Abstract
In this study, we show that aly/aly mice, which are devoid of lymph nodes and Peyer’s patches, rejected acutely fully allogeneic skin and heart grafts. They mounted potent inflammatory direct alloresponses but failed to develop indirect alloreactivity after transplantation. Remarkably, skin allografts were also rejected acutely by splenectomized aly/aly mice (aly/aly-spl−) devoid of all secondary lymphoid organs. In these recipients, the rejection was mediated by alloreactive CD8+ T cells presumably primed in the bone marrow. In contrast, cardiac transplants were not rejected in aly/aly-spl− mice. Actually, aly/aly-spl− mice having spontaneously accepted a heart allotransplant displayed donor-specific tolerance also accepted skin grafts from the same but not a third-party donor via a mechanism involving CD4+ regulatory T cells producing IL-10 cytokine. Therefore, direct priming of alloreactive T cells, as well as rejection and regulatory tolerance of allogeneic transplants, can occur in recipient mice lacking secondary lymphoid organs.
Introduction
Following allotransplantation, the immune response is initiated by T lymphocytes activated in either direct or indirect fashion (1). The direct alloresponse relies on the recognition by recipient T cells of intact donor MHC molecules displayed on donor APCs. This response is polyclonal as it involves up to 10% of the entire T cell repertoire owing to the high frequency of MHC determinants presented to T cells and the presentation of a multitude of peptides by allogeneic MHC molecules (2). On the other hand, host T cells activated via indirect allorecognition interact with processed donor-derived peptides presented by self-MHC molecules on recipient APCs (3). The indirect alloresponse is oligoclonal in that it is mediated by a few T cell clones recognizing dominant determinants on alloantigens (4, 5). While either of these pathways can lead to acute rejection of skin allografts (6), acute rejection of vascularized solid organ transplants is essentially mediated through the direct pathway. Alternatively, indirect alloreactivity is considered as the driving force behind chronic allograft rejection (7–10), which is characterized by graft tissue fibrosis and blood vessel obstruction (11, 12).
Cellular trafficking is an essential element of the process associated with alloimmunity and transplant rejection. Following skin transplantation, donor dendritic cells emigrate via lymphatics to the recipient draining lymph nodes (13) where they activate naïve recipient T cells thereby initiating the direct alloresponse (14–17). Alternatively, in the case of primarily vascularized organs such as hearts and kidneys, it is likely that donor APCs leaving the graft through the blood spread rapidly to various lymphoid organs where they can activate T cells. In addition, some studies suggest that, vascularized allografts can be rapidly infiltrated by some host pre-existing memory T cells (18). Indeed, unlike naive T cells whose homing is confined to lymphoid organs, memory T cells traffic regularly through peripheral tissues (19, 20). These memory T cells may be activated via direct recognition of MHC molecules on donor dendritic cells remaining in the graft and presumably endothelial cells expressing MHC class II and costimulatory molecules as a result of inflammation. This process could account for the direct activation of some alloreactive T cells following organ transplantation (21, 22). On the other hand, it is still unknown where and how donor antigens are acquired, processed and presented by recipient APCs to T cells for induction of the indirect alloresponse.
In this study, we investigated the role of secondary lymphoid organs to the T cell-mediated alloimmune responses, rejection and tolerance of mice transplanted with fully allogeneic conventional skin grafts or primarily vascularized skin or cardiac transplants. We show that aly/aly mice devoid of lymph nodes and Peyer’s patches develop direct but not indirect alloresponses and reject acutely both skin and cardiac allografts. In contrast, aly/aly mice which had been splenectomized (aly/aly-spl−) reject skin but not cardiac allografts. Remarkably, aly/aly-spl− mice having spontaneously accepted heart transplants developed donor-specific tolerance mediated by CD4+ T cells. Therefore, both transplant rejection and tolerance can be induced in the absence of secondary lymphoid organs.
Materials and Methods
Mice and transplantations
Six to eight weeks old aly/aly mice, used in this study, were autosomal recessive mutants of C57BL/6 mice displaying a point mutation in the gene encoding The NF-κB-inducing kinase (NIK) (23). These mice lack lymph nodes and Peyer’s patches. In turn, heterozygous aly/+ mice, displayed a normal immune system and secondary lymphoid organs. In some experiments, we used splenectomized aly/aly mice that are considered devoid of all secondary lymphoid organs. C57BL6 (B6, H-2b) as well as BALB/c (H-2d), C3H (H-2k) mice and B6 MHC class II KO mice were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were bred and maintained at MGH animal facilities under specific pathogen-free conditions. All animal care and handling were performed according to institutional guidelines. “Classical” non-vascularized full-thickness skin allografts (2 × 3 cm) were placed on the groin area of the recipients according to the technique previously described by Billingham and Medawar (24). Vascularized skin grafts were performed using a technique described by our laboratory. Briefly, a 2 × 3 cm full thickness flap was outlined in the groin and elevated. The epigastric vessels were dissected, the distal superficial and deep femoral vessels were ligated and the femoral artery and vein were separated. The artery was flushed with 10% heparinized saline until the venous flow in the pedicle became clear. Femoral artery and vein were then divided. For the recipient, the same size defect was created in the groin area. The femoral artery and vein, right below the inguinal ligament, were separated and prepared for anastomosis. End-to-end anastomosis was performed for arteries and end to side for the veins. After the patency of the vessels confirmed that the flap was sutured to the defect with interrupted sutures.
Vascularized heterotopic cardiac transplantation was performed as described by Corry et al. (25). Transplanted hearts were monitored daily by palpation through the abdominal wall. Heart beat intensity was graded on a scale of 0 (no palpable impulse) to 4 (strong impulse). Rejection was defined by the loss of palpable cardiac contractions and verified by autopsy and pathological examination.
T cells and T cells subsets isolation
T cells as well as CD4+ and CD8+ T cell subsets were isolated from the spleen and lymph nodes of transplanted and naïve mice by negative selection using commercially available T cell purification columns according to the manufacturer’s instructions (Accurate Chemical & Scientific Corp., Westbury, N.Y) (R & D Systems, Minneapolis, MN). Purified T cells were washed in HBSS and used in ELISPOT assays.
Preparation of sonicates
Stimulator spleen cells were suspended at 3 × 107 cells/ml in AIM-V containing 0.5 % FCS, and sonicated with 10 pulses of 1 second each. The resulting suspension was frozen in a dry ice/ethanol bath, thawed at room temperature and centrifuged at 300g for 10 minutes to remove remaining intact cells (1).
