippocampal neurogenesis.
Methods
Animals
Twenty-nine adult male Wistar rats (Charles River, Hollister, CA) were group-housed and maintained on a 12 hour/12 hour light/dark cycle with ad libitum access to food and water. All animal procedures were approved by The Scripps Research Institute Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health guidelines.
Nicotine Self-Administration
All rats underwent surgery for catheter implantation for intravenous nicotine self-administration (George et al. 2007). For surgery, rats were anesthetized with 2–3% of isoflurane mixed in oxygen. They were implanted with a silastic catheter (0.3x0.64mm OD; Dow Corning Co.) into the right external jugular vein under aseptic conditions. The distal end of the catheter was s.c. threaded over the shoulder of the rat where it exited the rat via a metal guide cannule (22G, Plastics One Inc.) that was anchored onto the back of the rat. After surgery, rats were given an analgesic (Flunixin, 2.5 mg/kg, s.c.). Antibiotic (Timentins, 20 mg, i.v.; SmithKline Beecham) was administered daily to the rats for at least 5 days. To extend catheter patency, the catheters were flushed once daily with 0.1 ml of an antibiotic solution of cefazolin (10.0 mg/mL; SavMart Pharmaceuticals) dissolved in heparinized saline (70 U/mL; Baxter Health Care Corp) before each self-administration session and with 0.1 ml of heparinized saline (70 U/mL) after each session. The patency of catheters in the rats was tested using the ultra short-acting barbiturate Brevital (methohexital sodium, 10 mg/ml, 2 mg/rat) whenever a catheter failure was suspected during the study.
Seventeen animals were surgically implanted with an intravenous jugular catheter. Twelve additional rats did not undergo intravenous surgeries and remained in their home cages as drug naive controls. Drug self-administration was performed in operant chambers fitted with levers for intravenous self-administration and nosepokes for food and water responses. Prior to and after recovery from intravenous surgery, rats were trained in the operant chambers to nosepoke for food pellets (45 mg; precision, Formula A/I from Research Diets, Lancaster, NH) and water (0.1 ml) on a fixed-ratio schedule (FR1). Pellets were dispensed between retracted two levers on the front wall of the chamber. Water was delivered into a metal dipper cup. When rats were split into extended access nicotine self-administration group, and when extended access sessions began, the rats were allowed to obtain intravenous nicotine through lever presses and food and water intake through nose-poke. Following acquisition of these operant responses, nicotine self-administration sessions were commenced, during which pressing the active lever resulted in an infusion of nicotine (nicotine hydrogen tartrate salt [Sigma, Natick, MA] dissolved in saline; pH 7.4; 0.03 mg/kg; FR1) in a volume of 0.1 ml over 1 second. Illumination of a white cue light above the active lever began at the onset of the nicotine infusion and ceased following a 20 second timeout period, during which responses were recorded but not reinforced. Pressing the inactive lever resulted in no scheduled consequences, but was also recorded.
To allow for acquisition of self-administration behavior, all rats were given access to nicotine for 1 hour per day over 12 days. Rats were then allowed to self-administer nicotine daily in sessions of either 1 hour [limited ‘short’ access (ShA); n = 8] or 21 hours [extended ‘long’ access (LgA); n = 9]. Extended access nicotine self-administration has been shown to induce nicotine dependence while limited access nicotine self-administration does not produce dependence-like behavior(Cohen et al. 2013; George et al. 2007; O'Dell et al. 2007). To model periodic deprivation, each week limited access and extended access rats self-administered nicotine for 4 days (Monday 10:00 AM through Friday 10:00 AM) and were subsequently deprived of nicotine for 3 days (Friday 10:00AM – Monday 10:00 AM; Figure 1a). Nicotine self-administration continued for 14 weeks. Following the final self-administration period of the 14th week, limited access rats were euthanized 28-48 hours (h) after their last self-administration session (n = 8), and extended access rats were either euthanized 2 h after the last self-administration session (n = 4) or 75 h after the last self-administration session (n = 5) by rapid decapitation under isoflurane anesthesia. Drug naïve controls were euthanized at identical time points. A potential limitation of the current study is the use of drug naïve controls that did not experience surgery for intravenous catheters and maintenance on antibiotics. However, we have previously reported that surgery for intravenous catheters and maintenance on antibiotics for an extended period of time does not alter proliferation and neurogenesis in the hippocampal dentate gyrus (Engelmann et al. 2013). Brain tissue was extracted from the skull and cut along the mid-sagital line and immediately immersed in 4% paraformaldehyde and stored in the same solution for 5 days after which all the brains were transferred to 30% sucrose solution. Brains remained in the sucrose solution at 4°C until processed for immunohistochemistry.
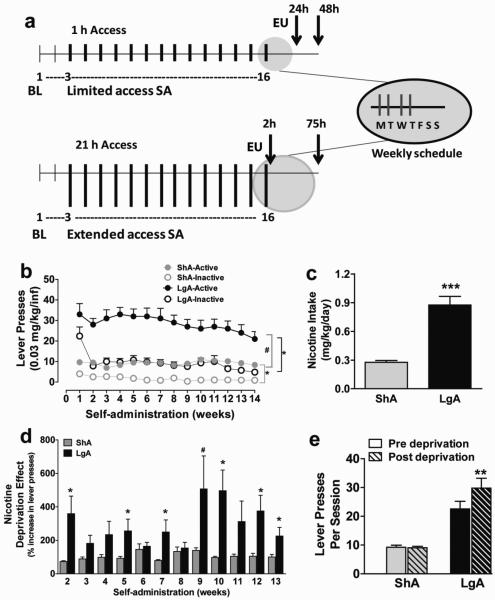
Figure 1.
