Abstract
Research in the field of ischemia-reperfusion injury continues to be plagued by the inability to translate research findings to clinically useful therapies. This may in part relate to the complexity of disease processes that result in intestinal ischemia but may also result from inappropriate research model selection. Research animal models have been integral to the study of ischemia-reperfusion-induced intestinal injury. However, the clinical conditions that compromise intestinal blood flow in clinical patients ranges widely from primary intestinal disease to processes secondary to distant organ failure and generalized systemic disease. Thus models that closely resemble human pathology in clinical conditions as disparate as volvulus, shock, and necrotizing enterocolitis are likely to give the greatest opportunity to understand mechanisms of ischemia that may ultimately translate to patient care. Furthermore, conditions that result in varying levels of ischemia may be further complicated by the reperfusion of blood to tissues that, in some cases, further exacerbates injury. This review assesses animal models of ischemia-reperfusion injury as well as the knowledge that has been derived from each to aid selection of appropriate research models. In addition, a discussion of the future of intestinal ischemia-reperfusion research is provided to place some context on the areas likely to provide the greatest benefit from continued research of ischemia-reperfusion injury.
Keywords: animal model, intestine, ischemia, mucosal injury, reperfusion
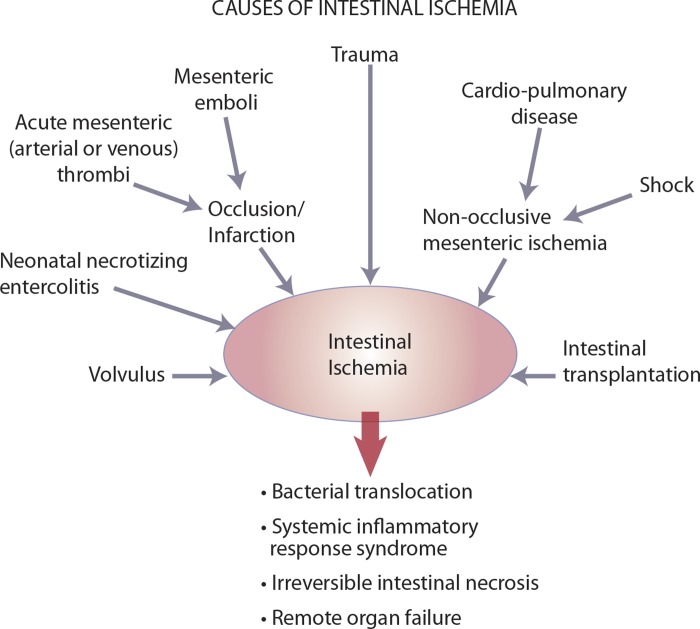
the survival rate for human and veterinary patients with ischemic intestinal injury is generally less than 50% (17, 69, 172, 183) because tissue hypoxia, inflammation, and cell infiltration result in loss of the mucosal barrier. This barrier is primarily composed of a single layer of columnar epithelial cells bound together by interepithelial junctions, which collectively prevents the translocation of bacteria and associated toxins into the systemic circulation (140). Intestinal ischemia is associated with a broad range of clinical conditions including neonatal necrotizing enterocolitis (125), acute mesenteric ischemia (AMI) (183), volvulus (155), trauma (41), cardiopulmonary disease (151), hemorrhagic shock (41, 53), and intestinal transplant rejection (112) (Fig. 1). Ischemic injury may be coupled with subsequent reperfusion of tissue that is known to exacerbate injury in some of these disease processes (112). Since intestinal ischemia is rarely preventable, most research in the field has focused on advancing techniques for early detection of ischemia and the development of novel therapeutic approaches that target the postischemic insult (the reperfusion period). Injury attributed to reperfusion is thought to be primarily attributable to reactive oxygen metabolites, associated with activation of oxidant-producing mucosal enzymes, release of lipid chemoattractants from injured cellular membranes, and subsequent infiltration of neutrophils (Fig. 2). Therefore, proposed therapeutic interventions to protect against reperfusion injury have targeted inhibition of oxidant injury and of neutrophil activation (144, 157). However, owing to the relative failure of clinical therapy directed at reperfusion injury following early studies, the importance of targeting reperfusion injury in cases of intestinal ischemic injury has been brought into question (105). The most recent clinical perspectives on the efficacy of these treatments are derived from studies of tissues more commonly affected by reperfusion, such as the myocardium following infarction (7). These too have failed to demonstrate the clinical utility of these therapies. Encouragingly, recent studies have recognized the modulation of cell death pathways as a novel target for therapeutic intervention (87, 181). Mechanisms of cell death mediated by necrosis and apoptosis have been shown to further exacerbate injury induced by ischemia and reperfusion. In fact, insufficient clearance of these dying cells has been shown to lead to increased inflammation and impaired tissue repair (181). As a result, there has been a shift of emphasis toward modulation of pathways of cell death (87, 181) and regenerative processes (113, 161). However, initial consideration of the animal model to select for study is of paramount importance, given the highly variable clinical presentation of diseases that involve ischemia. Animal models have been indispensable to the study of mechanisms of ischemia and reperfusion injury (22, 39, 134–137), but each model has distinct advantages and disadvantages so that none of these models can perfectly recapitulate the natural onset and progression of human disease (Tables 1 and 2). The aim of this review is to provide an in-depth assessment of key animal models used for the study of ischemia-reperfusion injury, with the goal of allowing investigators to understand and select from available models for translational research. In addition, the future of intestinal regenerative medicine in light of recent advances will be discussed.
Fig. 1.
Clinical conditions that cause ischemic intestinal injury.
Fig. 2.
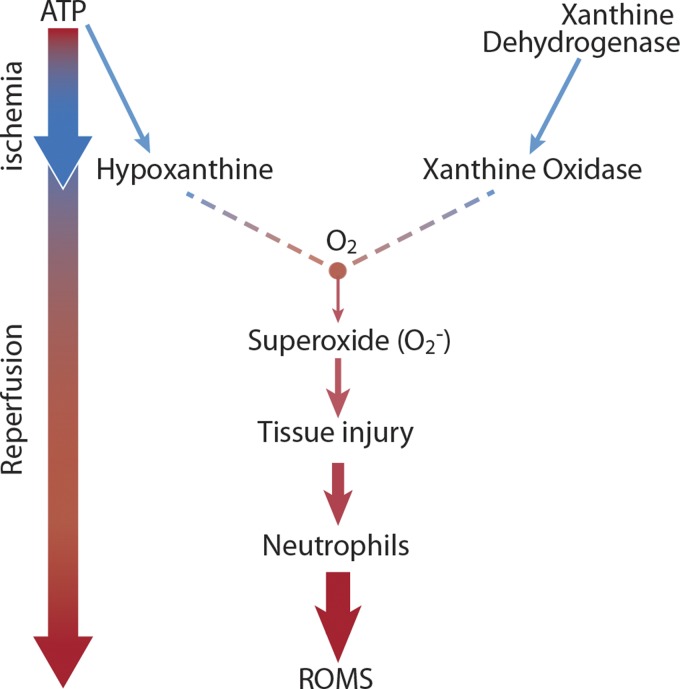
Mechanism of ischemia-reperfusion injury. ROMS, reactive oxygen metabolites.
