Abstract
In the early stages of sepsis, lymphocytes undergo apoptosis, resulting in lymphopenia and immunosuppression. The trigger for septic lymphopenia is unknown. Using the polymicrobial model of murine sepsis, we investigated the role of C5a receptors in septic lymphopenia. In wild type mice, CLP resulted in splenocyte apoptosis and significant lymphopenia after three days, which was not observed in C5aR1-deficient (C5aR1−/−) or C5aR2−/− mice. Our data show that mouse neutrophils exposed to recombinant mouse C5a cause release of histones in a dose-dependent and time-dependent manner. Histone levels in spleen were significantly elevated following CLP but were reduced by C5aR1 absence. Histones induced significant lymphocyte apoptosis in vitro. Antibody-mediated neutralization of histones prevented the development of lymphopenia in sepsis. Together, these results describe a new pathway of septic lymphopenia involving complement and extracellular histones. Targeting of this pathway may have therapeutic benefit for patients with sepsis or other serious illness.
Keywords: rodent, T cells, apoptosis, complement, cecal ligation, puncture
INTRODUCTION
Sepsis remains a significant clinical challenge, with over 750,000 cases annually in the United States resulting in 20–30% mortality (1). Lymphocyte apoptosis has been recognized as an important pathophysiologic mechanism during sepsis and is known to contribute to the resulting immunocompromise of sepsis (2). Lymphocyte apoptosis has been demonstrated in post-mortem studies of septic humans (3), and lymphopenia is related to high mortality rates (4). Many researchers now believe that the development of immunosuppression, rather than prolonged inflammation, is the predominant factor determining morbidity and mortality during sepsis (2).
Due to the fact that many septic patients are lymphopenic at the time of diagnosis, recent focus has been on the development of immunostimulant therapeutics to treat septic immunosuppression “after-the-fact” (5). As such, administration of immunostimulant cytokines (e.g., IL-7) or neutralization of inhibitory receptors (e.g., PD-1) have been tested in rodent models and have shown promise for restoring immunity post-sepsis (6, 7). However, these strategies may not be clinically viable for all human patients due to their immunostimulant nature, since they have the potential to exacerbate the hyperinflammatory phase of sepsis if administered too early in disease progression, the result of which could be intensified multi-organ failure. Therefore, a more complete understanding of the genesis of lymphopenia during sepsis is needed, which may present therapeutic options for patients in early stages of sepsis.
Precluding an early therapeutic option is the fact that the initial stimulus for lymphocyte apoptosis during sepsis remains unknown. Lymphocyte apoptosis during sepsis is known to occur through both mitochondrial (intrinsic) and receptor-mediated (extrinsic) pathways (8). As described by Hotchkiss et al., such findings suggest multiple pathways of lymphocyte apoptosis and lymphopenia during sepsis (2). The complement anaphylatoxin C5a, which is produced in large quantities during sepsis, is known to contribute to septic lethality (9). Our group has previously shown an important role for C5a in thymocyte apoptosis during sepsis (10). However, whether C5a contributes to mature peripheral lymphocyte apoptosis and the development of lymphopenia during sepsis is not known. In this report, we describe a novel mechanism of lymphocyte apoptosis and lymphopenia during sepsis involving complement and extracellular histones.
MATERIALS & METHODS
Animals
All procedures were performed within the U.S. National Institutes of Health guidelines and were approved by the University of Michigan Committee on the Use and Care of Animals. Male age-matched (8–9 weeks old) C57BL/6 (Wt) mice were purchased from the Jackson Laboratories (Bar Harbor, ME). C5aR1−/− and C5aR2−/− mice on the C57BL/6 background were bred in-house, and were generated as described (11, 12).
Reagents
Mixed calf thymus histones (Type II-A) were from Sigma (St. Louis, MO). Anti-histone antibody (clone BWA3 (13)) was purified from ascites fluid by protein A/G chromatography.
Cecal ligation and puncture (CLP)
Mid-grade CLP (~50% survival after 7 days) was used for this study, as described previously (14). For histone neutralization studies, mice received 400 µg of BWA3 or control antibody (Jackson Immunoresearch, West Grove, PA) i.v. at the time of CLP.