ELISPOT assays
Direct and indirect alloresponses by T cells were measured as previously described (1). Briefly, 96-well ELISPOT plates (Polyfiltronics, Rockland, MA) were coated with an anti-cytokine capture mAb in sterile PBS overnight. On the day of the experiment, the plates were washed twice with sterile PBS, blocked for 1.5 h with PBS containing 1 % BSA, then washed 3 times with sterile PBS. Responder cells or purified T cells were added to wells previously filled with either intact donor cells (direct response) or syngeneic APCs together with donor sonicates (indirect response) and cultured for 24 hours at 37°C, 5% CO2. After washing, biotinylated anti-lymphokine detection antibodies were added overnight as described elsewhere. The plates were developed using 800 ul AEC (Pierce, Rockford, IL, 10 mg dissolved in 1 ml dimethyl formamide) mixed in 24 ml 0.1M sodium acetate, pH 5.0, plus 12 ul H202. The resulting spots were counted and analyzed on a computer-assisted ELISA spot image analyzer (C.T.L., Cleveland, OH).
Monoclonal antibody treatments
For leukocyte costimulation blockade, recipient mice were injected intraperitoneally with 0.25 mg of anti-CD40L monoclonal antibodies (MR1) at the time of transplantation and at day 2, 4 and 6 post-transplantation, as previously described (26). For Treg depletion, recipient mice were treated one day prior transplantation with an anti-CD25 antibody (PC61, 1 mg given i.p) that has been previously shown to deplete CD25high FOXP3+ Tregs in vivo (27). CD4+ or CD8+ T cells were depleted from recipient mice with anti-CD4 (GK1.5) and anti-CD8 (53.6.72) monoclonal antibodies (1 mg given intraperitoneally at day −3 and −1 pre-transplant), respectively. NK cells were depleted using the monoclonal antibody NK1.1 (PK136, 0.6mg) given i.p on day −2 pre-transplantation. The depletions of CD4+, CD8+ and NK cells were greater than 95% (data not shown).
Histology
Cardiac transplants were fixed in 10% buffered formalin, embedded in paraffin, coronally sectioned and stained with hematoxylin and eosin (H&E) for evaluation of cellular infiltrates and myocyte damage (acute rejection) by light microscopy. For assessment of chronic rejection, cardiac grafts were stained with Verhoeff’s elastin (vessel arteriosclerosis scoring) or Mason’s trichrome (evaluation of fibrosis). Arteriosclerosis was assessed by light microscopy and the percentage of luminal occlusion and intimal thickening was determined using a scoring system, as previously described (28). Only vessels that display a clear internal elastic lamina were included in morphometric analysis (5–7 vessels per section). All arteries were scored by at least two examiners in a blinded fashion.
Statistics
All statistical analyses were performed using STATView software (Abacus Concepts, Inc., Berkeley, CA). P-values were calculated using paired t-test. P-values < 0.05 were considered statistically significant.
Results
Rejection of allografts and alloresponses in the absence of secondary lymphoid organs
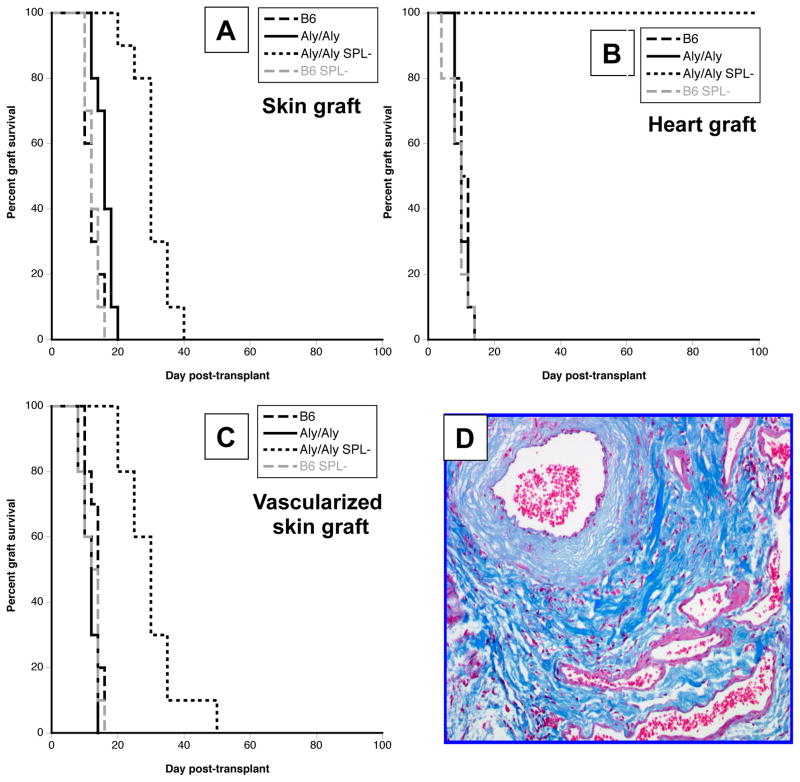
First, we investigated the rejection of fully allogeneic BALB/c (H-2d) skin and heart allografts in wild-type C57Bl/6 (B6) (wt) mice (H-2b), B6 aly/aly mice (lacking lymph nodes and Peyer’s patches) and B6 aly/aly mice that had been splenectomized (aly/aly-spl−). As shown in Figure 1A and B, control wt B6 mice rejected acutely skin and cardiac transplants within 10–12 days after placement. A slight prolongation of skin graft survival was observed in aly/aly mice (MST:16 days) (Fig. 1A and B). In contrast, in aly/aly-spl− mice, while skin allografts were also acutely rejected (although in a delayed fashion, MST:30 days), cardiac allografts survived indefinitely (Fig. 1A and B). Additionally, mice that had been splenectomized (wt-spl−) rejected skin and heart allografts at the same pace as control wt B6 mice (Fig. 1A and B). Of note, histological examination of accepted hearts explanted from aly/aly-spl− mice revealed pathological features characteristics of mild chronic rejection detectable at day 50 post-transplantation (Fig. 1D). Therefore, rejection of cardiac allografts requires the presence of either recipient’s lymph nodes or spleen while skin allografts can be rejected in the absence of any secondary lymphoid organ.
Figure 1. Allograft rejection in aly/aly and aly/aly-splenectomized mice.