(a) Schematic of the experimental design. Each vertical line (tick) represents one week of self-administration session (thin line, baseline (BL); thick line, limited or extended access. Top panel represents the experimental design for limited access (ShA) animals. All animals received baseline sessions for two weeks followed by limited (1 hour) or extended (21 hours) access sessions for 14 weeks (indicated in the main panel). Each week was composed of 4 consecutive daily sessions of self-administration followed by 3 days of abstinence (indicated as weekly schedule). Top panel represents the experimental design for limited access (ShA) animals. ShA animals were euthanized (EU) at two different time points, 24 (n = 4) and 48 (n = 4) hours, after their last self-administration session. All ShA animals were combined for immunohistochemical analysis. Bottom panel represents the experimental design for extended access (LgA) animals. LgA animals were euthanized at two different time points, 2 (n = 4) and 75 (n = 5) hours after their last self-administration session and the two LgA groups were not combined for immunohistochemical analysis. (b) Weekly 4 day average of nicotine intake (mean ± SEM) in rats that self-administered nicotine under a fixed-ratio (FR)1 schedule in either 21 h extended access (LgA) or 1 h limited access (ShA) sessions. LgA rats had higher number of active lever presses when compared with ShA rats. Total number of nicotine infusions per session per week (active operant responses) was significantly higher than the total number of inactive operant responses per session in LgA and ShA animals. * p < 0.05 compared with inactive responses; # p < 0.05 compared with ShA active responses. (c) Total nicotine consumed over the period of 14 weeks (data is the average of 4 sessions per week for 14 weeks); *** p < 0.0001 compared with ShA. (d) Percent increase in the number of lever presses during the first hour of the post-deprivation of LgA/ShA session (day 1 after 3 days of deprivation) compared with the previous pre-deprivation session (day 4 during the previous week of self-administration). LgA animals are in black and ShA animals are in gray. *p < 0.05; #p = 0.06 compared with ShA animals. (e) Average number of lever presses pre- (day 4) and post-deprivation (day 1) over the entire 14 week period. **p<0.01 compared with pre-deprivation sessions. n = 8–9 per group.
Immunohistochemistry and Microscopic Analysis
Fixed brain tissue containing the left hemisphere was cut into serial sections (40 μm) on a freezing microtome and was collected in 4 vials (containing 0.1% NaN3 in 1X phosphate-buffered saline (PBS)) and stored at 4°C until processed for immunohistochemistry. One eighth of the brain region was used for immunohistochemical analysis. Unilateral brain sections containing the medial prefrontal cortex (3.7 to 2.2 mm from bregma) and hippocampus (−1.88 to −6.8 mm from bregma; (Paxinos and Watson 1997) were slide mounted, coded, and processed for immunohistochemistry as described previously (Mandyam et al. 2004). Four sections through the mPFC and eight sections through the hippocampus were examined for each rat. The distance between the adjacent sections was maintained at 320 microns. Immunohistochemistry was performed with primary antibodies directed against Ki-67(rabbit monoclonal anti-Ki-67; 1:1000; LabVision), neurogenic differentiation factor 1 [NeuroD; a basic helix-loop-helix (bHLH) transcription factor with significant roles in neuronal differentiation] (rabbit polyclonal anti-NeuroD1; 1:1000; Santacruz Biotechnology), Olig2 (a bHLH transcription factor and a proneural factor with significant roles in gliogenesis; rabbit polyclonal anti-Olig2, 1:10,000; generous gift from Drs. Charles Stiles and John Alberta, Harvard Medical School), and activated caspase-3 (AC3, rabbit polyclonal anti AC-3; Cell Signaling).
Absolute cell counting rather than unbiased stereological estimates (Noori and Fornal 2011) were quantified in sections through the mPFC and hippocampus using a Zeiss Axiophot Microscope equipped with MicroBrightField Stereo Investigator software, a three-axis Mac 5000 motorized stage, a Zeiss digital charge-coupled device ZVS video camera, PCI color frame grabber, and computer workstation. Live video images were used to draw contours delineating the mPFC and granule cell layer (a region in the dentate gyrus of the hippocampus including the granule cell neurons and the subgraular zone) and were traced at 25x magnification. The contours were realigned at high magnification of 400x - 600x. Following determination of mounted section thickness (cut section thickness 40 μm; measured mounted section thickness and antibody penetration 28 μm), z plane values and selection of contours, absolute cell counting with systematic random sampling was performed at 400x (mPFC cells – cells within the cingulate, prelimbic and infralimbic cortices) and 600x (subgranular zone (SGZ) cells – cells touching and within three cell widths inside and outside the hippocampal granule cell-hilus border) magnification. A 150 × 150 μm frame was placed over the regions of interest using the Stereo Investigator stereology platform. The frame was moved systematically over the tissue to cover the entire contoured area and labeled cells (Ki-67 in the mPFC and subgranular zone and NeuroD in the SGZ) in each region of the contour were marked and analyzed. Immunoreactive cells were quantified unilaterally from sections representing mPFC and hippocampus and were summed up for each brain region to give the total number of cells per mm2 of the brain region.