Blood and spleen collection
At time points after the induction of CLP, heparinized blood was collected by cardiac puncture. Leukocyte counts were determined on a hemocytometer. Differential counts were determined with blood smears. Greater than 200 cells were analyzed per blood sample.
Single-cell suspensions of spleens were prepared as described (15). Erythrocytes were lysed in a 0.1 M ammonium chloride solution. Cells were labeled with fluorochrome-conjugated antibodies to detect CD4 and CD8 (both from eBioscience, San Diego, CA) and analyzed on a BD LSR-II flow cytometer equipped with FACSDiva software (both from BD biosciences, San Jose, CA).
TUNEL
Sections were labeled for TUNEL analysis according to the manufacturer recommendations using a commercially available kit (Promega, Madison, WI), and mounted in ProLong Gold anti-fade reagent containing DAPI (Life Technologies, Carlsbad, CA). Images were acquired on a Nikon A-1 confocal system with Nikon Elements software. The average number of TUNEL+ cells per field was determined in a blinded fashion from ≥10 fields over several sections per spleen.
Apoptosis assays
Apoptosis was determined by PE-Annexin V and 7-AAD (both from BD biosciences). Cells were analyzed by flow cytometry as described above.
Neutrophils and macrophages were harvested by peritoneal lavage 4 hrs and 4 days, respectively, after i.p. administration of 2.4% thioglycollate.
Determination of tissue histone content and histone release assays
Spleens were harvested and snap frozen in LN2 at time points following CLP. Tissues were mechanically homogenized in PBS containing protease inhibitors (Roche, Indianapolis, IN). Total protein estimations were determined by the BCA assay (Sigma). Histone ELISAs were from Roche. Purified mixed calf thymus histones were used to generate standard curves, as described (16). For histone release assays, peritoneal neutrophils (3 × 106 per ml) were incubated with recombinant mouse C5a (R&D Systems, Minneapolis, MN) for the indicated time and dose at 37°C.
Statistical analysis
Data are expressed as mean ± SEM. Significant differences between sample means were determined by Student’s t test or one-way ANOVA followed by Tukey’s multiple comparisons test, where appropriate. p values < 0.05 were considered to be significant.
RESULTS AND DISCUSSION