Wild-type C57BL/6 (B6), aly/aly, aly/aly splenectomized (aly/aly SPL-) and splenectomized B6 (B6 SPL-) were transplanted with either a BALB/c conventional skin allograft (panel A), a BALB/c heart transplant (panel B) or a primarily vascularized BALB/c skin allograft (panel C). The results are shown as percent graft survival over time after transplantation. Four to eight mice were tested in each group. Graft survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Panel D shows a representative photomicrograph of a BALB/c heart transplant harvested from an aly/aly mouse 50 days after its placement.
Unlike conventional skin grafts, cardiac transplants are vascularized at the time of transplantation, a feature which contributes to their lower immunogenicity and greater susceptibility to tolerance induction (29). This prompted us to test whether primary vascularization of skin grafts would prolong their survival in aly/aly-spl− mice. As shown in Figure 1C, aly/aly-spl− mice rejected vascularized skin allografts at the same pace as conventional skin allografts (Fig. 1A).
Direct but no indirect alloresponses are induced in transplanted aly/aly mice
Next, we measured T cell alloresponses in aly/aly mice transplanted with BALB/c skin allografts. Direct and indirect alloresponses by T cells collected from the recipient’s spleen were evaluated 10 days after transplantation using a previously described ELISPOT assay (1). As expected, transplanted wt B6 mice mounted potent direct and indirect alloresponses mediated primarily by pro-inflammatory T cells secreting IL-2 and γIFN and a few T cells producing type 2 IL-4 cytokine but not IL-10 (Fig. 2A). In contrast, in aly/aly mice, the direct alloresponse was dominated by T cells secreting IL-4 and IL-10 cytokines. In addition, aly/aly mice failed to mount an indirect alloresponse (Fig. 2B).
Figure 2. Direct and indirect T cell alloresponses in transplanted mice.
The frequencies of T cells activated either via direct (panel A) or indirect (panel B) allorecognition and secreting pro-inflammatory cytokines type 1 (IL-2 and γIFN) or type 2 (IL-4 and IL-10) cytokines were measured by ELISPOT. Alloresponses were measured using spleen T cells collected from naïve B6 mice (no Tx) and from B6 and aly/aly mice recipient of a BALB/c skin allograft (10 days post-transplantation). The results are expressed as spots per million T cells for each cytokine ± SD. 5–8 mice were tested in each group.
CD8+ T cells reject skin allografts in aly/aly splenectomized mice
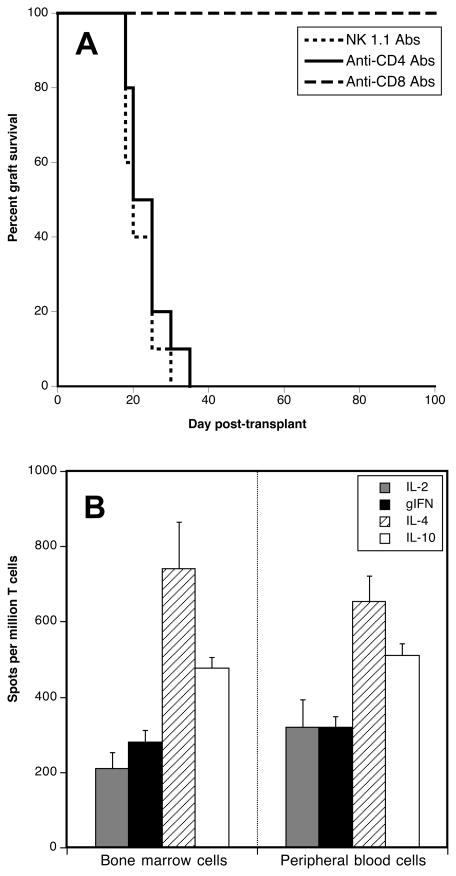
To identify the cells causing transplant rejection in recipients devoid of secondary lymphoid organs, aly/aly-spl− mice were injected with anti-CD4 (GK1.5) or anti-CD8 (56.8.72) or NK1.1 (anti-NK cells) depleting antibodies, as described in the methods section. As shown in Figure 3A, while depletion of CD4+ and NK cells had no effect, treatment with anti-CD8 mAbs resulted in long-term survival of skin allografts. Therefore, CD8+ T cells mediate skin allograft rejection in aly/aly-spl− mice.
Figure 3. Mechanisms involved in alloskin graft rejection by splenectomized aly/aly mice.
Panel A. Splenectomized aly/aly mice were injected twice intraperitoneally with anti-CD4 (GK1.5), anti-CD8 (53.6.72) (1 mg) or NK1.1 (PK136, 0.6 mg) monoclonal antibodies at d-3 and d-1 prior to placement of a BALB/c skin allograft. The results are shown as percent graft survival over time after transplantation. Four to eight mice were tested in each group. Graft survival was analyzed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. Panel B. Direct alloresponses were measured using either bone marrow or peripheral blood T cells collected from aly/aly splenectomized mice recipient of a BALB/c skin allograft (10 days post-transplantation). The frequencies of T cells secreting pro-inflammatory cytokines type 1 (IL-2 and γIFN) or type 2 (IL-4 and IL-10) cytokines were measured by ELISPOT. The results are expressed as spots per million T cells for each cytokine ± SD. 3–5 mice were tested in each group.
Detection of alloresponses in the blood and bone marrow of transplanted aly/aly splenectomized mice
Since aly/aly-spl− mice lacking all secondary lymphoid organs reject skin allografts through a process involving CD8+ T cells, we investigated whether an allospecific response could be detected in other lymphoid tissues. To test this, T cells from the peripheral blood and bone marrow of aly/aly-spl− mice were tested 10 days after BALB/c skin grafting for the presence of a direct alloresponse. As shown in Figure 3B, direct alloresponses were detected with T cells isolated from bone marrow or peripheral blood. No response was detected in non-transplanted aly/aly-spl− mice (data not shown). These results demonstrate that transplanted aly/aly-spl− can mount an allospecific T cell response despite the absence of lymph nodes and spleen.