Table 1.
Comparison of models of ischemia
| Complete Vascular Occlusion |
||||
|---|---|---|---|---|
| SMA Ligation | SMA Embolization | Low-Flow | Segmental Vascular Occlusion | |
| Advantages |
|
|
||
| Disadvantages |
|
|
||
SMA, superior mesenteric artery.
Table 2.
Comparison of large and small animal models of digestive disease
| Advantages | Disadvantages | |
|---|---|---|
| Large animal (pig, dog, cat) |
|
|
| Small animal (rodent) |
|
|
Mechanisms of Ischemia
Ischemia is the reduction or complete occlusion of blood flow to a target organ that results in a state of tissue oxygen deprivation. In the intestine, overt obstruction of the blood supply can result from mesenteric vascular occlusion derived from thrombus formation or emboli secondary to cardiopulmonary disease (143, 166). Compromise of normal blood flow to the intestine can also be associated with severe intestinal distension and mechanical obstruction as occurs in cases of intestinal strangulation associated with hernia, volvulus and intussusception (4, 183). Additionally, ischemic injury is thought to contribute to the pathogenesis of neonatal necrotizing enterocolitis, the most common life-threatening gastrointestinal emergency in neonatal patients (69, 125, 127). Other major sources of intestinal ischemic injury result from diseases that reduce systemic blood flow such as cardiopulmonary diseases and shock states (53, 151). Finally, the interruption of blood flow is an important component of the process of intestinal transplantation that inevitably results in a period of ischemia (106, 112) (Fig. 1).
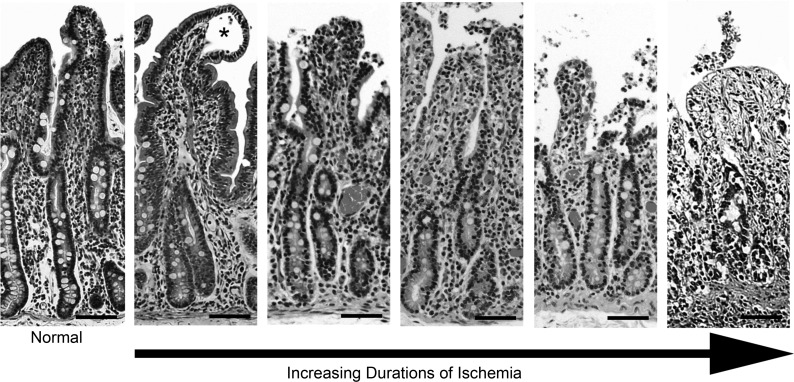
Decreases in blood flow compromise the oxygen supply required for normal cellular function and result in both cellular damage and death. The intestinal epithelium has a particularly high energy demand, largely as a result of basolateral Na+-K+-ATPase used to drive secretion and absorption, and is therefore particularly sensitive to reductions in blood flow. The process of epithelial cell loss that results from ischemic injury is well documented in many research animal species (39, 135, 137, 147). Cells closest to the intestinal lumen on the villus tips in the small intestine and intercrypt surface epithelium in the colon initially lose their attachment to the basement membrane with progressive loss of cells extending toward the crypt base as the duration of ischemia increases (39, 135) (Fig. 3). Interestingly, the cells closest to the luminal surface normally function in a state of “physiological hypoxia” due to an oxygen gradient that is derived from the mucosal vascular architecture (146, 171). Although there are species differences in the anatomy of the mucosal vasculature (Fig. 4), a general construct of countercurrent exchange exists whereby oxygen diffuses from the arterial blood supply that ascends the mucosa toward the lumen and venous drainage flowing in the opposite direction, progressively decreasing the arterial concentration of oxygen as the vessels near the luminal surface (2, 9). This creates a steep oxygen gradient that makes the villus tips hypoxic (33, 159). A similarly decreasing gradient of oxygen from the crypt base to the luminal surface exists within the colon (171). Therefore, further decreases in oxygen concentrations, as occurs with intestinal ischemia, impact the cells at the villus tips and colonic surface epithelium first. This initially creates a distinct lesion identified histopathologically, called Gruenhagen's space (16, 39) (Fig. 3). Originally, this space was solely attributed to the accumulation of cytoplasmic fluid from ischemically damaged cells (39). More recent studies using in vitro techniques and a novel in vivo human study have shown that contraction of the myofibroblasts within the lamina propria also contribute to the creation of this initial defect (46, 141). In fact, the creation of the space was shown to occur within 30 min of complete ischemia and reseal by 60 min if the blood supply was subsequently returned (46). This demonstrates the remarkable capacity of the intestine to repair rapidly in cases where vascular compromise can be reversed. However, in most cases the duration of ischemia is likely to be lengthier and the tight junctions that serve to anchor the cells together break down, resulting in cell loss into the intestinal lumen. Epithelial cell separation from the basement membrane and complete cell loss then continue and progress toward the crypt base in a time-dependent manner (39). It has been specifically demonstrated in a porcine model of jejunal mesenteric vascular occlusion that by 60 min, epithelium is lost from the upper third of the villus and by 120 min there is near complete loss of villus epithelium (22). Any exposure of the basement membrane causes marked compromise of barrier function, allowing luminal bacteria and toxins to gain access to the underlying vasculature within the lamina propria (75, 111, 140). The sequelae are septicemia and multiple organ dysfunction, which are responsible for the high morbidity and mortality rates associated with severe intestinal ischemic injury (86, 143, 166, 183).
Fig. 3.
Epithelial loss associated with ischemic intestinal injury in porcine jejunum. As the duration of ischemia increases there is progressive loss of epithelium that begins at the villus tip and continues toward the crypt base. Asterisk indicates Gruenhagen's space. Scale bar 50 μm.
Fig. 4.
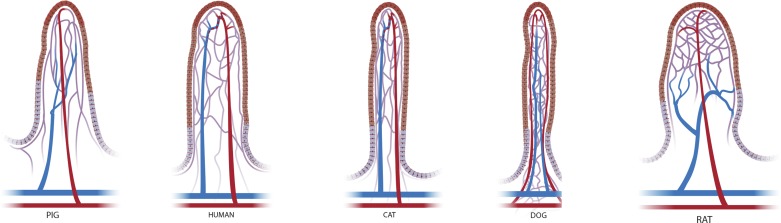
Species differences in villus microvascular architecture.
Models of Ischemia
Complete vascular occlusion.