Role for C5a receptors in the development of septic lymphopenia
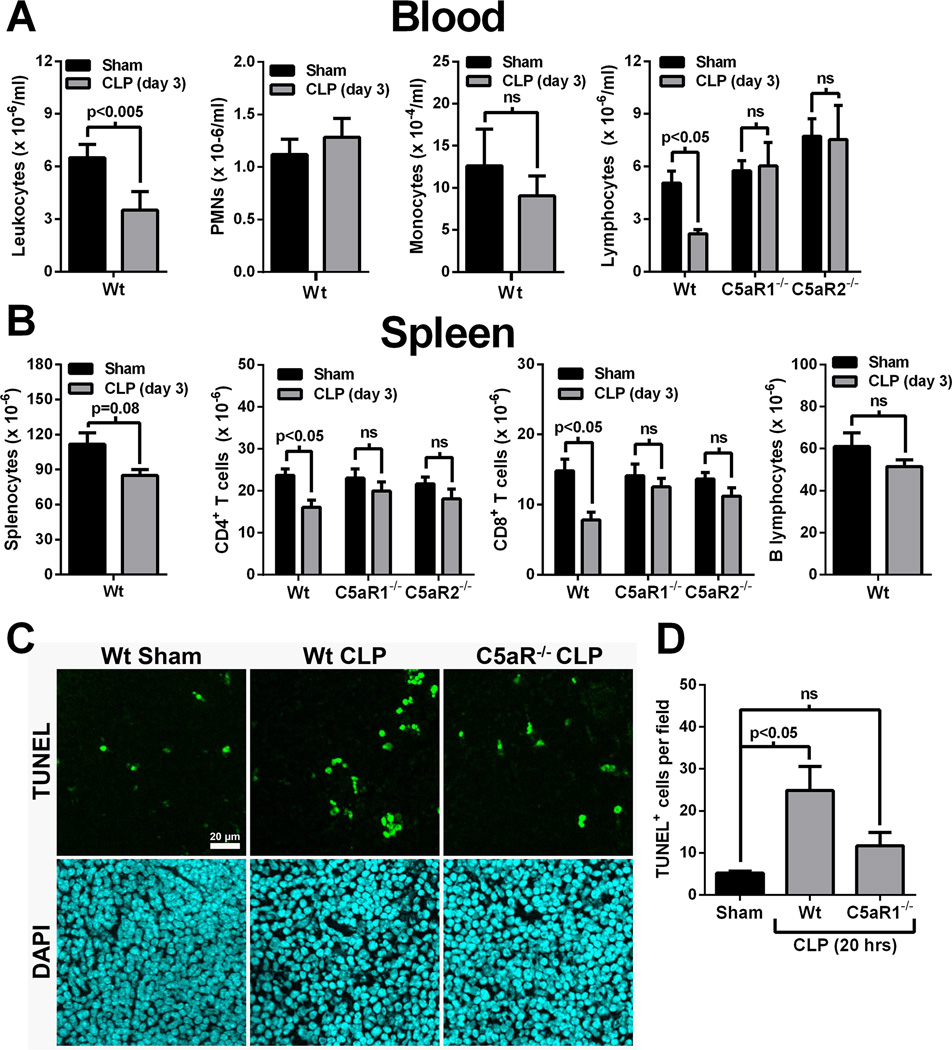
Three days following CLP, blood leukocyte numbers were significantly reduced, compared to sham mice (Fig. 1A, left panel). Leukocyte differential analyses revealed that PMN and monocyte numbers were not affected at this time point after CLP (Fig 1A, middle panels). In contrast, blood lymphocyte numbers in CLP mice were reduced by 57% compared to sham animals (Fig. 1A, right panel). However, CLP did not cause reductions in blood lymphocyte numbers from C5aR1−/− and C5aR2−/− mice (Fig. 1A, right panel). In the spleen, the numbers of splenocytes were modestly reduced following CLP, although this did not reach statistical significance (Fig. 1B, left panel). Splenic CD4+ and CD8+ lymphocytes were reduced in Wt mice by 32% and 42%, respectively, 3 days after CLP (Fig. 1B). However, CLP did not significantly reduce the numbers of CD4+ or CD8+ splenocytes in C5aR1−/− or C5aR2−/− mice (Fig. 1B, middle panels). Splenic B cell numbers were not affected three days after CLP (Fig. 1B, right panel). Together, these results suggest a role for both C5a receptors in the development of T cell lymphopenia following CLP. Since C5aR1 and C5aR2 are known to act in concert in many inflammatory conditions (16–18), we focused on the role of C5aR1 in subsequent studies.
Figure 1.
CLP-induced lymphocyte lymphopenia is C5a receptor-dependent. A) Blood leukocyte numbers 3 days after CLP in Wt mice or Wt, C5aR1−/−, and C5aR2−/− mice (n=5–10 mice per group). B) Splenic leukocyte numbers 3 days after CLP in Wt mice or Wt, C5aR1−/−, and C5aR2−/− mice (n=5 mice per group). C) Representative TUNEL labeling of spleen sections from Wt or C5aR1−/− mice 20 hrs after CLP (or sham Wt). The scale bar is for all images. D) Aggregate data from TUNEL labeling expressed as the number of TUNEL+ cells per microscopic field (n=5 mice per group).
Lymphocyte apoptosis is a prominent feature of sepsis, and is a significant factor in the development of septic lymphopenia (2). CLP induced significant splenic apoptosis in Wt mice after 20 hrs, as measured by TUNEL labeling (Fig.1C and 1D). Far fewer apoptotic cells were observed in C5aR1−/− mice at the same time point following CLP (Fig.1C and 1D). These results suggest that C5aR1 contributes to splenocyte apoptosis following CLP sepsis.