Aly/aly splenectomized mice mount memory alloresponses
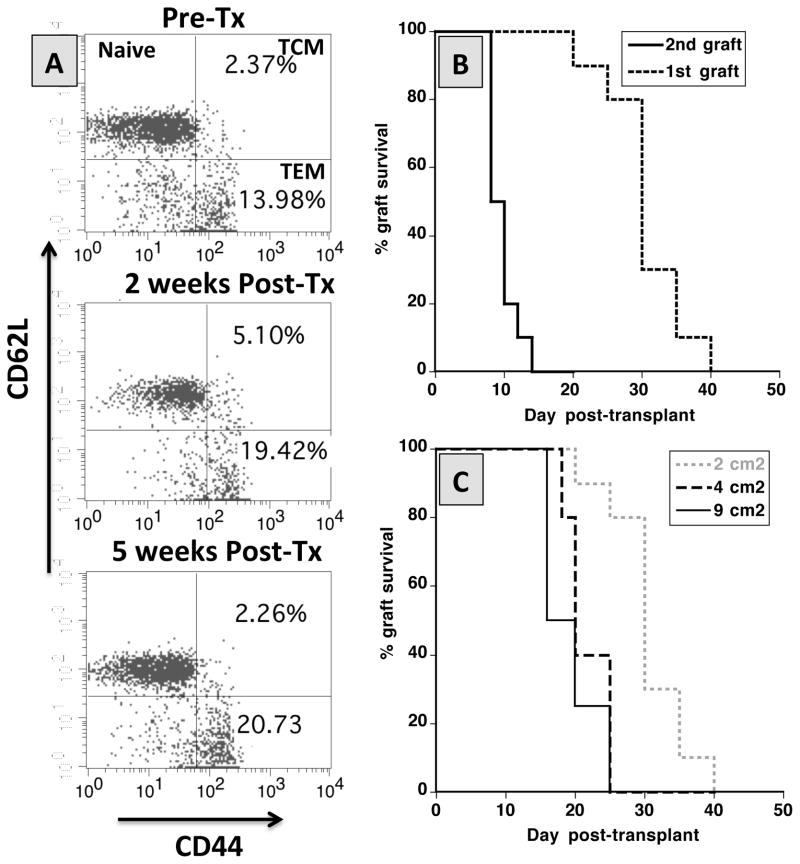
Next, we investigated whether aly/aly-spl− mice can mount a donor-specific memory alloresponse following allotransplantation. To test this, aly/aly-spl− mice having rejected a BALB/c skin graft were retransplanted (20 days after rejection) with a skin allograft derived from the same or a third-party C3H (H-2k) donor. First, we observed an expansion of T cells expressing CD44 (mostly CD44+CD62L− effector memory T cells), which is characteristic of a memory phenotype (Fig. 4A). Consistent with this observation, these mice rejected a second BALB/c skin graft in an accelerated fashion (Fig. 4B). No accelerated rejection was observed after placement of control third-party C3H allografts (data not shown). Therefore, despite their lack of secondary lymphoid organs, aly/aly-spl− can mount a donor-specific memory alloimmune response resulting in second set graft rejection.
Figure 4. Anamnestic T cell responses and rejection of alloskin grafts of different sizes by splenectomized-aly/aly mice.
Panel A. The frequencies of naïve (CD44−CD62L+), central memory T cells (TCM, CD44+CD62L+) and effector memory T cells (TEM, CD44−CD62L−) were determined by FACS among lymphocytes isolated from the peripheral blood of non-transplanted aly-aly splenectomized mice (top panel) and of aly-aly splenectomized mice tested 2 weeks (middle panel) and 4 weeks (lower panel) after placement of a BALB/c skin allograft. The results are representative of 5 mice tested individually. Panel B. aly/aly splenectomized mice were transplanted with a first skin graft at d0 and with a second graft at d20 i.e. after rejection of the first graft. Ten mice were tested. Panel C. Splenectomized aly/aly mice were transplanted with BALB/c skin allografts of different sizes (2 cm2, 4 cm2 or 9 cm2). In both panel B and C, the results are shown as percent graft survival over time after transplantation. Graft survival was analyzed using the Kaplan-Meier method. Three to eight mice were tested in each group
Differential rejection of heart and skin grafts does not rely on graft size
Minor antigen-mismatched (H-Y) heart transplants are less susceptible to rejection than skin grafts due to their larger size presumably causing T cell exhaustion (30). However, this finding may only be relevant to transplants rejected via oligoclonal alloresponses such as the anti-H-Y response. Nevertheless, given the overall paucity of lymphocytes in aly/aly-spl− mice, we reasoned that the graft size could influence the rejection process. To address this question, aly/aly-spl− mice were transplanted with skin allografts of increasing sizes i.e. 2 cm2 (size of control grafts), 4 cm2 and 9 cm2. Bigger sized grafts were rejected significantly faster than the initial 2 cm2-sized grafts (Fig. 4C). Therefore, not only that larger skin grafts did not exhaust T cells in aly/aly/SPL− mice but also they were more immunogenic.
CD4+ T cell-mediated tolerance to allografts in aly/aly splenectomized mice
Finally, aly/aly-spl− mice, which had accepted a BALB/c heart for 50 days, were transplanted with a skin allograft from the same (BALB/c, H-2d) or a third-party (C3H, H-2k) donor. Strikingly, BALB/c skin allografts enjoyed indefinite survival (> 250 days) while C3H third-party allografts were rejected within 20–25 days post-transplantation (Figure 5). Therefore, despite the absence of secondary lymphoid organs, aly/aly-spl− developed donor-specific tolerance. Of note, heart transplants placed in aly/aly-spl− mice displayed histological features of cardiac allograft vasculopathy detectable at day 50–75 post-transplantation (data not shown). Therefore, ongoing chronic rejection of cardiac allografts did not prevent tolerance of skin grafts.
Figure 5. Splenectomized aly/aly mice having spontaneously accepted a BALB/c heart transplant develop donor-specific tolerance.
Splenectomized aly/aly mice having spontaneously accepted a BALB/c heart for 50 days were then transplanted with a skin graft from the same (BALB/c) or a third-party (C3H) donor. The results are shown as percent skin graft survival over time after skin transplantation (d0). Graft survival was analyzed using the Kaplan-Meier method. Three to five mice were tested in each group. The insert shows a picture of a representative aly/aly spl− mouse having accepted a BALB/c skin allograft for 280 days.