For Olig2 analysis, mPFC sections were examined with the same microscope and software system. Live video images were used to draw contours delineating the area in the cingulate, prelimbic and infralimbic cortices, and each region was traced separately at 25x magnification. The contours were realigned at high magnification at 200x. Following determination of mounted section thickness, z plane values and selection of contours, an optical fractionator analysis was used to determine unbiased estimates of Olig2 cell amounts per mPFC. A counting frame of appropriate dimensions, denoting forbidden and nonforbidden boundaries, was superimposed on the video monitor, and the optical fractionator analysis was performed at 200x. Cells were identified as Olig2 immunoreactive cells based on standard morphology (Ligon et al. 2006), and only cells with a focused nucleus within the nonforbidden regions of the counting frame were counted. Over 30 cells (with cell bodies falling entirely within the borders of the frame) were counted using a 150 × 150 μm counting grid, and a 2 μm top and bottom guard zone. Total number of Olig2 cells per mm2 was calculated for each section.
For AC3 analysis absolute cell counting in the granule cell layer of the dentate gyrus was performed with a Zeiss Primostar microscope at 400x magnification. The total number of cells per section were added and the total number of cells per animal was multiplied by 16 to extrapolate the number of cells within the entire dentate gyrus.
Statistical Analyses
All statistical tests were performed using SPSS software. The effects of access (ShA versus LgA) and lever (active versus inactive) on the mean number of lever presses were examined using a two-way mixed analysis of variance (ANOVA). The effects of nicotine dependence and deprivation (pre- versus post-deprivation) on the mean amount of nicotine intake (mg/kg) were also evaluated using a repeated measures two-way ANOVA, with access (limited vs. extended) as a between-subject factor and deprivation as a within-subject factor. To determine the difference between groups (drug naïve, limited access, extended access, withdrawal from extended access) in the mean number of immunoreactive Ki-67, NeuroD, Olig2 and AC3 cells, one-way independent measures ANOVA was performed. All significant ANOVAs were followed by Newman-Keuls multiple comparison test for post-hoc analysis. For both the dependent groups (pre- and post-deprivation), the number of immunoreactive NeuroD cells were correlated with the total amount of nicotine intake infused pre-deprivation and the total amount of nicotine infused post-deprivation using Pearson’s correlation tests. The significance criterion was set to 0.05 for all tests.
Results
Extended access rats self-administer more nicotine than limited access rats
Rats were given either limited access (ShA; 1 h/day, 4 days/week) or extended access (LgA; 21 h/day, 4 days/week) to self-administer nicotine. Repeated measures two-way ANOVA indicated that there was a significant effect of access (F1,15 = 23.605, p < 0.001), lever responses (F1,15 = 87.067, p < 0.001) and their interaction (F1, 15 = 17.282, p < 0.01; Figure 1a) on the mean number of lever presses. Both groups pressed the active lever significantly more than the inactive lever (each p < 0.001) and LgA rats self-administered significantly more nicotine than ShA rats (p < 0.001; Figure 1a, b). Repeated measures two-way ANOVA indicated that the number of nicotine infusions on post deprivation days was significantly higher compared with the nicotine infusions on the last day pre deprivation in LgA rats, but not in ShA rats (effect of deprivation F1,88 = 26.5, p < 0.001; and a significant access × deprivation interaction, where lever presses were greater post deprivation during weeks 2 to 13 in LgA rats , F10,88 = 2.5, p = 0.009). Nicotine deprivation was associated with a 200-500% increase in active lever presses during the first hour in LgA rats but not in ShA rats. Post hoc analysis revealed a higher response during the first hour post deprivation during weeks 2, 5, 7, 10, 12, 13 in LgA rats (p < 0.05; Figure 1c) and during the entire 14 week period (p = 0.02; Figure 1d) compared with pre-deprivation responses in LgA animals.
Nicotine does not alter levels of proliferating progenitors and premyelinating oligodendrocytes in the mPFC
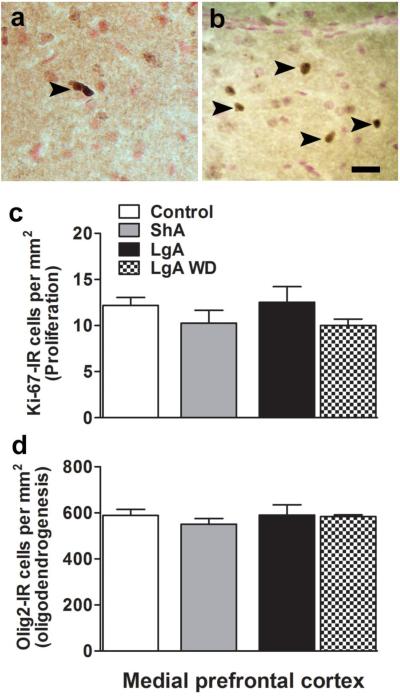
Separate cell counting was performed for each limited access group (24 h and 48 h) and the data (Ki-67, Olig2, NeuroD analysis) indicated no significant difference between the groups. Therefore, cell quantification for the limited access animals were pooled and reported as one group for the entire study. Limited access nicotine self-administration also did not alter the levels of Ki-67 labeled cells and did not alter the levels of Olig2 labeled premyelinating oligodendrocytes in the mPFC when compared with naïve and extended access animals. Extended access nicotine self-administration with periodic deprivation did not alter the levels of Ki-67 labeled cells or Olig2 labeled cells when analyzed immediately following nicotine self-administration (2 h group), and after 3 days of deprivation (75 h group; Figure 2a-d) when compared with naïve and limited access animals.
Figure 2.
Schematic representation of Ki-67 (a) and Olig2 immunoreactive cells (b) in the mPFC. Arrowhead points to positively stained cells. Scale bar in (b) is 30um. Quantitative analysis of Ki-67 (c) and Olig2 (d) labeled cells per mm2 of the mPFC by stereology. ShA animals from both 24h and 48h groups were combined; n = 8. LgA animals from 2h and 75h groups are represented separately; n = 4-5 each group. n = 12 in drug naïve controls.