C5a does not directly induce lymphocyte apoptosis
We hypothesized that C5a may directly induce lymphocyte apoptosis. Normal splenocytes or splenocytes harvested from septic mice (5 or 18 hrs after CLP) were exposed to various concentrations of C5a (125–1000 ng/ml) and cell viability was determined after 14 hrs. Results showed that C5a did not induce significant cell death in vitro in any of the splenocyte preparations (Supplementary Figure 1), thus ruling out a direct role for C5a in lymphocyte death.
Role for extracellular histones in septic lymphopenia
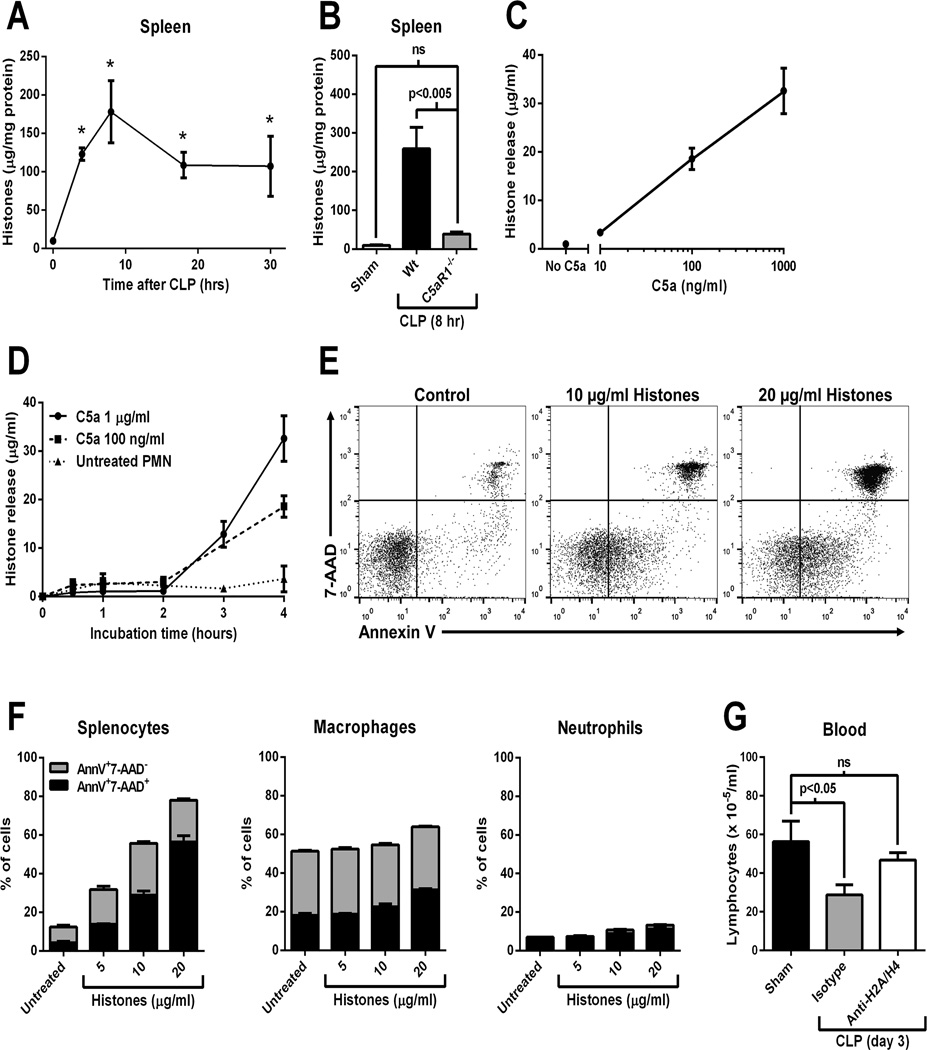
Evidence has accumulated that histones function as damage-associated molecular patterns (DAMPs) when present in the extracellular space (16, 19–21). High levels of extracellular histones in plasma are known to be present during sepsis in humans and animals (20, 22). Extracellular histones contribute to septic mortality, as evidenced by the observation that antibody-mediated neutralization of histones is protective during several models of sepsis in mice (20). C5a is known to induce the presence of extracellular histones during acute lung inflammation in vivo (16, 23) through direct effects on neutrophils via the release of neutrophil extracellular traps (NETs) (23). In the current study, levels of histones detected in spleen homogenates were dramatically elevated following CLP (Fig. 2A), suggesting that histones were accumulating in the spleen during sepsis. High histone levels in spleen following CLP were abolished in C5aR1−/− mice (Fig. 2B). Figure2C and 2D document histone release from neutrophils in vitro as a function of dose of C5a (10–1000 ng/ml) and of time (0–4 hours). Extracellular histones are known to be cytotoxic for a variety of cell types, including lymphocytes (16, 19). In fact, extracellular histones have recently been shown to directly induce lymphocyte apoptosis which was dependent on p38, mitochondrial injury, and caspase-3 activation (24). In our studies, treatment of splenocytes with extracellular histones in vitro resulted in apoptosis/necrosis in a dose-dependent manner (Fig.2E and 2F, left panel). To determine whether these effects were lymphocyte-specific, we treated enriched macrophage and neutrophil populations with histones under the same conditions in vitro. Results showed that histones did not induce significant levels of apoptosis in these populations (Fig. 2F, middle and