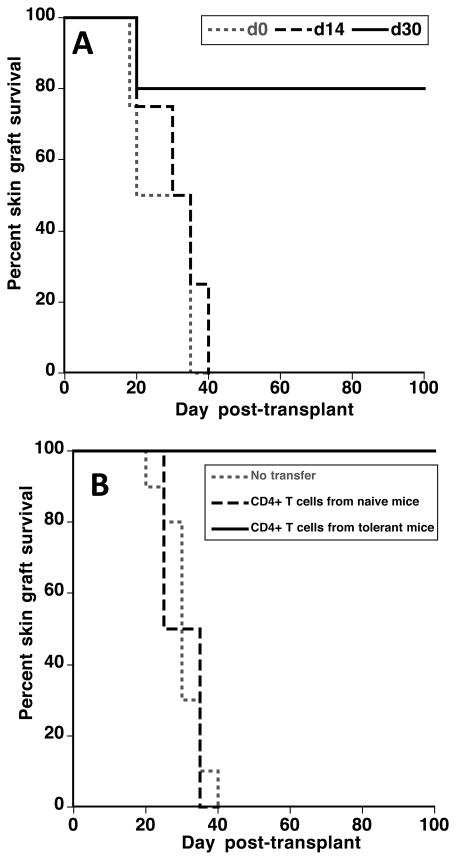
Additional experiments were conducted to investigate the mechanisms underlying transplant tolerance in aly/aly-spl− mice. First, BALB/c skin allografts were placed on aly/aly-spl− mice either at the time of (d0) or 14 or 30 days (d14 and d30) after heart transplantation of BALB/c hearts. Skin grafts placed at d0 or d14 were rejected acutely while the majority of those transplanted at d30 enjoyed long-term survival (Figure 6A). Therefore, tolerance requires 2–4 weeks to become established in this model. Next, naïve aly/aly-spl− mice were injected with 106 CD4+ T cells isolated from the peripheral blood of tolerant or control aly-aly-spl− mice 3 days prior to placement of a BALB/c skin graft. CD4+ T cells from tolerant mice achieved long-term survival of skin allografts in most recipients (Fig. 6B). Finally, aly-aly-spl− mice were injected either with anti-CD4 antibodies (GK1.5) or with anti-CD25 antibody (PC61) using a regimen known to deplete CD4+CD25highFoxp3+ Tregs (27), 3 days prior to and 20 and 47 days after BALB/c heart transplantation. All mice were transplanted with a BALB/c skin graft 50 days after cardiac transplantation. Mice recipients with anti-CD4 antibodies accepted heart but not skin transplants (n = 4, MST: 32 days). In contrast, anti-CD25 mAb-treated mice accepted both transplants (n= 5, MST > 100 days). Altogether, these experiments show that while transplant tolerance is dependent upon CD4+ T cells it apparently does not rely on the presence of CD4+CD25highFoxp3+ Tregs.
Figure 6. Mechanisms involved in tolerance of skin allografts in aly-aly splenectomized mice.
Panel A. Splenectomized aly/aly mice were transplanted with a BALB/c heart and received a skin allograft from the same donor at the same time (d0) or fourteen (d14) or thirty (d30) days later. The results are shown as percent skin graft survival over time after skin transplantation. Graft survival was analyzed using the Kaplan-Meier method. Three to five mice were tested in each group. Panel B. Splenectomized aly-aly mice were injected with 106 CD4+ T cells isolated from the peripheral blood of tolerant (mice having accepted a BALB/c heart for over 100 days) or control naïve aly-aly-spl− mice, 3 days prior to placement of a BALB/c skin graft. The results are shown as percent skin graft survival over time after skin transplantation (d0). Graft survival was analyzed using the Kaplan-Meier method. Three to six mice were tested in each group.
Discussion
A single spontaneous autosomal mutation in the NIK gene on chromosome 11 results in lack of lymph nodes and Peyer’s patches in aly/aly mice (23). These mice display a disorganized thymic architecture and severe defects in the spleen associated with the absence of germinal centers, an atrophic white pulp and no marginal zone (31). These multiple defects result in alymphoplasia and compromised cell-mediated and humoral immunity (31, 32). Despite such severe immunodeficiency, it is remarkable that aly/aly mice acutely rejected skin and cardiac allografts at the same pace as normal mice. Our observations are in disagreement with former studies from F. Lakkis et al. in the same aly/aly mouse model showing similar rejection of heart transplants but indefinite survival of skin allografts (33). On the other hand, our results corroborate previous reports from two other laboratories showing the rejection of skin and lung allografts in aly/aly mice as well as LTβR KO mice, which are also lacking lymph nodes (34–37).
Direct but no indirect T cell alloresponses were detected in skin-grafted aly/aly mice. This presumably reflects the fact that T cell indirect allosensitization occurs through traditional priming by peptides processed and presented by self-APCs in draining lymph nodes (38). On the other hand, direct allorecognition is unconventional in that it involves TCR interaction with intact allogeneic MHC molecules present on foreign APCs and triggers a polyclonal response engaging up to 10% of the T cell repertoire (1, 39, 40). We surmise that in skin-grafted aly/aly mice donor APCs leaving the graft upon its revascularization (d4-5 post-transplant) can initiate a direct alloresponse given the exceptionally high frequency of alloreactive T cells present in the recipient. On the other hand, indirect allosensitization requires “classical” T cell priming in regional lymph nodes. This is supported by our recent report showing that primarily vascularized skin allografts whose DCs emigrate through blood vessels elicit direct but no indirect alloresponses (29).
Unexpectedly, aly/aly-spl− mice devoid of all secondary lymphoid organs rejected skin allografts acutely (Fig. 1). This process was primarily mediated by CD8+ T cell activated directly. Moreover, transplanted mice displayed a significant generation/expansion of effector memory T cells and rejected a second skin graft from the same donor in an accelerated fashion. Where do T cells from transplanted aly/aly-spl− mice encounter alloantigens? There is accumulating evidence sugegsting that naïve alloreactive T cells can be primed either in secondary or tertiary lymphoid organs (TLO) located outside the graft or in tertiary lymphoid structures (TLS) being formed within the graft itself during inflammation (41–44). However, it is unlikely that naïve T cells of skin-grafted aly/aly-spl− mice become sensitized in TLO or TLS because aly/aly-spl− mice are devoid of TLO (with the exception of cryptopatches in the intestinal lamina propria (42)) and never form TLS in the skin, even upon lymphotoxin alpha transgenesis (45). Another possibility is that the direct alloresponse is mediated by “natural” alloreactive memory T cells (TMEMs) already present in mice before transplantation and reactivated at the graft site (18). Unlike naïve T cells, TMEMs recirculate through peripheral non-lymphoid tissues where they can be reactivated locally upon antigen presentation (19, 20). Indeed, our study shows the presence of 10–14% memory T cells (TMEMs) in the blood of non-transplanted aly/aly-spl− mice, including presumably some alloreactive cells. However, these pre-existing TMEMs are unlikely to mediate the alloresponse based upon previous studies showing their inability to trigger alloimmunity and allograft rejection upon adoptive transfer in aly/aly mice (18). Finally, since high frequencies of donor-specific activated inflammatory T cells were revealed in the bone marrow of transplanted aly/aly-spl− mice, we surmise that this could represent the site of allosensitization. This hypothesis is supported by a recent study demonstrating that bone marrow can serve as a priming site for T cell responses to blood-borne antigen (46).