Complete ischemia by temporary vascular occlusion (with atraumatic vascular clamps) or permanent vascular occlusion (by ligation) of the superior mesenteric artery (SMA, technically the cranial mesenteric artery in animals) in rodent models is currently the most commonly used method of inducing intestinal ischemic injury. The SMA is approached through a midline incision and occluded for varying lengths of time (87). In most of these studies, heparin is injected intravenously to prevent thrombus formation within the SMA, which enables the circulation to be reestablished once the atraumatic vascular clamps are removed for measures of reperfusion injury. However, an early study by Megison et al. (116) noted a highly variable degree of injury and often high mortality of rats in studies in which occlusion of the SMA was performed. That study noted that SMA occlusion alone was unreliable in creating consistent and reproducible injury, whereas SMA occlusion combined with ligation of collateral arcades created a greater degree of injury and a higher but consistent level of mortality in the animals. These findings lent support to a similar study performed in cats in which SMA occlusion reduced blood flow to the mucosa an average of 35% in the proximal duodenum, 61% in the distal duodenum, 71% in the jejunum and ileum, and 63% in the proximal colon. No reduction of blood flow was identified in the mid and distal colon (142). Thus the effect of SMA occlusion is highly dependent on the intestinal segment being evaluated. These findings may be complicated further by the fact that different segments of the intestine may be more or less sensitive to ischemic injury. For example, differences in resistance to ischemic injury have been identified between the jejunum, ileum, and large colon, with studies indicating a progressive increase in resistance to ischemic injury in aboral segments (37, 107, 147). Furthermore, a porcine study of small bowel transplantation demonstrated microscopic injury in the ileum only after 5 h of ischemic injury compared with 1.5 h in the jejunum (35). Little reperfusion injury was noted in either segment. It should be noted, however, that this study only evaluated injury histologically.
Unfortunately, many studies of ischemic injury either assess injury in a single segment of intestine, many times the ileum, or do not specify which segment is being evaluated (Table 3). Additionally, few studies have focused on the colon despite the fact that colonic ischemia is a more common clinical entity in people than small intestinal ischemia (4, 51, 73, 122). The colon does, in fact, respond differently to insult. For example, a study compared regional differences in susceptibility to injury between the small intestine and colon and demonstrated that the colon was more resistant to ischemia in rats (107).
Table 3.
Experimental details of in vivo ischemia-reperfusion models in cats
| Type of Experimental IR Injury | Segment of Intestine | Reference |
|---|---|---|
| SMA complete occlusion | Ileum | 95 |
| SMA complete occlusion | Jejunum + ileum | 52 |
| Mesenteric vascular occlusion | Small intestine segment not specific | 80 |
| Low-flow SMA occlusion | Ileum | 67, 70, 82, 83, 90, 97, 100, 101, 128, 129, 136–138, 153, 164, 165, 180, 186, 187 |
| Low-flow SMA occlusion | Jejunum + ileum | 98 |
| Low-flow SMA occlusion | Jejunum | 130 |
| Low-flow SMA occlusion | Small intestine does not specify segment | 91, 99, 184 |
IR, ischemia-reperfusion.
A more recent technique used to achieve complete irreversible ischemia was established in a porcine model by embolization of the SMA. The SMA was accessed either percutaneously (96) or via endovascular catheterization (1, 25, 148) and a solution of either butyl-2-cyanoacrylate (96) or polyvinyl alcohol particles and gel foam (1, 25, 148) was injected intravascularly. These models are particularly useful for imaging studies aimed at improving diagnosis in patients suspected of having AMI. With this approach, the abdominal contents are not exposed or manipulated in creating the vascular obstruction. Since bowel manipulation alone creates inflammation that otherwise would not be present in clinical cases of AMI, minimally invasive approaches to the SMA may be optimal for studies modeling AMI.
The critical assessment of the type of ischemia model with regard to studies of AMI cannot be overstated. Acute mesenteric ischemia is a complex of diseases that can be classified on the basis of whether the mechanism of obstruction is occlusive or nonocclusive. Occlusive intestinal ischemia is further subdivided into acute or chronic and whether arteries or veins are affected. Nonocclusive mesenteric ischemia is a disease process that is more poorly understood but occurs with patent mesenteric arteries and is associated with disease processes such as congestive heart failure, aortic insufficiency, renal or hepatic disease, and patients following cardiac surgery (172). Therefore, other approaches to create ischemic injury such as the low-flow model likely better recapitulate this form of injury.
Low-flow ischemia.
Most of the original low-flow ischemia studies were performed in cats (Table 3). This model likely best recreates the type of intestinal injury that would be expected following hemorrhagic shock or other events that dramatically decreases the volume of blood delivered to an intestinal segment. In the low-flow ischemia model, blood flow is reduced to 20% of baseline levels (∼25–35 mmHg). With this method, the abdomen is approached through a midline incision and a segment of ileum is typically isolated. An arterial circuit is then established between the superior mesenteric and femoral arteries and pressure cannulas are used within the vessels to measure vascular pressure. Reduced blood pressure within the SMA (25–30 mmHg) is achieved by use of an adjustable vascular clamp. The majority of injury associated with this model has been attributed to reperfusion as a result of increases in xanthine oxidase and neutrophil activation, making the ischemic event more of a priming mechanism for subsequent injury. This may or may not adequately represent clinical scenarios, in which injury caused by ischemia may be so severe that reperfusion injury is of limited significance. Again, careful selection of models depending on the human condition being studied is critical.
Segmental mesenteric vascular occlusion.
An additional model of ischemia is segmental mesenteric vascular occlusion, in which the blood flow within a discrete loop of intestine is interrupted by clamping the local mesenteric vascular supply and cross-clamping the bowel. Within a single animal, multiple loops can be subjected to varying lengths of ischemia with or without reperfusion, thereby titrating the degree of injury within each loop. This model can be readily performed in large animals such as the pig and in rodents (73, 74) and has the advantage of providing multiple treatment groups and controls within a single animal (Tables 4, 6, and 7). However, this model of strangulating obstruction relies on complete occlusion of the arterial and venous blood supply, whereas clinically the venous circulation is commonly compromised prior to the arterial blood supply owing to differences in vessel wall thickness and compliance (135). This results in continued arterial supply of oxygenated blood for a variable period of time, until the tissue becomes so congested in the absence of venous drainage that the arterial vascular supply ultimately collapses. This complex form of ischemic injury includes elements of ischemia as well as excessive interstitial pressure, which together likely affect the level of epithelial sloughing.
Table 4.
Experimental details of in vivo ischemia-reperfusion models in dogs
Perhaps the most interesting and significant application of the mesenteric vascular occlusion model of intestinal ischemia has been its application in human subjects (46, 47). In these studies, a normal segment of jejunum from patients undergoing Roux-en-Y or similar intestinal reconstruction were isolated and subjected to 30–45 min of mesenteric vascular occlusion, with varying lengths of reperfusion, prior to removal from the patient (46, 47, 75, 115). The limitation of these studies is that severe ischemic damage as applies to most patients suffering from ischemia-reperfusion injury cannot be induced in these clinical studies.
With regard to each of these models of ischemia, the research animal species used may contribute significantly to the outcome. As previously noted, variations of the mucosal vascular supply, particularly in the small intestine, exist between species (Fig. 4). The pig and human villus microvascular architecture are very similar, arborizing at the tip of the villus into a fountain-like pattern and converging into one or two venules located beside the centrally located arteriole (9, 33). In contrast, in the rat, the flattened leaf-shaped villi are supplied by a single arteriole that passes unbranched from the base to the tip of the villus where it bifurcates into a capillary network, a so-called “netted bag” pattern (38). The arterioles begin to converge and form two venules as the vasculature approaches the base of the villus (33, 38). In the mouse, two arterioles supply oxygenated blood to the villus and then divide into a capillary network that rejoins to form a single venule at the tip of the villus. The venule then travels down the center toward the base of the villus (34). These differences may impact the physiological function of the intestinal mucosa between species and potentially their susceptibility or response to injury. Multiple in vivo and in vitro intestinal permeability studies have demonstrated significant differences between humans and rodents and greater correlation between humans and pigs (15, 45, 126). In particular, Bijlsma et al. (15) hypothesize that the differences in permeability between rodents and humans are due to the interspecies variation in villus blood vessel architecture resulting in varying countercurrent exchange efficiency that directly impacts villus epithelial cell function.