right panels), suggesting that lymphocytes are especially susceptible to histones-induced apoptosis. Importantly, antibody-mediated neutralization of histones (H2A and H4) protected blood lymphocyte numbers 3 days after CLP (Fig. 2G), thus demonstrating an in vivo role for extracellular histones in septic lymphopenia. Taken together, these results suggest that C5aR1 promoted histone release and accumulation in spleen during CLP. Histones induced lymphocyte apoptosis/necrosis and promoted the development of septic lymphopenia.
Figure 2.
Role for extracellular histones in septic lymphopenia. A) Levels of histones in whole spleen homogenates at time points following CLP (n=3–5 mice per group). *p<0.05 vs. time 0. B) Levels of histones in spleen homogenates 8 hrs after CLP in Wt and C5aR−/− mice (or sham Wt, n=5 mice per group). C) Dose-response (4 hours) and D) time course data demonstrating in vitro release of histones from elicited peritoneal neutrophils exposed to C5a. E) Apoptosis of splenocytes exposed to purified calf thymus histones in vitro for 90 min. Apoptosis was determined by Annexin V binding and use of the vital dye 7-AAD. Representative flow cytometry plots are shown. F) Aggregate data from 3 experiments using splenocytes, or enriched populations of peritoneal macrophages, or peritoneal neutrophils. G) Blood lymphocyte numbers 3 days after CLP or sham surgery. CLP mice received anti-histone (H2A/H4) neutralizing antibody (400 µg i.v.) or a control antibody at the time of CLP (n=6 mice per group).
Complement- and extracellular histone-dependent septic lymphopenia
Both receptor- (extrinsic) and mitochondrial- (intrinsic) mediated pathways of apoptosis are known to occur in lymphocytes from septic patients and from rodents after CLP (8, 25). In the current study, C5a was an important inducer of septic lymphopenia via extracellular histones. Extracellular histones are known to induce lymphocyte apoptosis through the mitochondrial pathway (24). However, other pathways of C5a-induced apoptosis (i.e., receptor-mediated) are not ruled out by the current study. Indeed, while C5aR1 deficiency or histone neutralization was protective, it failed to fully return levels to sham controls in many cases (e.g., Fig. 1B, Fig. 1D, Fig. 2E), although the differences were not statistically significant. Ultimately, further study is needed to identify the drivers of receptor-mediated apoptosis during sepsis.