Why do Aly/aly-spl− mice reject acutely allogeneic skin but not heart transplants? Skin allografts are notoriously more immunogenic and less susceptible to tolerogenesis than heart transplants (29). We have recently shown that this is due partly to the fact that cardiac allografts are immediately vascularized after their placement while conventional skin allografts are not (29). However, our observation that primarily vascularized skin allografts are acutely rejected in aly/aly mice (Fig. 1C) does not support the view that graft vascularization accounts for the longer survival of cardiac allografts in aly/aly-spl−. Alternatively, presentation of some highly immunogenic skin-specific antigens previously described by Steinmuller and others could account for the rejection of skin but not heart allografts in aly/aly-spl− mice (47).
While aly/aly-spl− mice did not reject acutely cardiac allotransplants, histological examination of these grafts revealed the presence of cardiac allograft vasculopathy (CAV) typical of chronic rejection. No alloantibodies were detected in these mice (data not shown). It is conceivable that the few inflammatory T cells activated directly (Figure 3B:200–300 γIFN-producing cells per 106 T cells) in these mice were insufficient to ensure acute rejection of heart transplants but elicited CAV, a phenomenon previously documented with adoptively transferred alloreactive T cell clones (30, 48). On the other hand, we surmise that chronic rejection may be associated with the high frequency of activated T cells secreting type 2 cytokines (IL-4 and IL-10) that were detected in these mice. The contribution of these T cells in chronic allograft rejection has been previously documented in several studies (49–51). Likewise, we recently reported that donor-specific T cells secreting type 2 cytokines (IL-10) could prevent early acute rejection of skin allografts while causing cardiac allograft vasculopathy in a MHC class I-disparate mouse transplant model (8).
Remarkably, aly/aly-spl− mice transplanted with cardiac transplants acquired donor-specific tolerance and accepted skin allografts from the same but not a third-part donor. Two observations show the contribution of CD4+ T cells to transplant tolerance in aly/aly-spl− mice: 1) pre-treatment of recipient mice with anti-CD4 mAbs prevented tolerance induction (data not shown) and, 2) long-term survival of skin grafts was achieved in naïve aly/aly-spl− mice via adoptive transfer of CD4+ T cells from tolerant mice (Fig. 6). Therefore, unlike previously reported (33), graft acceptance in this model does not result from immunologic ignorance but relies on an active tolerance process. It is noteworthy that pre-treatment of aly/aly-spl− recipients with an anti-CD25 mAb, which depletes CD4+FoxP3+ regulatory T cells (Tregs), had no effects on transplant tolerance induction. This is not surprising since it is established that aly/aly mice are basically devoid of FoxP3+ Tregs (52). In addition, studies from Bromberg’ laboratory support the requirement of lymph nodes for Treg-mediated tolerance (53). Therefore, it is unlikely that Tregs are not responsible for tolerance to allografts in aly/aly-spl− recipients. On the other hand, it is possible that Tr1 cells mediate tolerance. Indeed, such CD4+ FoxP3− Tr1 cells secreting IL-10 are regularly activated following systemic antigen administration via blood or oral routes (54–57). Furthermore, donor-specific FoxP3− Tr1 cells have been shown to prevent allograft rejection (58–60). Therefore, we surmise that IL-10-producing alloreactive Tr1 cells present in tolerant aly/aly-spl− mice represent the cells preventing acute rejection of skin allografts by CD8+ T cells (8).
In summary, our study demonstrates beyond doubt that both rejection and tolerance of allografts can occur in the absence of secondary lymphoid organs. Both aly/aly and aly-aly-spl− mice mount direct but not indirect alloresponses. It is still unclear where T cells actually interact with alloantigens on donor APCs after skin transplantation of aly-aly spl− mice. We cannot rule out the possibility that allorecognition by some preexisting memory T cells occurs in the graft. However, this hypothesis is not supported by the alloresponse kinetics as well as previous observations in the same model. Alternatively, since we detect potent direct alloresponses in the bone marrow of transplanted aly-aly-spl− mice and that priming of naïve T cells has been previously documented in this tissue, it represents a plausible site for initiation of the alloresponse in transplanted mice lacking secondary lymphoid organs.
Acknowledgments
This work was supported by grants from the NIH to Gilles Benichou, NIH R21AI100278 and R03AI094235.