Nicotine dependence and deprivation increases the levels of immature neurons in the subgranular zone of the dentate gyrus of the hippocampus
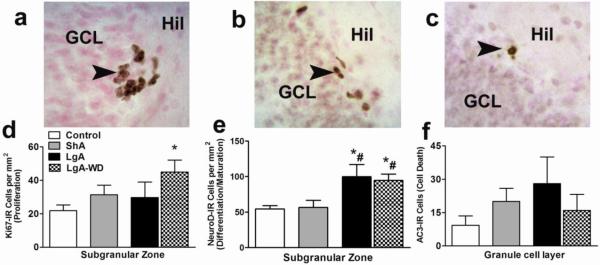
Limited access nicotine self-administration did not alter the levels of Ki-67 labeled cells and did not alter the levels of NeuroD labeled immature neurons in the SGZ when compared with naïve animals. Extended access nicotine self-administration and deprivation differentially affected proliferation versus immature neuron levels in the SGZ. One-way ANOVA indicated a significant effect of nicotine plus deprivation on the mean number of Ki-67 immunoreactive cells (F3,26 = 2.8, p = 0.054). Post-hoc analysis indicated a significantly higher number of Ki-67 cells in extended access rats post-deprivation (75 h group; LgA-WD) when compared with drug-naïve controls (p < 0.05; Figure 3d). Extended access nicotine self-administration and deprivation significantly increased the number of NeuroD cells in the SGZ. One-way ANOVA indicated a significant effect of nicotine on the mean number of NeuroD immunoreactive cells (F3,26 = 6.3, p = 0.003). Post-hoc analysis indicated a significantly higher number of NeuroD cells in LgA rats pre-deprivation (2 h group) and post-deprivation (75 h group; LgA-WD) when compared with drug-naïve controls and ShA rats (p< 0.05; Figure 3e). Limited and extended access nicotine self-administration and deprivation did not alter the number of AC-3 immunoreactive cells in the SGZ (Figure 3f) when compared with naïve animals.
Figure 3.
Schematic representation of Ki-67 (a), NeuroD (b) and AC-3 (c) immunoreactive cells in the hippocampal dentate gyrus. Arrowheads point to immunoreactive cells. Quantitative analysis of Ki-67 (d), NeuroD (e) and AC-3 (f) labeled cells in the hippocampal dentate gyrus. Ki-67 and NeuroD cells are represented as cells per mm2 of the granule cell layer. AC-3 cells are represented as total number of cells. ShA animals from both 24h and 48h groups were combined; n = 8. LgA animals from 2h and 75h groups are represented separately; n = 4-5 each group. n = 12 in drug naïve controls.* p < 0.05 compared with controls, # p < 0.05 compared with ShA animals.
Nicotine intake positively correlates with the number of immature neurons in extended access rats
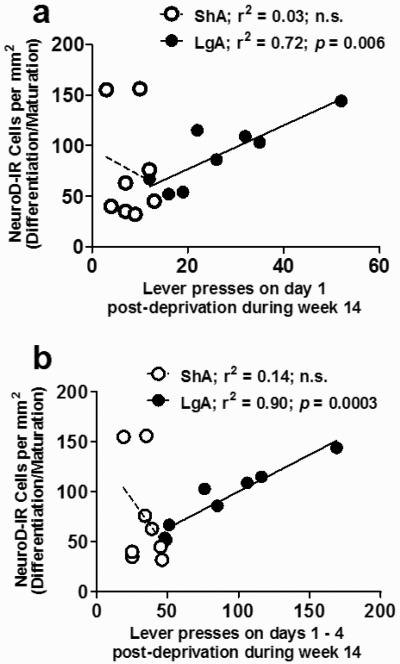
Because the number of NeuroD immunoreactive immature neurons in 24 h and 48 h limited access and 2 h and 75 h extended access animals were not differently affected by nicotine and deprivation (Figure 3e), and because the transient expression of NeuroD occurs in differentiating progenitors between 1 day and 7 days of cell birth (Seki 2002), the animals in both groups were combined for further analysis. More specifically, in limited access and extended access we determined whether the number of NeuroD cells correlated with nicotine seeking behavior experienced during the last week of self-administration (when NeuroD expression was initiated in differentiating neural progenitors). In limited access rats, there were no significant correlations between the number of NeuroD immunoreactive immature neurons and the number of nicotine lever presses on day 1 post-deprivation (Figure 4a; ShA; r2 = 0.03, n.s.), or the number of nicotine lever presses on days 1-4 post-deprivation (Figure 4b; ShA; r2 = 0.14, n.s.) during the last week of self-administration. In extended access rats, there were strong positive correlations between the number of NeuroD immunoreactive immature neurons and the number of nicotine lever presses on day 1 post-deprivation (Figure 4a; LgA; r2 = 0.72, p = 0.006), or the number of nicotine lever presses on days 1-4 post-deprivation (Figure 4b; LgA; r2 = 0.90, p = 0.0003) during the last week of self-administration.
Figure 4.
Linear regression analysis of nicotine self-administration-induced changes in the levels of immature neurons (NeuroD). (a) Total number of NeuroD-labeled cells per mm2 in extended access animals (2h and 75h LgA animals combined; black circles) and limited access animals (24h and 48h ShA animals combined; white circles) plotted against the number of active lever presses on day 1 post-deprivation during week 14 of self-administration. (b) Total number of NeuroD-labeled cells per mm2 in extended access and limited access animals plotted against the number of lever presses on days 1 to 4 combined during the last week (week 14) of self-administration. n = 8-9 per group.