Mechanisms of Reperfusion Injury
The concept of progressive injury that occurs following the restoration of normal vascular circulation to an ischemically compromised region seems counterintuitive. After all, reestablishing blood flow is ultimately critical to rescue and maintain cell function. However, ischemia creates an environment primed for the production of injurious metabolites, the influx of inflammatory cells and epithelial cell apoptosis and necrosis. Early studies of reperfusion in cats and rodents attributed the exacerbation of injury during this period to the production of reactive oxygen metabolites that directly contribute to injury as well as generation of neutrophils chemoattractants (26, 48, 65, 66, 68, 70, 77, 78, 82, 98, 99, 101, 128–130, 136–138, 153, 164). During ischemic conditions, as tissues continue to utilize adenosine triphosphate as a source of energy, the metabolic by-product hypoxanthine accumulates. In addition, in this oxygen-deprived environment xanthine dehydrogenase, which would normally function to metabolize hypoxanthine, is converted to xanthine oxidase by locally produced tissue proteases (130). When tissue perfusion is reestablished, xanthine oxidase utilizes the now freely available and abundant oxygen as an electron acceptor as it metabolizes hypoxanthine, producing superoxide (66–68, 70) (Fig. 2). The direct injury induced by oxygen free radicals combined with their role in the activation and chemoattraction of neutrophils creates the severe tissue injury attributed to reperfusion.
Neutrophils inflict tissue injury by a multitude of proposed mechanisms that include occlusion and increased permeability of the microvasculature (44, 83), release of reactive oxygen metabolites (48, 130, 165), cytotoxic enzyme release (186), and mechanical injury induced by migration (58). The vast majority of the studies used to determine these findings used a feline low-flow ischemia model. Upon activation, neutrophils release the contents of their granules that include potent proteases such as elastase, myeloperoxidase, protease-3, and metalloproteinases as well as reactive oxygen species including H2O2 and hypochlorous acid (108, 153, 186). These compounds are bactericidal and aid in neutrophil migration as connective tissues are lysed but concomitantly cause extensive collateral damage to surrounding matrix proteins and nearby cells. Additional injury is created by the mechanical damage induced by their migration across the epithelial monolayer, which has been shown to increase paracellular permeability (58). The detrimental role of neutrophil accumulation on epithelial barrier function postischemia was supported by findings in a porcine model of complete mesenteric vascular occlusion (58) in which pretreatment of animals with anti CD11/CD18 monoclonal antibody, targeting neutrophil adhesion, significantly reduced neutrophil infiltration and improved measures of epithelial barrier function (58).
Model-Dependent Reperfusion Injury
The significance of reperfusion to intestinal injury following an ischemic event has been and continues to be controversial. This is partly due to the fact that the degree of reperfusion injury appears to depend on the type of ischemia (complete vs. incomplete vascular occlusion) and duration of ischemic injury as well as the research animal species and segment of affected intestine.
Complete vascular occlusion.
Currently, the complete vascular occlusion method for induction of ischemic injury in rodents is the most commonly used approach for the study of ischemia-reperfusion injury (Tables 5 and 6). Rodents are advantageous to use because of their relatively low cost, ease of maintenance, and rapid reproduction rate. In recent ischemia-reperfusion research, mouse models have been used to determine genes associated with oxidative stress (12) as well as to provide evidence of increased epithelial proliferation and barrier function in response to endogenous factors such as keratinocyte growth factor administration (30). Furthermore, mice are highly amenable to genetic manipulation. Attenuation of reperfusion injury in mice genetically modified to overexpress superoxide dismutase has provided further proof of the deleterious role of reactive oxygen metabolites in reperfusion (48). Additionally, other transgenic mouse models have been used to demonstrate the protective role of mu opioid receptor signaling (61) and the detrimental effects of cytokines such as IL-17A (104) in ischemia-reperfusion injury. A large number of studies have also utilized rat models. Aside from transgenic murine models, the role of a host of factors has been successfully investigated in rodents, highlighting the beneficial effects of epidermal growth factor (11, 59), heparin-binding epidermal growth factor (36, 50, 114), antiapoptotic signaling (86), and xanthine oxidase blockade (157) to prevent injury or improve healing. Other studies using rats have shown that atenolol (31), a beta-blocker, and l-arginine (62), a substrate of nitric oxide biosynthesis, both attenuate enteric nervous system dysfunction that occurs as a result of ischemia-reperfusion injury. However, multiple studies have demonstrated that the degree of injury attributed to reperfusion following mesenteric vascular occlusion is variable. The degree of reperfusion injury appears to depend on the duration of preceding ischemia and the segment of intestine (107, 135). Evidence of reperfusion injury has been shown to occur following intervals between 30 and 60 min of ischemia but not when the duration of ischemia is shorter or longer (107, 135). Additionally, the colon appears to be more resistant to reperfusion injury compared with the small intestine and susceptibility to injury along the length of the small intestine is variable, with the ileum appearing to be more resistant to reperfusion injury than the jejunum. The reason for these differences remains poorly understood (35, 37, 107), although the lack of oxidant-producing enzymes in the colon may be one reason that injury differs from the small intestine (76, 120, 121).
Table 5.
Experimental details of in vivo ischemia-reperfusion models in mice
| Type of Experimental IR Injury | Segment of Intestine | Reference |
|---|---|---|
| SMA occlusion | Jejunum | 12, 181 |
| SMA occlusion | Jejunum + ileum | 185 |
| SMA occlusion | Colon | 144 |
| SMA occlusion* | Jejunum + ileum | 104 |
| SMA occlusion* | Jejunum | 103 |
| SMA occlusion* | Small intestine segment not specified | 48, 162 |
| SMA occlusion + collateral vessel ligation* | Ileum | 61 |
Transgenic animal use.
Table 6.
Experimental details of in vivo ischemia-reperfusion models in rats
| Type of Experimental IR Injury | Segment of Intestine | Reference |
|---|---|---|
| Mesenteric vascular occlusion | Proximal colon | 73, 74 |
| SMA occlusion | Proximal colon | 107, 122, 179 |
| SMA occlusion | Jejunum + ileum | 11, 107, 114, 157 |
| SMA occlusion | Ileum | 134, 135, 150 |
| SMA occlusion | Jejunum | 30, 31, 60, 62, 86, 88, 131 |
| SMA occlusion | Ileum | 158, 168, 169 |
| SMA occlusion | Small intestine does not specify segment | 59, 181 |
| SMA + jejunal and colic artery occlusion | Jejunum | 87, 116 |
| SMA + jejunal and colic artery occlusion | Ileum | 173 |
Low-flow ischemia.