Literature cited
- 1.Benichou G, Valujskikh A, Heeger PS. Contributions of direct and indirect T cell alloreactivity during allograft rejection in mice. J Immunol. 1999;162:352–358. [PubMed] [Google Scholar]
- 2.Lechler RI, Lombardi G, Batchelor JR, Reinsmoen N, Bach FH. The molecular basis of alloreactivity. Immunology today. 1990;11:83–88. doi: 10.1016/0167-5699(90)90033-6. [DOI] [PubMed] [Google Scholar]
- 3.Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med. 1992;175:305–308. doi: 10.1084/jem.175.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benichou G, Fedoseyeva E, Lehmann PV, Olson CA, Geysen HM, McMillan M, Sercarz EE. Limited T cell response to donor MHC peptides during allograft rejection. Implications for selective immune therapy in transplantation. J Immunol. 1994;153:938–945. [PubMed] [Google Scholar]
- 5.Liu Z, Sun YK, Xi YP, Hong B, Harris PE, Reed EF, Suciu-Foca N. Limited usage of T cell receptor V beta genes by allopeptide-specific T cells. J Immunol. 1993;150:3180–3186. [PubMed] [Google Scholar]
- 6.Gould DS, Auchincloss H., Jr Direct and indirect recognition: the role of MHC antigens in graft rejection. Immunology today. 1999;20:77–82. doi: 10.1016/s0167-5699(98)01394-2. [DOI] [PubMed] [Google Scholar]
- 7.Yamada A, Laufer TM, Gerth AJ, Chase CM, Colvin RB, Russell PS, Sayegh MH, Auchincloss H., Jr Further analysis of the T-cell subsets and pathways of murine cardiac allograft rejection. Am J Transplant. 2003;3:23–27. doi: 10.1034/j.1600-6143.2003.30105.x. [DOI] [PubMed] [Google Scholar]
- 8.Illigens BM, Yamada A, Anosova N, Dong VM, Sayegh MH, Benichou G. Dual effects of the alloresponse by Th1 and Th2 cells on acute and chronic rejection of allotransplants. Eur J Immunol. 2009;39:3000–3009. doi: 10.1002/eji.200838980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee RS, Yamada K, Houser SL, Womer KL, Maloney ME, Rose HS, Sayegh MH, Madsen JC. Indirect recognition of allopeptides promotes the development of cardiac allograft vasculopathy. Proc Natl Acad Sci U S A. 2001;98:3276–3281. doi: 10.1073/pnas.051584498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayegh MH, Carpenter CB. Role of indirect allorecognition in allograft rejection. International reviews of immunology. 1996;13:221–229. doi: 10.3109/08830189609061749. [DOI] [PubMed] [Google Scholar]
- 11.Hayry P, Isoniemi H, Yilmaz S, Mennander A, Lemstrom K, Raisanen-Sokolowski A, Koskinen P, Ustinov J, Lautenschlager I, Taskinen E, et al. Chronic allograft rejection. Immunological reviews. 1993;134:33–81. doi: 10.1111/j.1600-065x.1993.tb00639.x. [DOI] [PubMed] [Google Scholar]
- 12.Weiss MJ, Madsen JC, Rosengard BR, Allan JS. Mechanisms of chronic rejection in cardiothoracic transplantation. Frontiers in bioscience : a journal and virtual library. 2008;13:2980–2988. doi: 10.2741/2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg AS, Singer A. Cellular basis of skin allograft rejection: an in vivo model of immune-mediated tissue destruction. Annu Rev Immunol. 1992;10:333–358. doi: 10.1146/annurev.iy.10.040192.002001. [DOI] [PubMed] [Google Scholar]
- 14.Austyn JM, Larsen CP. Migration patterns of dendritic leukocytes. Implications for transplantation. Transplantation. 1990;49:1–7. doi: 10.1097/00007890-199001000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CP, Austyn JM, Morris PJ. The role of graft-derived dendritic leukocytes in the rejection of vascularized organ allografts. Recent findings on the migration and function of dendritic leukocytes after transplantation. Annals of surgery. 1990;212:308–315. doi: 10.1097/00000658-199009000-00009. discussion 316–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen CP, Morris PJ, Austin JM. Migration of dendritic leukocytes from cardiac allografts into host spleens. A novel pathway for initiation of rejection. J Exp Med. 1990;171:307–314. doi: 10.1084/jem.171.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lechler RI, Batchelor JR. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982;155:31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051–4058. doi: 10.4049/jimmunol.176.7.4051. [DOI] [PubMed] [Google Scholar]
- 19.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 20.Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E, Hengartner H, Zinkernagel RM. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc Natl Acad Sci U S A. 2000;97:13263–13268. doi: 10.1073/pnas.230417497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen CP, Barker H, Morris PJ, Austyn JM. Failure of mature dendritic cells of the host to migrate from the blood into cardiac or skin allografts. Transplantation. 1990;50:294–301. doi: 10.1097/00007890-199008000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Oberbarnscheidt MH, Walch JM, Li Q, Williams AL, Walters JT, Hoffman RA, Demetris AJ, Gerard C, Camirand G, Lakkis FG. Memory T cells migrate to and reject vascularized cardiac allografts independent of the chemokine receptor CXCR3. Transplantation. 2011;91:827–832. doi: 10.1097/TP.0b013e31820f0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyawaki S, Nakamura Y, Suzuka H, Koba M, Yasumizu R, Ikehara S, Shibata Y. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur J Immunol. 1994;24:429–434. doi: 10.1002/eji.1830240224. [DOI] [PubMed] [Google Scholar]
- 24.Billingham RE, Medawar PD. The technique of free skin grafting in mammals. J Exp Biol. 1951;28:385. [Google Scholar]
- 25.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C, Cho HR, Aruffo A, Hollenbaugh D, Linsley PS, Winn KJ, Pearson TC. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 27.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc Natl Acad Sci U S A. 2004 doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Russell ME, Hancock WW, Akalin E, Wallace AF, Glysing-Jensen T, Willett TA, Sayegh MH. Chronic cardiac rejection in the LEW to F344 rat model. Blockade of CD28-B7 costimulation by CTLA4Ig modulates T cell and macrophage activation and attenuates arteriosclerosis. J Clin Invest. 1996;97:833–838. doi: 10.1172/JCI118483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kant CD, Akiyama Y, Tanaka K, Shea S, Connolly SE, Germana S, Winn HJ, Leguern C, Tocco G, Benichou G. Primary vascularization of allografts governs their immunogenicity and susceptibility to tolerogenesis. J Immunol. 2013;191:1948–1956. doi: 10.4049/jimmunol.1202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He C, Schenk S, Zhang Q, Valujskikh A, Bayer J, Fairchild RL, Heeger PS. Effects of T cell frequency and graft size on transplant outcome in mice. J Immunol. 2004;172:240–247. doi: 10.4049/jimmunol.172.1.240. [DOI] [PubMed] [Google Scholar]
- 31.Shinkura R, Kitada K, Matsuda F, Tashiro K, Ikuta K, Suzuki M, Kogishi K, Serikawa T, Honjo T. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nature genetics. 1999;22:74–77. doi: 10.1038/8780. [DOI] [PubMed] [Google Scholar]