Discussion
The present study sought to relate nicotine self-administration and two distinct stages of development of adult hippocampal neurogenesis in a model of extended access to self-administration of nicotine with periodic deprivation, in which rats were exposed to weekly cycles composed of 4 days of extended (21h) or limited (1h) nicotine self-administration followed by 3 days of abstinence. As previously demonstrated (Cohen et al. 2013; George et al. 2007), in extended access (but not limited access) conditions nicotine intake is increased on day 1 post-abstinence compared with the previous self-administration session, and the increase tapers off by day 4 post-abstinence. This deprivation effect is assumed to represent the consequence of withdrawal symptoms on the motivation to consume nicotine (Cohen et al. 2013). Indeed, withdrawal symptoms have been shown to be important for the escalation of tobacco smoking in humans (Dierker and Mermelstein 2010; DiFranza et al. 2002; Doubeni et al. 2010). We used the 4 days on-3 days off nicotine self-administration schedule in the current study because of several design advantages: it allows comparison between the state of neurogenesis before and after withdrawal and, as tobacco use among smokers is interspersed with periods of deprivation (Borland et al. 2012), the periodic deprivations may accurately model effects of withdrawal in dependent nicotine users. Thus, we varied rats’ access to nicotine self-administration and examined whether extended access with deprivation affects proliferation and differentiation of hippocampal neural progenitors despite its recurrence over fourteen weeks. In contrast to studies that found daily administration of nicotine impairs several aspects of hippocampal neurogenesis (proliferation, immature neurons and neurogenesis), our findings demonstrate that extended access self-administration with deprivation produces increases in the levels of proliferation and immature neurons compared with limited access nicotine intake.
The main findings of the study are: repeated cycles of extended access (21 h/day, 4 days) to nicotine self-administration followed by 3 days of deprivation produces a high level of nicotine intake compared with limited access (1 h/day) schedule of self-administration, particularly on the sessions following abstinence. The higher amount of nicotine intake in extended access animals resulted in increases in the levels of immature neurons in the dentate gyrus of the hippocampus, as measured by expression of NeuroD. Concomitantly, extended access nicotine intake with deprivation produced increases in the levels of proliferation (Ki-67) and immature neurons when compared with limited access animals and drug naïve controls. In extended access animals, the number of immature neurons also positively correlated with the amount of nicotine self-administered the first day after deprivation in the last week of nicotine self-administration. The number of immature neurons also positively and significantly correlated with the amount of nicotine self-administered in the entire last week of nicotine self-administration. In contrast, limited access nicotine self-administration did not alter the levels of progenitors in the proliferation and differentiation stage of hippocampal neurogenesis. Additionally, the amount of nicotine administered during limited access intake did not correlate with the number of immature neurons. Extended access and limited access nicotine self-administration did not alter the number of oligodendrocyte progenitors in the mPFC. Consequently, these observations in limited access and extended access animals suggest that nicotine dependence may have adverse consequences in the hippocampus via an increase in neurogenesis.
The generation of myelin and myelin associated proteins by oligodendrocytes in the adult brain has been hypothesized to play a critical role in the maintenance of brain function (Fields 2005; 2010). For example, myelination in the adult brain occurs through the proliferation and differentiation of oligodendrocyte progenitors followed by maturation into premyelinating oligodendrocytes and myelin forming cells (Baumann and Pham-Dinh 2001). Furthermore, in the postnatal brain, Olig2 is expressed in premyelinating oligodendrocyte progenitors and in mature and terminally differentiating myelinating oligodendrocytes, where it appears to have ongoing biological functions (Rivers et al. 2008). Notably, recent evidence supports the significant role of myelination in the neuropathology of opiate and nicotine addiction (Bora et al. 2012; Cao et al. 2013; Eschenroeder et al. 2012; Lin et al. 2013), where clinical and preclinical findings demonstrate significant reduction of myelin in opiate and nicotine exposed subjects. However, it is not known whether nicotine exposure affects the various developmental processes that maintain myelination in the adult brain. The present study demonstrates that nicotine self-administration either with limited access or extended access fails to alter the levels of progenitors in the mPFC that were proliferating and differentiating into premyelinating oligodendrocytes. Thus, it appears that chronic nicotine exposure does not alter developmental stages of the premyelinating progenitors in the PFC. Whether the newly generated premyelinating oligodendrocytes eventually mature to attain a myelin phenotype after limited or extended access nicotine exposure remains to be determined and warrants detailed investigation.