As noted above, in models utilizing the low-flow method of ischemia, the majority of tissue damage occurs during reperfusion. In vivo feline studies have shown a direct correlation between elevated xanthine oxidase levels and tissue damage as well as microvascular injury (65, 68, 164, 184). However, important differences exist between species that influence their susceptibility to reperfusion injury. The robust reperfusion injury identified in cats and rodents as a result of mucosal xanthine oxidase-induced oxidant release is not found in other species (22). In fact, humans and pigs lack mucosal xanthine oxidase at birth, and levels remain low even into adulthood, making the clinical relevance of the feline and rodent studies questionable (14). Furthermore, no definitive clinical utility has been identified for xanthine oxidase inhibitors in patients with intestinal ischemia-reperfusion injury (105, 132). Perhaps with the development of the novel human model of ischemia-reperfusion injury (47) the controversy regarding the contribution of xanthine oxidase to reperfusion injury will be resolved.
Another important species difference is the role of neutrophils in the induction and perpetuation of reperfusion injury. The resident mucosal population of neutrophils, as opposed to neutrophils recruited from circulation during onset of injury, appears to play a significant role in reperfusion injury in rodents and cats (48, 101, 130, 153). In a low-flow cat model, acute vs. chronic pretreatment with a CD18-specific monoclonal antibody enabled the investigators to assess the role of resident mucosal neutrophils, with chronic administration of the antibody depleting mucosal neutrophil reserves. These studies indicated that it was the resident neutrophils, rather than those infiltrating from the microvasculature, that were responsible for the majority of the reperfusion injury documented (101, 130, 153). However, humans have a relatively small population of resident neutrophils (22), a finding that was also found to be the case in horses. Interestingly, equine ischemia studies have shown very limited evidence of reperfusion injury (21, 120). Together, differences in oxidant enzyme expression and neutrophil populations likely affect the reliability of basic studies to translate findings to the treatment of humans with ischemia-reperfusion injury.
Segmental mesenteric vascular occlusion.
Most of the segmental mesenteric vascular occlusion studies have been performed in pigs (Table 7), although other species have been used (Tables 3, 4, and 6). This is likely because the larger size of the pig facilitates temporary occlusion of smaller vessels, such as those found within the mesentery, without compromising the integrity of the vessels, thereby allowing reperfusion. There are other advantages to using the porcine model for the study of reperfusion injury. Pigs share a similar pattern of xanthine oxidase expression as humans (22), whereas in rodents enzyme expression is four- to fivefold higher (14, 22). Additionally, pigs, like humans, have a relatively small population of resident neutrophils, unlike rodents and cats (22).
Table 7.
Experimental details of in vivo ischemia-reperfusion models in pigs
| Type of Experimental IR Injury | Segment of Intestine | Reference |
|---|---|---|
| SMV occlusion | Jejunum, ileum, colon | 170 |
| Mesenteric vascular occlusion | Ileum | 3, 18–20, 22–24, 28, 58, 89, 96, 102, 110, 117–119, 133, 154, 160, 170 |
| Mesenteric vascular occlusion | Jejunum + ileum | 35 |
| SMA embolism | Jejunum + ileum | 148 |
| SMA embolism | Small intestine + colon | 96 |
| SMA embolism | Small intestine segment not specified | 1, 25 |
SMV, superior mesenteric vein.
Table 8.
Experimental details of in vivo ischemia-reperfusion models in humans
The larger size of pigs is also advantageous for models requiring surgical manipulation, such as Thiry-Vella loops in which an isolated cannulated segment of intestine can be subject to ischemic injury (19), or where research involves tissue transplantation (182) or testing advanced diagnostic imaging (28). Similar surgical manipulations in rodents have been fraught with complications (6).
Although evidence supports the use of pigs to more closely recapitulate human intestine, obvious morphological differences exist that may influence the use of pigs for the study of reperfusion injury (10, 45, 92, 149). In many mammalian species, including humans, the small intestinal epithelium is composed of stem cells that reside within the crypt base and four postmitotic cell types: absorptive enterocytes, goblet cells, enteroendocrine cells, and Paneth cells. However, the existence of Paneth cells in pigs remains controversial and there is currently no definitive evidence to prove or disprove the existence of a similarly functioning cell type (63, 123, 124). Paneth cells are unique in that they reside adjacent to the intestinal stem cells within the small intestinal crypt base and are thought to migrate toward the crypt base following complete maturation, in contrast to other maturing cell lineages that migrate toward the lumen. Paneth cells are thought to play an integral role in modulating stem cell self-renewal or differentiation especially during times of insult (40, 57, 152), making the study of regenerative events following ischemic injury potentially more complex in porcine models. However, there is recent evidence that Paneth cells may actually contribute to the injury induced by ischemia-reperfusion injury (72, 93, 104). Work in rodent models attribute injury within the gut, systemic inflammation, and remote organ injury following intestinal ischemia-reperfusion injury to Paneth cell degranulation. In fact, it appears that Paneth cell depletion may attenuate ischemia-reperfusion injury (104).
Knowledge regarding the advantages and disadvantages to each model is critical to appropriate experimental design and data interpretation.
Future Directions
New insight into mechanisms of cell death.
Recent insight into the process of cell death by apoptosis and necrosis in both rodent and human models of intestinal ischemia-reperfusion injury have begun to shed light into the role of these pathways in the process of injury and subsequent repair (32, 46, 115, 156, 181). An important component to the combined injury attributed to ischemia and reperfusion is the direct impact on the intestinal epithelial cells. Controlled cell death is critical to the maintenance of normal barrier function and serves to maintain epithelial continuity along the luminal surface while expelling dying cells. Under homeostatic conditions, cells mature as they gradually migrate along the crypt-villus axis. As they near the luminal surface a normal process of desquamation occurs that results in epithelial cell shedding. Briefly, this process is thought to be driven by an interaction between the extracellular matrix and integrins and cadherins, the transmembrane proteins that anchor cells to the basement membrane and that signal cell survival through pathways including focal adhesion kinase (p125fak), phosphatidylinositol 3′-kinase/Akt, and mitogen-activated protein kinases (ERK, JNK, p38, MAPK) (49, 54, 55, 84, 174). Changes in these surface proteins and signaling pathways occur as cells approach the villus tip that signal a normal process of cell shedding into the intestinal lumen (29). During ischemia and reperfusion, cell injury results in death by both apoptotic and necrotic processes (75, 87, 109). Sustained tissue injury appears to be associated with inflammation that is perpetuated by the presence of excessive apoptotic and necrotic cells mediated by the complement system (79, 162, 178, 185). However, the exact pathophysiological mechanisms that occur are complex and incompletely understood (181). Nonetheless, inhibition of apoptosis as well as the facilitated clearance of apoptotic cells appears to reduce bacterial translocation and promote repair by minimizing prolonged inflammation (46, 86, 115, 181). On the basis of research dedicated to understanding ischemia-reperfusion injury, it seems that the attenuation or modulation of the inflammatory response would be critical to future therapeutic approaches aimed at reducing tissue destruction. This is discussed in a few recent reviews: one that addresses the contribution of oxidative stress to the pathogenesis of gastrointestinal disease (13) and another that summarizes the therapeutic potential for neutrophil elastase inhibitors to decrease inflammatory tissue injury (81). These reviews note that despite the large amount of preclinical research dedicated to these particular approaches to modulation of inflammation, the challenges to translate findings to clinical application remain. However, compared with the field of gastrointestinal research, more current and novel therapeutic strategies appear to be available for ischemic cardiovascular disease (81, 139). Nevertheless, little progress has been made in improving clinical outcome in either field. Therefore a better understanding of reparative processes may provide an alternative means to improve care.