We observed dramatically increased levels of histones in spleen already 4 hrs after CLP (Fig. 2A). The exact source of extracellular histones during sepsis is currently unknown, but we have recently shown that cell-associated (NETs) and cell-free (supernatant) histones are released by neutrophils in response to C5a (23). Intravascular NETs are known to be present 4 hrs after i.p. LPS administration in mice (26). It may be that histone presence in spleen following CLP is the result of release of histones by intravascular neutrophils/NETs, with passive accumulation in the spleen. In the current report, we have demonstrated histone release from neutrophils in a dose- and time-dependent manner. However, the exact mechanism of histone release and splenic accumulation of histones during sepsis remains to be determined.
It is likely that a potential drug therapy targeting the generation of lymphopenia will not be effective for many septic patients, due to the fact that most patients are lymphopenic prior to diagnosis and admission to the hospital. However, targeting C5a or extracellular histones may be useful for a subset of patients that have benefitted from an early diagnosis. Additionally, lymphocyte apoptosis and lymphopenia can result during other serious conditions such as severe trauma, ischemia/reperfusion injury, or hemorrhagic shock (27, 28). Indeed, robust complement activation and the presence of extracellular histones in plasma have been described in these conditions (29, 30). Such patients may have the opportunity to receive drug treatment to prevent lymphopenia as opposed to “after-the-fact” strategies to restore immunity.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge support from the UMMS Dept. of Pathology flow cytometry core facility. The authors thank Sue Scott for excellent support in the preparation of this manuscript.
ABBREVIATIONS
- C5aR1−/−
C5a receptor 1-deficient
- C5aR2−/−
C5a receptor 2-deficient
- CLP
cecal ligation and puncture
- DAMP
damage-associated molecular pattern
- NET
neutrophil extracellular trap
- Wt
wild type
Footnotes
This work was supported by grants from the National Institutes of Health, GM-29507 and GM-61656 (PAW) and NHLBI-T32-HL007517-29 (JJG).
REFERENCES
- 1.Angus DC, Van der Poll T. Severe sepsis and septic shock. N. Engl. J. Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Chien YC, Chung KP, Cheng JS, Chang HT, Yu CJ. Lymphopenia is associated with worse outcomes in patients with severe sepsis. Am. J. Respir. Crit. Care Med. 2012;185:A5992. [Google Scholar]
- 5.Boomer JS, Shuherk-Shaffer J, Hotchkiss RS, Green JM. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit. Care. 2012;16:R112. doi: 10.1186/cc11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unsinger J, McGlynn M, Kasten KR, Hoekzema AS, Watanabe E, Muenzer JT, McDonough JS, Tschoep J, Ferguson TA, McDunn JE, Morre M, Hildeman DA, Caldwell CC, Hotchkiss RS. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J. Immunol. 2010;184:3768–3779. doi: 10.4049/jimmunol.0903151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmamdam P, Inoue S, Unsinger J, Chang KC, McDunn JE, Hotchkiss RS. Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survivial during sepsis. J. Leukoc. Biol. 2010;88:233–240. doi: 10.1189/jlb.0110037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 9.Czermak BJ, Sarma JV, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat. Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 10.Riedemann NC, Guo RF, Laudes IJ, Keller K, Sarma JV, Padgaonkar V, Zetoune FS, Ward PA. C5a receptor and thymocyte apoptosis in sepsis. FASEB J. 2002;16:887–888. doi: 10.1096/fj.02-0033fje. [DOI] [PubMed] [Google Scholar]
- 11.Hopken UE, Lu B, Gerard NP, Gerard C. Impaired inflammatory responses in the reverse arthus reaction through genetic deletion of the C5a receptor. J. Exp. Med. 1997;186:749–756. doi: 10.1084/jem.186.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerard NP, Lu B, Liu P, Craig S, Fujiwara Y, Okinaga S, Gerard C. An anti-inflammatory function for the complement anaphylatoxin C5a-binding protein, C5L2. J. Biol. Chem. 2005;280:39677–39680. doi: 10.1074/jbc.C500287200. [DOI] [PubMed] [Google Scholar]