Neurogenesis in the dentate gyrus of the hippocampus via birth and differentiation of neural stem cells has been conceptualized as a process contributing to neural plasticity, and the functional significance of this phenomenon in brain regeneration and repair is still under intense investigation. Adult hippocampal neurogenesis has been identified in many different non-mammalian and mammalian species (Gould 2007), including humans (Eriksson et al. 1998). A number of computational models of adult hippocampal neurogenesis have been developed (Aimone et al. 2009; Becker 2005; Becker et al. 2009; Garthe et al. 2009; Weisz and Argibay 2009), and studies have proposed that immature neurons engage in pattern integration (contextual discrimination), or the associating of events (memory resolution). These studies also suggest that immature neurons may play a role in cognitive-behavioral tasks, such as drug self-administration and relapse to drug seeking. In this context, it now well-established that levels of immature neurons and neurogenesis are altered by drugs of abuse, including alcohol, morphine, heroin, cocaine, methamphetamine and nicotine (Eisch and Harburg 2006; Mandyam and Koob 2012; Nixon 2006). With respect to nicotine, several studies have previously demonstrated that chronic daily nicotine impairs generation of immature neurons and neurogenesis across forms of administration. These routes of administration include self-administration (Abrous et al. 2002; Wei et al. 2012), and experimenter delivered administration, such as, intraperitoneal injection (Shingo and Kito 2005), subcutaneous osmotic mini-pumps (Scerri et al. 2006) and intracerebroventricular delivery (Van Kampen and Eckman 2010). Furthermore, such impairment is well-evidenced by dose dependent decreases in several exogenous and endogenous markers of hippocampal neurogenesis, such as 5-bromo-2’-deoxyuridine (Abrous et al. 2002; Scerri et al. 2006; Van Kampen and Eckman 2010; Wei et al. 2012), PSA-NCAM (Abrous et al. 2002; Shingo and Kito 2005), and NeuN (Abrous et al. 2002; Shingo and Kito 2005; Wei et al. 2012). Importantly, it appears that these negative effects of daily nicotine intake on the developmental stages of neural progenitors are only induced with chronic exposure to relatively high doses of nicotine (Scerri et al. 2006) . Acute binge nicotine (Mudo et al. 2007) and short access to nicotine self-administration with unit dose <0.04 mg/kg did not affect the levels of proliferation and immature neurons in the hippocampus (Abrous et al., 2002, and present results with limited access rats). In rats given extended access to self-administer nicotine, the number of immature neurons strongly and positively correlated with nicotine intake on day 1 post-deprivation and total nicotine intake on days 1-4 post-deprivation. Thus, it is the withdrawal from nicotine that may be affecting the synaptic plasticity of, and hence the information encoded by newborn neurons (Nixon and Crews 2004; Noonan et al. 2008; Recinto et al. 2012).
Recent in vivo evidence demonstrates that systemic nicotine administration potently influences inhibitory circuitry in the dentate gyrus. For example, nicotine affects synaptic plasticity of granule cell neurons by inhibiting γ-Aminobutyric acid (GABA)ergic inhibitory interneurons via nAChRs, consequently disinhibiting granule cell neurons (Zhang et al. 2010). Notably, GABA (released from GABAergic inhibitory interneurons and signaling through the GABAA receptor on immature neurons) in the dentate gyrus has been charged with dictating the “tempo” for activity-dependent regulation of adult hippocampal neurogenesis (Esposito et al. 2005; Ge et al. 2006; Ge et al. 2007; Overstreet Wadiche et al. 2005; Wang et al. 2005). Therefore, chronic nicotine exposure could be associated with reduced generation of immature neurons in the dentate gyrus via altering GABAergic signaling from the interneurons. Alternately, deprivation from chronic nicotine may produce a rebound effect via enhancing GABAergic signaling from interneurons, thus producing increases in the levels of immature neurons. Recurrent alternation between chronic extended access to nicotine self-administration and deprivation may then be modifying synaptic plasticity of immature neurons as visualized by the increases in NeuroD numbers (present results). Consequently, chronic nicotine and deprivation-induced increases in the number of NeuroD cells could affect the synaptic transmission in the hippocampus. These alterations could contribute to the ability of nicotine to alter hippocampal neural signaling that leads to changes in synaptic plasticity that underlies learning and memory (Zhang et al. 2010).
In conclusion, these findings demonstrate that periodic deprivation with extended access to nicotine self-administration produces aberrant effects on immature neurons compared with limited access to nicotine self-administration (Abrous et al. 2002; Wei et al. 2012). Moreover, these results provide support for the hypothesis that immature (young adult-generated) granule cell neurons may serve to modulate nicotine-induced alterations in hippocampal synaptic plasticity which modifies the overall tone of activity in the dentate gyrus in response to periods of self-administration and deprivation. The aberrant changes may consequently contribute to or assist with the increased motivation for nicotine observed in nicotine dependent subjects.
Acknowledgements
Funds from grants DA022473, AA020098 and AA06420 (CDM) and DA004398 (GFK) from the National Institute of Health and the Pearson Center for Alcoholism and Addiction Research supported the study. We acknowledge the technical assistance of Anne Phan-Huy from University of California San Diego, Jan Kirby Zabala from the Life Sciences Summer Internship Program at The Scripps Research Institute and Ariel Feifel for assistance with processing brain tissue and immunohistochemistry. We appreciate the technical support of Elena Crawford for immunohistochemical analyses and StereoInvestigator and the editorial assistance of McKenzie Fannon. This is publication number 26032 from The Scripps Research Institute.