Advances in understanding epithelial renewal.
Recent advances in the field of intestinal stem cell biology and regenerative medicine have improved our understanding of epithelial renewal (145). The recent discovery of biomarkers to clearly distinguish intestinal stem cell populations has contributed to the rapid advancement of the field (8, 64, 167, 175, 176). Interestingly, rodent models of intestinal ischemia have shown architectural preservation of the intestinal epithelial stem cells (56, 88, 134, 173). Additionally, the ability of the intestinal epithelial stem cell compartment to expand after intestinal resection, radiation injury, and doxorubicin treatment has been demonstrated in rodent models (42, 43, 85, 177). Together this makes the intestinal epithelial stem cells a promising therapeutic target to hasten mucosal epithelial regeneration following ischemia-reperfusion injury. It is our hope that this new knowledge and advanced technology will facilitate targeted therapies to improve treatment and outcome of this devastating disease.
Summary.
Ischemia and reperfusion contribute to intestinal injury in a multitude of clinical gastroenterological conditions. Despite this, advances in the field have remained modest. This review aimed to shed light on the most commonly used animal models for the study of ischemia and reperfusion to aid in interpretation of study results and perhaps influence model selection in the future. For example, if investigation of reperfusion injury were of particular interest, a feline model of ischemia would be helpful, whereas strangulating intestinal ischemia would be more appropriately studied via a porcine mesenteric vascular occlusion model. Appropriate modeling of disease states or model selection are arguably components of the failure to translate benchtop discoveries to clinical application.
REFERENCES
- 1.Acosta S, Nilsson TK, Malina J, Malina M. l-lactate after embolization of the superior mesenteric artery. J Surg Res 143: 320–328, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Aharinejad S, Lametschwandtner A, Franz P, Firbas W. The vascularization of the digestive tract studied by scanning electron microscopy with special emphasis on the teeth, esophagus, stomach, small and large intestine, pancreas, and liver. Scanning Microsc 5: 811–849, 1991. [PubMed] [Google Scholar]
- 3.Ahdieh N, Blikslager AT, Bhat BG, Coleman RA, Argenzio RA, Rhoads JM. l-Glutamine and transforming growth factor-alpha enhance recovery of monoacylglycerol acyltransferase and diacylglycerol acyltransferase activity in porcine postischemic ileum. Pediatr Res 43: 227–233, 1998. [DOI] [PubMed] [Google Scholar]
- 4.American Gastroenterological Association Medical Position Statement: guidelines on intestinal ischemia. Gastroenterology 118: 951–953, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa K, Takeyoshi I, Akao Y, Totsuka O, Matsumoto K, Morishita Y. Bradykinin B2 receptor antagonist FR173657 ameliorates small bowel ischemia-reperfusion injury in dogs. Dig Dis Sci 50: 27–36, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Avansino JR, Chen DC, Woolman JD, Hoagland VD, Stelzner M. Engraftment of mucosal stem cells into murine jejunum is dependent on optimal dose of cells. J Surg Res 132: 74–79, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Bainey KR, Armstrong PW. Clinical perspectives on reperfusion injury in acute myocardial infarction. Am Heart J 167: 637–645, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449: 1003–1007, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Bellamy JE, Latshaw WK, Nielsen NO. The vascular architecture of the porcine small intestine. Can J Comp Med 37: 56–62, 1973. [PMC free article] [PubMed] [Google Scholar]
- 10.Bendixen E, Danielsen M, Larsen K, Bendixen C. Advances in porcine genomics and proteomics—a toolbox for developing the pig as a model organism for molecular biomedical research. Brief Funct Genomics 9: 208–219, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Berlanga J, Prats P, Remirez D, Gonzalez R, Lopez-Saura P, Aguiar J, Ojeda M, Boyle JJ, Fitzgerald AJ, Playford RJ. Prophylactic use of epidermal growth factor reduces ischemia/reperfusion intestinal damage. Am J Pathol 161: 373–379, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertoletto PR, Ikejiri AT, Somaio Neto F, Chaves JC, Teruya R, Bertoletto ER, Taha MO, Fagundes DJ. Oxidative stress gene expression profile in inbred mouse after ischemia/reperfusion small bowel injury. Acta Cir Bras 27: 773–782, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94: 329–354, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianciardi P, Scorza R, Ghilardi G, Samaja M. Xanthine oxido-reductase activity in ischemic human and rat intestine. Free Radic Res 38: 919–925, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Bijlsma PB, Peeters RA, Groot JA, Dekker PR, Taminiau JAJM, Van Der Meer R. Differential in vivo and in vitro intestinal permeability to lactulose and mannitol in animals and humans: a hypothesis. Gastroenterology 108: 687–696, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Blikslager AT. Life in the gut without oxygen: adaptive mechanisms and inflammatory bowel disease. Gastroenterology 134: 346–348, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Blikslager AT. Treatment of gastrointestinal ischemic injury. Vet Clin North Am Equine Pract 19: 715–727, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Blikslager AT, Pell SM, Young KM. PGE2 triggers recovery of transmucosal resistance via EP receptor cross talk in porcine ischemia-injured ileum. Am J Physiol Gastrointest Liver Physiol 281: G375–G381, 2001. [DOI] [PubMed] [Google Scholar]
- 19.Blikslager AT, Rhoads JM, Bristol DG, Roberts MC, Argenzio RA. Glutamine and transforming growth factor-alpha stimulate extracellular regulated kinases and enhance recovery of villous surface area in porcine ischemic-injured intestine. Surgery 125: 186–194, 1999. [PubMed] [Google Scholar]
- 20.Blikslager AT, Roberts MC, Argenzio RA. Prostaglandin-induced recovery of barrier function in porcine ileum is triggered by chloride secretion. Am J Physiol Gastrointest Liver Physiol 276: G28–G36, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Blikslager AT, Roberts MC, Gerard MP, Argenzio RA. How important is intestinal reperfusion injury in horses? J Am Vet Med Assoc 211: 1387–1389, 1997. [PubMed] [Google Scholar]