- 32.Shinkura R, Matsuda F, Sakiyama T, Tsubata T, Hiai H, Paumen M, Miyawaki S, Honjo T. Defects of somatic hypermutation and class switching in alymphoplasia (aly) mutant mice. International immunology. 1996;8:1067–1075. doi: 10.1093/intimm/8.7.1067. [DOI] [PubMed] [Google Scholar]
- 33.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nature medicine. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 34.Yamanokuchi S, Ikai I, Nishitai R, Matsushita T, Sugimoto S, Shiotani T, Yamaoka Y. Asialo GM1 positive CD8+ T cells induce skin allograft rejection in the absence of the secondary lymphoid organs. The Journal of surgical research. 2005;129:57–63. doi: 10.1016/j.jss.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Luo ZJ, Tanaka T, Kimura F, Miyasaka M. Analysis of the mode of action of a novel immunosuppressant FTY720 in mice. Immunopharmacology. 1999;41:199–207. doi: 10.1016/s0162-3109(99)00004-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhou P, Hwang KW, Palucki D, Kim O, Newell KA, Fu YX, Alegre ML. Secondary lymphoid organs are important but not absolutely required for allograft responses. Am J Transplant. 2003;3:259–266. doi: 10.1034/j.1600-6143.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 37.Gelman AE, Li W, Richardson SB, Zinselmeyer BH, Lai J, Okazaki M, Kornfeld CG, Kreisel FH, Sugimoto S, Tietjens JR, Dempster J, Patterson GA, Krupnick AS, Miller MJ, Kreisel D. Cutting edge: Acute lung allograft rejection is independent of secondary lymphoid organs. J Immunol. 2009;182:3969–3973. doi: 10.4049/jimmunol.0803514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Ashwell JD, Chen C, Schwartz RH. High frequency and nonrandom distribution of alloreactivity in T cell clones selected for recognition of foreign antigen in association with self class II molecules. J Immunol. 1986;136:389–395. [PubMed] [Google Scholar]
- 40.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 41.Nasr IW, Reel M, Oberbarnscheidt MH, Mounzer RH, Baddoura FK, Ruddle NH, Lakkis FG. Tertiary lymphoid tissues generate effector and memory T cells that lead to allograft rejection. Am J Transplant. 2007;7:1071–1079. doi: 10.1111/j.1600-6143.2007.01756.x. [DOI] [PubMed] [Google Scholar]
- 42.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Dong Y, Sun JZ, Taylor RT, Guo C, Alegre ML, Williams IR, Newell KA. Donor lymphoid organs are a major site of alloreactive T-cell priming following intestinal transplantation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6:2563–2571. doi: 10.1111/j.1600-6143.2006.01516.x. [DOI] [PubMed] [Google Scholar]
- 44.Hautz T, Zelger BG, Nasr IW, Mundinger GS, Barth RN, Rodriguez ED, Brandacher G, Weissenbacher A, Zelger B, Cavadas P, Margreiter R, Lee WP, Pratschke J, Lakkis FG, Schneeberger S. Lymphoid neogenesis in skin of human hand, nonhuman primate, and rat vascularized composite allografts. Transplant international : official journal of the European Society for Organ Transplantation. 2014;27:966–976. doi: 10.1111/tri.12358. [DOI] [PubMed] [Google Scholar]
- 45.Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT, Romani N, Lira SA. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- 46.Feuerer M, Beckhove P, Garbi N, Mahnke Y, Limmer A, Hommel M, Hammerling GJ, Kyewski B, Hamann A, Umansky V, Schirrmacher V. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nature medicine. 2003;9:1151–1157. doi: 10.1038/nm914. [DOI] [PubMed] [Google Scholar]
- 47.Steinmuller D, Tyler JD, Waddick KG, Burlingham WJ. Epidermal alloantigen and the survival of mouse skin allografts. Transplantation. 1982;33:308–313. doi: 10.1097/00007890-198203000-00019. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Demir Y, Valujskikh A, Heeger PS. Antigen location contributes to the pathological features of a transplanted heart graft. The American journal of pathology. 2004;164:1407–1415. doi: 10.1016/S0002-9440(10)63227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mhoyan A, Wu GD, Kakoulidis TP, Que X, Yolcu ES, Cramer DV, Shirwan H. Predominant expression of the Th2 response in chronic cardiac allograft rejection. Transplant international : official journal of the European Society for Organ Transplantation. 2003;16:464–473. doi: 10.1007/s00147-003-0590-6. [DOI] [PubMed] [Google Scholar]
- 50.Koksoy S, Kakoulidis TP, Shirwan H. Chronic heart allograft rejection in rats demonstrates a dynamic interplay between IFN-gamma and IL-10 producing T cells. Transpl Immunol. 2004;13:201–209. doi: 10.1016/j.trim.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Csencsits K, Wood SC, Lu G, Magee JC, Eichwald EJ, Chang CH, Bishop DK. Graft rejection mediated by CD4+ T cells via indirect recognition of alloantigen is associated with a dominant Th2 response. Eur J Immunol. 2005;35:843–851. doi: 10.1002/eji.200425685. [DOI] [PubMed] [Google Scholar]
- 52.Kajiura F, Sun S, Nomura T, Izumi K, Ueno T, Bando Y, Kuroda N, Han H, Li Y, Matsushima A, Takahama Y, Sakaguchi S, Mitani T, Matsumoto M. NF-kappa B-inducing kinase establishes self-tolerance in a thymic stroma-dependent manner. J Immunol. 2004;172:2067–2075. doi: 10.4049/jimmunol.172.4.2067. [DOI] [PubMed] [Google Scholar]
- 53.Ochando JC, Yopp AC, Yang Y, Garin A, Li Y, Boros P, Llodra J, Ding Y, Lira SA, Krieger NR, Bromberg JS. Lymph node occupancy is required for the peripheral development of alloantigen-specific Foxp3+ regulatory T cells. J Immunol. 2005;174:6993–7005. doi: 10.4049/jimmunol.174.11.6993. [DOI] [PubMed] [Google Scholar]
- 54.Faria AM, Weiner HL. Oral tolerance. Immunological reviews. 2005;206:232–259. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liblau RS, Tisch R, Shokat K, Yang X, Dumont N, Goodnow CC, McDevitt HO. Intravenous injection of soluble antigen induces thymic and peripheral T-cells apoptosis. Proc Natl Acad Sci U S A. 1996;93:3031–3036. doi: 10.1073/pnas.93.7.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thorstenson KM, Khoruts A. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J Immunol. 2001;167:188–195. doi: 10.4049/jimmunol.167.1.188. [DOI] [PubMed] [Google Scholar]
- 57.Valujskikh A, VanBuskirk AM, Orosz CG, Heeger PS. A role for TGFbeta and B cells in immunologic tolerance after intravenous injection of soluble antigen. Transplantation. 2001;72:685–693. doi: 10.1097/00007890-200108270-00022. [DOI] [PubMed] [Google Scholar]
- 58.Groux H, Bigler M, de Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J Exp Med. 1996;184:19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roncarolo MG, Gregori S, Lucarelli B, Ciceri F, Bacchetta R. Clinical tolerance in allogeneic hematopoietic stem cell transplantation. Immunological reviews. 2011;241:145–163. doi: 10.1111/j.1600-065X.2011.01010.x. [DOI] [PubMed] [Google Scholar]
- 60.Gagliani N, Jofra T, Valle A, Stabilini A, Morsiani C, Gregori S, Deng S, Rothstein DM, Atkinson M, Kamanaka M, Flavell RA, Roncarolo MG, Battaglia M. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13:1963–1975. doi: 10.1111/ajt.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]