References
- Abrous DN, Adriani W, Montaron MF, Aurousseau C, Rougon G, Le Moal M, Piazza PV. Nicotine self-administration impairs hippocampal plasticity. J Neurosci. 2002;22:3656–62. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Wiles J, Gage FH. Computational influence of adult neurogenesis on memory encoding. Neuron. 2009;61:187–202. doi: 10.1016/j.neuron.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- Becker S. A computational principle for hippocampal learning and neurogenesis. Hippocampus. 2005;15:722–38. doi: 10.1002/hipo.20095. [DOI] [PubMed] [Google Scholar]
- Becker S, Macqueen G, Wojtowicz JM. Computational modeling and empirical studies of hippocampal neurogenesis-dependent memory: Effects of interference, stress and depression. Brain Res. 2009;1299:45–54. doi: 10.1016/j.brainres.2009.07.095. [DOI] [PubMed] [Google Scholar]
- Bora E, Yucel M, Fornito A, Pantelis C, Harrison BJ, Cocchi L, Pell G, Lubman DI. White matter microstructure in opiate addiction. Addict Biol. 2012;17:141–8. doi: 10.1111/j.1369-1600.2010.00266.x. [DOI] [PubMed] [Google Scholar]
- Borland R, Partos TR, Yong HH, Cummings KM, Hyland A. How much unsuccessful quitting activity is going on among adult smokers? Data from the International Tobacco Control Four Country cohort survey. Addiction. 2012;107:673–82. doi: 10.1111/j.1360-0443.2011.03685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–44. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Wang J, Dwyer JB, Gautier NM, Wang S, Leslie FM, Li MD. Gestational nicotine exposure modifies myelin gene expression in the brains of adolescent rats with sex differences. Transl Psychiatry. 2013;3:e247. doi: 10.1038/tp.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Center for Disease Control and Prevention. Vital signs: current cigarette smoking among adults aged ≥ 18 years—United States, 2009. Morbidity and Mortality Weekly Report. 2010;59:1135–1140. [PubMed] [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology. 2012;37:2153–60. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A, Treweek J, Edwards S, Leao RM, Schulteis G, Koob GF, George O. Extended access to nicotine leads to a CRF receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict Biol. 2013 doi: 10.1111/adb.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–65. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Dierker L, Mermelstein R. Early emerging nicotine-dependence symptoms: a signal of propensity for chronic smoking behavior in adolescents. J Pediatr. 2010;156:818–22. doi: 10.1016/j.jpeds.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Rigotti NA, Fletcher K, Ockene JK, McNeill AD, Coleman M, Wood C. Development of symptoms of tobacco dependence in youths: 30 month follow up data from the DANDY study. Tob Control. 2002;11:228–35. doi: 10.1136/tc.11.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doubeni CA, Reed G, Difranza JR. Early course of nicotine dependence in adolescent smokers. Pediatrics. 2010;125:1127–33. doi: 10.1542/peds.2009-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–86. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- Engelmann AJ, Aparicio MB, Kim A, Sobieraj JC, Yuan CJ, Grant Y, Mandyam CD. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Eschenroeder AC, Vestal-Laborde AA, Sanchez ES, Robinson SE, Sato-Bigbee C. Oligodendrocyte responses to buprenorphine uncover novel and opposing roles of mu-opioid- and nociceptin/orphanin FQ receptors in cell development: implications for drug addiction treatment during pregnancy. Glia. 2012;60:125–36. doi: 10.1002/glia.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–86. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist. 2005;11:528–31. doi: 10.1177/1073858405282304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields RD. Neuroscience. Change in the brain's white matter. Science. 2010;330:768–9. doi: 10.1126/science.1199139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–93. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–66. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, O'Dell LE, Richardson HN, Koob GF. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–5. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Grundey J, Thirugnanasambandam N, Kaminsky K, Drees A, Skwirba AC, Lang N, Paulus W, Nitsche MA. Neuroplasticity in cigarette smokers is altered under withdrawal and partially restituted by nicotine exposition. J Neurosci. 2012;32:4156–62. doi: 10.1523/JNEUROSCI.3660-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrist A, Beech RD, King SL, Zanardi A, Cleary MA, Caldarone BJ, Eisch A, Zoli M, Picciotto MR. Alteration of hippocampal cell proliferation in mice lacking the beta 2 subunit of the neuronal nicotinic acetylcholine receptor. Synapse. 2004;54:200–6. doi: 10.1002/syn.20081. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology (Berl) 2006;187:385–96. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Gust SW, Skoog K, Keenan RM, Fenwick JW. Symptoms of tobacco withdrawal. A replication and extension. Arch Gen Psychiatry. 1991;48:52–9. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89:1461–70. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keenan RM, Yellin A. Effect of tobacco withdrawal on sustained attention. Addict Behav. 1989;14:577–80. doi: 10.1016/0306-4603(89)90079-8. [DOI] [PubMed] [Google Scholar]
- Ide Y, Fujiyama F, Okamoto-Furuta K, Tamamaki N, Kaneko T, Hisatsune T. Rapid integration of young newborn dentate gyrus granule cells in the adult hippocampal circuitry. Eur J Neurosci. 2008;28:2381–92. doi: 10.1111/j.1460-9568.2008.06548.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–66. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jaffe JH, Kanzler M. Smoking as an addictive disorder. NIDA Res Monogr. 1979:4–23. [PubMed] [Google Scholar]
- Kaneko N, Okano H, Sawamoto K. Role of the cholinergic system in regulating survival of newborn neurons in the adult mouse dentate gyrus and olfactory bulb. Genes Cells. 2006;11:1145–59. doi: 10.1111/j.1365-2443.2006.01010.x. [DOI] [PubMed] [Google Scholar]