- 22.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Is reperfusion injury an important cause of mucosal damage after porcine intestinal ischemia? Surgery 121: 526–534, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Blikslager AT, Roberts MC, Rhoads JM, Argenzio RA. Prostaglandins I2 and E2 have a synergistic role in rescuing epithelial barrier function in porcine ileum. J Clin Invest 100: 1928–1933, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blikslager AT, Roberts MC, Young KM, Rhoads JM, Argenzio RA. Genistein augments prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol 278: G207–G216, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Block T, Isaksson HS, Acosta S, Bjorck M, Brodin D, Nilsson TK. Altered mRNA expression due to acute mesenteric ischaemia in a porcine model. Eur J Vasc Endovasc Surg 41: 281–287, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Boros M, Takaichi S, Hatanaka K. Ischemic time-dependent microvascular changes and reperfusion injury in the rat small intestine. J Surg Res 59: 311–320, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Brath E, Miko I, Nemeth N, Kovacs J, Peto K, Furka I. Effects of allopurinol and preconditioning on apoptosis due to ischemia-reperfusion on a double jejunum-segment canine model. Acta Cir Bras 26: 186–193, 2011. [DOI] [PubMed] [Google Scholar]
- 28.Bruhn RS, Distelmaier MS, Hellmann-Sokolis M, Naami A, Kuhl CK, Hohl C. Early detection of acute mesenteric ischemia using diffusion-weighted 3.0-T magnetic resonance imaging in a porcine model. Invest Radiol 48: 231–237, 2013. [DOI] [PubMed] [Google Scholar]
- 29.Bullen TF, Forrest S, Campbell F, Dodson AR, Hershman MJ, Pritchard DM, Turner JR, Montrose MH, Watson AJ. Characterization of epithelial cell shedding from human small intestine. Lab Invest 86: 1052–1063, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Cai Y, Wang W, Liang H, Sun L, Teitelbaum DH, Yang H. Keratinocyte growth factor improves epithelial structure and function in a mouse model of intestinal ischemia/reperfusion. PLoS One 7: e44772, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campos VF, Miranda-Ferreira R, Taha NS, Teixeira GD, Souza WT, Carmo CE, Silva-Neto LA, Gomes IT, Monteiro HP, Montero EF, Fagundes DJ, Caricati-Neto A, Taha MO. Atenolol to treat intestinal ischemia and reperfusion in rats. Transplant Proc 44: 2313–2316, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol 190: 255–266, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Casley-Smith JR, Gannon BJ. Intestinal microcirculation: spatial organization and fine structure. In: Physiology of the Intestinal Circulation, edited by Granger DN and Shepherd AP. New York: Raven, 1984, p. 9–31. [Google Scholar]
- 34.Casley-Smith JR. Endothelial fenestrae in intestinal villi: differences between the arterial and venous ends of the capillaries. Microvasc Res 3: 49–68, 1971. [DOI] [PubMed] [Google Scholar]
- 35.Chan KL, Chan KW, Tam PK. Segmental small bowel allograft—ischemic injury and regeneration. J Pediatr Surg 33: 1703–1706, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Chen CL, Yu X, James IO, Zhang HY, Yang J, Radulescu A, Zhou Y, Besner GE. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest 92: 331–344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen SH, Tang YB, Chen HC. Survival of transferred ileum after ischemia time longer than 1 hour: a clinical result different from animal studies. J Am Coll Surg 217: 300–305, 2013. [DOI] [PubMed] [Google Scholar]
- 38.Chen YM, Zhang JS, Duan XL. Changes of microvascular architecture, ultrastructure and permeability of rat jejunal villi at different ages. World J Gastroenterol 9: 795–799, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg 101: 478–483, 1970. [DOI] [PubMed] [Google Scholar]
- 40.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 75: 289–311, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Corcos O, Nuzzo A. Gastro-intestinal vascular emergencies. Best Pract Res Clin Gastroenterol 27: 709–725, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Dekaney CM, Fong JJ, Rigby RJ, Lund PK, Henning SJ, Helmrath MA. Expansion of intestinal stem cells associated with long-term adaptation following ileocecal resection in mice. Am J Physiol Gastrointest Liver Physiol 293: G1013–G1022, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Dekaney CM, Gulati AS, Garrison AP, Helmrath MA, Henning SJ. Regeneration of intestinal stem/progenitor cells following doxorubicin treatment of mice. Am J Physiol Gastrointest Liver Physiol 297: G461–G470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke 22: 1276–1283, 1991. [DOI] [PubMed] [Google Scholar]
- 45.Delahunty T, Hollander D. A comparison of intestinal permeability between humans and three common laboratory animals. Comp Biochem Physiol A Comp Physiol 86: 565–567, 1987. [DOI] [PubMed] [Google Scholar]
- 46.Derikx JP, Matthijsen RA, de Bruine AP, van Bijnen AA, Heineman E, van Dam RM, Dejong CH, Buurman WA. Rapid reversal of human intestinal ischemia-reperfusion induced damage by shedding of injured enterocytes and reepithelialisation. PLoS One 3: e3428, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Derikx JP, Matthijsen RA, de Bruine AP, van Dam RM, Buurman WA, Dejong CH. A new model to study intestinal ischemia-reperfusion damage in man. J Surg Res 166: 222–226, 2011. [DOI] [PubMed] [Google Scholar]
- 48.Deshmukh DR, Mirochnitchenko O, Ghole VS, Agnese D, Shah PC, Reddell M, Brolin RE, Inouye M. Intestinal ischemia and reperfusion injury in transgenic mice overexpressing copper-zinc superoxide dismutase. Am J Physiol Cell Physiol 273: C1130–C1135, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Dufour G, Demers MJ, Gagne D, Dydensborg AB, Teller IC, Bouchard V, Degongre I, Beaulieu JF, Cheng JQ, Fujita N, Tsuruo T, Vallee K, Vachon PH. Human intestinal epithelial cell survival and anoikis. Differentiation state-distinct regulation and roles of protein kinase B/Akt isoforms. J Biol Chem 279: 44113–44122, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg 42: 214–220, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Feuerstadt P, Brandt LJ. Colon ischemia: recent insights and advances. Curr Gastroenterol Rep 12: 383–390, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Filez L, Stalmans W, Penninckx F, Kerremans R. Influences of ischemia and reperfusion on the feline small-intestinal mucosa. J Surg Res 49: 157–163, 1990. [DOI] [PubMed] [Google Scholar]
- 53.Fishman JE, Sheth SU, Levy G, Alli V, Lu Q, Xu D, Qin Y, Qin X, Deitch EA. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg 260: 1112–1120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fouquet S, Lugo-Martinez VH, Faussat AM, Renaud F, Cardot P, Chambaz J, Pincon-Raymond M, Thenet S. Early loss of E-cadherin from cell-cell contacts is involved in the onset of anoikis in enterocytes. J Biol Chem 279: 43061–43069, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol 124: 619–626, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuller MK, Faulk DM, Sundaram N, Mahe MM, Stout KM, von Furstenberg RJ, Smith BJ, McNaughton KK, Shroyer NF, Helmrath MA, Henning SJ. Intestinal stem cells remain viable after prolonged tissue storage. Cell Tissue Res 354: 441–450, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garabedian EM, Roberts LJ, McNevin MS, Gordon JI. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem 272: 23729–23740, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Gayle J, Jones SL, Argenzio RA, Blikslager AT. Neutrophils increase paracellular permeability of restituted ischemic-injured porcine ileum. Surgery 132: 461–470, 2002. [DOI] [PubMed] [Google Scholar]