- 13.Monestier M, Fasy TM, Losman MJ, Novick KE, Muller S. Structure and binding properties of monoclonal antibodies to core histones from autoimmune mice. Mol. Immunol. 1993;30:1069–1075. doi: 10.1016/0161-5890(93)90153-3. [DOI] [PubMed] [Google Scholar]
- 14.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA. Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 2009;4:31–36. doi: 10.1038/nprot.2008.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grailer JJ, Steeber DA. Vascular endothelail growth factor receptor inhibitor SU5416 suppresses lymphocyte generation and immune responses in mice by increasing plasma corticosterone. PLoS One. 2013;8:e75390. doi: 10.1371/journal.pone.0075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosmann M, Grailer JJ, Russkamp NF, Ruemmler R, Monestier M, Zetoune FS, Sarma JV, Ward PA. Extracellular histones are essential effectors of C5aR and C5L2-dependent tissue damage and inflammation in acute lung injury. FASEB J. 2013;27:5010–5021. doi: 10.1096/fj.13-236380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bosmann M, Haggadone MD, Zetoune FS, Sarma JV, Ward PA. The interaction between C5a and both C5aR and C5L2 receptors is required for production of G-CSF during acute inflammation. Eur. J. Immunol. 2013;43:1907–1913. doi: 10.1002/eji.201243075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang M, Mackay CR, Zetoune FS, Gerard NP, Cianflone K, Kohl J, Gerard C, Sarma JV, Ward PA. Functional roles for C5a receptors in sepsis. Nat. Med. 2008;14:551–557. doi: 10.1038/nm1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT. Extracellular histones are major mediators of death in sepsis. Nat. Med. 2009;15:1318–1321. doi: 10.1038/nm.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT. Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J. Immunol. 2011;187:2626–2631. doi: 10.4049/jimmunol.1003930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allam R, Darisipudi MN, Tschopp J, Anders HJ. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur. J. Immunol. 2013 doi: 10.1002/eji.201243224. Epub ahead of print: [DOI] [PubMed] [Google Scholar]
- 22.Ostrowski SR, Berg RM, Windelov NA, Meyer MA, Plovsing RR, Moller K, Johansson PI. Coagulopathy, catecholamines, and biomarkers of endothelial damage in experimental human endotoxemia and in patients with severe sepsis: a prospective study. J. Crit. Care. 2013;28:586–596. doi: 10.1016/j.jcrc.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Grailer JJ, Canning BA, Kalbitz M, Haggadone MD, Dhond RM, Andjelkovic AV, Zetoune FS, Ward PA. Role for the NLRP3 inflammasome during acute lung injury. J. Immunol. 2014 doi: 10.4049/jimmunol.1400368. Epub: May 2, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu ZG, Ni SY, Chen GM, Cai J, Guo ZH, Chang P, Li YS. Histones-mediated lymphocyte apoptosis during sepsis is dependent on p38 phosphorylation and mitochondrial permeability transition. PLoS One. 2013;8:e77131. doi: 10.1371/journal.pone.0077131. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J. Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 26.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12:324–333. doi: 10.1016/j.chom.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Teodorczyk-Injeyan JA, Cembrzynska-Nowak M, Lalani S, Peters WJ, Mills GB. Immune deficiency following thermal trauma is associated with apoptotic cell death. J. Clin. Immunol. 1995;15:318–328. doi: 10.1007/BF01541322. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Liang L, Wu W, Gao Y, Chen ZB, Liang ZY, Liang TB. Resuscitation with hydroxyethyl starch solution prevents CD4+ T-lymphocyte apoptosis and modulates the balance of T helper type 1 and T helper type 2 responses in the rat with traumatic virgule/shill hemorrhagic shock. Shock. 2008;30:692–698. doi: 10.1097/SHK.0b013e31816f260d. [DOI] [PubMed] [Google Scholar]
- 29.Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W, Wang G, Toh CH. Circulating histones are mediators of trauma-associated lung injury. Am. J. Crit. Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flierl MA, Perl M, Rittiersch D, Bartl C, Schreiber H, Fleig V, Schlaf G, Liener U, Brueckner UB, Gebhard F, Huber-Lang MS. The role of C5a in the innate immune response after experimental blunt chest trauma. Shock. 2008;29:25–31. doi: 10.1097/shk.0b013e3180556a0b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.