- Ligon KL, Kesari S, Kitada M, Sun T, Arnett HA, Alberta JA, Anderson DJ, Stiles CD, Rowitch DH. Development of NG2 neural progenitor cells requires Olig gene function. Proc Natl Acad Sci U S A. 2006;103:7853–8. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Wu G, Zhu L, Lei H. Heavy smokers show abnormal microstructural integrity in the anterior corpus callosum: a diffusion tensor imaging study with tract-based spatial statistics. Drug Alcohol Depend. 2013;129:82–7. doi: 10.1016/j.drugalcdep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Koob GF. The addicted brain craves new neurons: putative role for adult-born progenitors in promoting recovery. Trends Neurosci. 2012;35:250–60. doi: 10.1016/j.tins.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. Journal of Neuroscience Research. 2004;76:783–94. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Saghatelyan A, Grailhe R, Scoriels L, Gheusi G, Gabellec MM, Lledo PM, Changeux JP. Nicotinic receptors regulate the survival of newborn neurons in the adult olfactory bulb. Proc Natl Acad Sci U S A. 2004;101:9822–6. doi: 10.1073/pnas.0403361101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addict Behav. 2006;31:833–44. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt PS, Cobb AR, Moissinac L, Hirshman E. Evidence that episodic memory impairment during tobacco abstinence is independent of attentional mechanisms. J Gen Psychol. 2010;137:331–42. doi: 10.1080/00221309.2010.499395. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–46. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Mudo G, Belluardo N, Mauro A, Fuxe K. Acute intermittent nicotine treatment induces fibroblast growth factor-2 in the subventricular zone of the adult rat brain and enhances neuronal precursor cell proliferation. Neuroscience. 2007;145:470–83. doi: 10.1016/j.neuroscience.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Nakauchi S, Sumikawa K. Endogenously released ACh and exogenous nicotine differentially facilitate long-term potentiation induction in the hippocampal CA1 region of mice. Eur J Neurosci. 2012;35:1381–95. doi: 10.1111/j.1460-9568.2012.08056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–95. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–22. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self-administration normalizes deficits in proliferation and enhances maturity of adult-generated hippocampal neurons. J Neurosci. 2008;28:2516–26. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Fornal CA. The appropriateness of unbiased optical fractionators to assess cell proliferation in the adult hippocampus. Front Neurosci. 2011;5:140. doi: 10.3389/fnins.2011.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, Markou A, Zorrilla EP, Koob GF. Extended access to nicotine self-administration leads to dependence: Circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–93. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- Overstreet Wadiche L, Bromberg DA, Bensen AL, Westbrook GL. GABAergic signaling to newborn neurons in dentate gyrus. J Neurophysiol. 2005;94:4528–32. doi: 10.1152/jn.00633.2005. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3° Academic Press; San Diego: 1997. [Google Scholar]
- Pich EM, Pagliusi SR, Tessari M, Talabot-Ayer D, Hooft van Huijsduijnen R, Chiamulera C. Common neural substrates for the addictive properties of nicotine and cocaine. Science. 1997;275:83–6. doi: 10.1126/science.275.5296.83. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Recinto P, Samant AR, Chavez G, Kim A, Yuan CJ, Soleiman M, Grant Y, Edwards S, Wee S, Koob GF, George O, Mandyam CD. Levels of neural progenitors in the hippocampus predict memory impairment and relapse to drug seeking as a function of excessive methamphetamine self-administration. Neuropsychopharmacology. 2012;37:1275–87. doi: 10.1038/npp.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers LE, Young KM, Rizzi M, Jamen F, Psachoulia K, Wade A, Kessaris N, Richardson WD. PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice. Nat Neurosci. 2008;11:1392–401. doi: 10.1038/nn.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri C, Stewart CA, Breen KC, Balfour DJ. The effects of chronic nicotine on spatial learning and bromodeoxyuridine incorporation into the dentate gyrus of the rat. Psychopharmacology (Berl) 2006;184:540–6. doi: 10.1007/s00213-005-0086-4. [DOI] [PubMed] [Google Scholar]
- Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res. 2002;69:772–83. doi: 10.1002/jnr.10366. [DOI] [PubMed] [Google Scholar]
- Shingo AS, Kito S. Effects of nicotine on neurogenesis and plasticity of hippocampal neurons. J Neural Transm. 2005;112:1475–8. doi: 10.1007/s00702-005-0370-2. [DOI] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: performance decrements assessed on a computerized test battery. Drug Alcohol Depend. 1989;23:259–66. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Jarvis MJ. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 1995;117:2–10. doi: 10.1007/BF02245088. discussion 14-20. [DOI] [PubMed] [Google Scholar]
- Valentine JD, Hokanson JS, Matta SG, Sharp BM. Self-administration in rats allowed unlimited access to nicotine. Psychopharmacology (Berl) 1997;133:300–4. doi: 10.1007/s002130050405. [DOI] [PubMed] [Google Scholar]
- Van Kampen JM, Eckman CB. Agonist-induced restoration of hippocampal neurogenesis and cognitive improvement in a model of cholinergic denervation. Neuropharmacology. 2010;58:921–9. doi: 10.1016/j.neuropharm.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–9. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Wei Z, Belal C, Tu W, Chigurupati S, Ameli NJ, Lu Y, Chan SL. Chronic nicotine administration impairs activation of cyclic AMP-response element binding protein and survival of newborn cells in the dentate gyrus. Stem Cells Dev. 2012;21:411–22. doi: 10.1089/scd.2010.0326. [DOI] [PubMed] [Google Scholar]
- Weisz VI, Argibay PF. A putative role for neurogenesis in neuro-computational terms: inferences from a hippocampal model. Cognition. 2009;112:229–40. doi: 10.1016/j.cognition.2009.05.001. [DOI] [PubMed] [Google Scholar]
- WHO World Health Organization. 2002 Smoking statistics Retrieved from http://www.wpro.who.int/media_centre/fact_sheets/fs_20020528.htm.
- Zhang TA, Tang J, Pidoplichko VI, Dani JA. Addictive nicotine alters local circuit inhibition during the induction of in vivo hippocampal synaptic potentiation. J Neurosci. 2010;30:6443–53. doi: 10.1523/JNEUROSCI.0458-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]