- 59.Geng Y, Li J, Wang F, Li Q, Wang X, Sun L, Li W. Epidermal growth factor promotes proliferation and improves restoration after intestinal ischemia-reperfusion injury in rats. Inflammation 36: 670–679, 2013. [DOI] [PubMed] [Google Scholar]
- 60.Ghadie MM, Miranda-Ferreira R, Taha NS, Maroso AS, Moreti RJ, Andraus MP, Zempulski P, Monteiro HP, Simoes MJ, Fagundes DJ, Caricati-Neto A, Taha MO. Study of heparin in intestinal ischemia and reperfusion in rats: morphological and functional evaluation. Transplant Proc 44: 2300–2303, 2012. [DOI] [PubMed] [Google Scholar]
- 61.Goldsmith JR, Perez-Chanona E, Yadav PN, Whistler J, Roth B, Jobin C. Intestinal epithelial cell-derived mu-opioid signaling protects against ischemia reperfusion injury through PI3K signaling. Am J Pathol 182: 776–785, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomez JS, Miranda-Ferreira R, Taha NS, Rodrigues LW, Bento DR, Fernandes DT, Chihara RT, Simoes MJ, Oliveira-Junior IS, Monteiro HP, Fagundes DJ, Caricati-Neto A, Taha MO. Study of l-arginine in intestinal lesions caused by ischemia-reperfusion in rats. Transplant Proc 44: 2309–2312, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8: e66465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gracz AD, Fuller MK, Wang F, Li L, Stelzner M, Dunn JC, Martin MG, Magness ST. CD24 and CD44 mark human intestinal epithelial cell populations with characteristics of active and facultative stem cells. Stem Cells 31: 2024–2030, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Granger DN, Benoit JN, Suzuki M, Grisham MB. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 257: G683–G688, 1989. [DOI] [PubMed] [Google Scholar]
- 66.Granger DN, Hollwarth ME, Parks DA. Ischemia-reperfusion injury: role of oxygen-derived free radicals. Acta Physiol Scand Suppl 548: 47–63, 1986. [PubMed] [Google Scholar]
- 67.Granger DN, McCord JM, Parks DA, Hollwarth ME. Xanthine oxidase inhibitors attenuate ischemia-induced vascular permeability changes in the cat intestine. Gastroenterology 90: 80–84, 1986. [DOI] [PubMed] [Google Scholar]
- 68.Granger DN, Rutili G, McCord JM. Superoxide radicals in feline intestinal ischemia. Gastroenterology 81: 22–29, 1981. [PubMed] [Google Scholar]
- 69.Gregory KE, Deforge CE, Natale KM, Phillips M, Van Marter LJ. Necrotizing enterocolitis in the premature infant: neonatal nursing assessment, disease pathogenesis, and clinical presentation. Adv Neonatal Care 11: 155–164; quiz 165–166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol Gastrointest Liver Physiol 251: G567–G574, 1986. [DOI] [PubMed] [Google Scholar]
- 71.Grogaard B, Parks DA, Granger DN, McCord JM, Forsberg JO. Effects of ischemia and oxygen radicals on mucosal albumin clearance in intestine. Am J Physiol Gastrointest Liver Physiol 242: G448–G454, 1982. [DOI] [PubMed] [Google Scholar]
- 72.Grootjans J, Hodin CM, de Haan JJ, Derikx JP, Rouschop KM, Verheyen FK, van Dam RM, Dejong CH, Buurman WA, Lenaerts K. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology 140: 529–539.e3, 2011. [DOI] [PubMed] [Google Scholar]
- 73.Grootjans J, Hundscheid IH, Buurman WA. Goblet cell compound exocytosis in the defense against bacterial invasion in the colon exposed to ischemia-reperfusion. Gut Microbes 4: 232–235, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grootjans J, Hundscheid IH, Lenaerts K, Boonen B, Renes IB, Verheyen FK, Dejong CH, von Meyenfeldt MF, Beets GL, Buurman WA. Ischaemia-induced mucus barrier loss and bacterial penetration are rapidly counteracted by increased goblet cell secretory activity in human and rat colon. Gut 62: 250–258, 2013. [DOI] [PubMed] [Google Scholar]
- 75.Grootjans J, Lenaerts K, Derikx JP, Matthijsen RA, de Bruine AP, van Bijnen AA, van Dam RM, Dejong CH, Buurman WA. Human intestinal ischemia-reperfusion-induced inflammation characterized: experiences from a new translational model. Am J Pathol 176: 2283–2291, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grosche A, Morton AJ, Graham AS, Sanchez LC, Blikslager AT, Polyak MM, Freeman DE. Ultrastructural changes in the equine colonic mucosa after ischaemia and reperfusion. Equine Vet J Suppl 39: 8–15, 2011. [DOI] [PubMed] [Google Scholar]
- 77.Grum CM, Gross TJ, Mody CH, Sitrin RG. Expression of xanthine oxidase activity by murine leukocytes. J Lab Clin Med 116: 211–218, 1990. [PubMed] [Google Scholar]
- 78.Haglind E, Haglund U, Lundgren O, Stenberg B. Mucosal lesions of the small intestine after intestinal vascular obstruction in the rat. Acta Chir Scand 151: 147–150, 1985. [PubMed] [Google Scholar]
- 79.Hart ML, Ceonzo KA, Shaffer LA, Takahashi K, Rother RP, Reenstra WR, Buras JA, Stahl GL. Gastrointestinal ischemia-reperfusion injury is lectin complement pathway dependent without involving C1q. J Immunol 174: 6373–6380, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Henninger DD, Granger DN, Aw TY. Enterocyte respiration rates in feline small intestine exposed to graded ischemia. Am J Physiol Gastrointest Liver Physiol 268: G116–G120, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Henriksen PA. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr Opin Hematol 21: 23–28, 2014. [DOI] [PubMed] [Google Scholar]
- 82.Hernandez LA, Grisham MB, Granger DN. A role for iron in oxidant-mediated ischemic injury to intestinal microvasculature. Am J Physiol Gastrointest Liver Physiol 253: G49–G53, 1987. [DOI] [PubMed] [Google Scholar]
- 83.Hernandez LA, Grisham MB, Twohig B, Arfors KE, Harlan JM, Granger DN. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol Heart Circ Physiol 253: H699–H703, 1987. [DOI] [PubMed] [Google Scholar]
- 84.Hofmann C, Obermeier F, Artinger M, Hausmann M, Falk W, Schoelmerich J, Rogler G, Grossmann J. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology 132: 587–600, 2007. [DOI] [PubMed] [Google Scholar]
- 85.Hua G, Thin TH, Feldman R, Haimovitz-Friedman A, Clevers H, Fuks Z, Kolesnick R. Crypt base columnar stem cells in small intestines of mice are radioresistant. Gastroenterology 143: 1266–1276, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang CY, Hsiao JK, Lu YZ, Lee TC, Yu LC. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab Invest 91: 294–309, 2011. [DOI] [PubMed] [Google Scholar]
- 87.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, Nomura M, Itoh G. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut 42: 530–537, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Itoh H, Yagi M, Hasebe K, Fushida S, Tani T, Hashimoto T, Shimizu K, Miwa K. Regeneration of small intestinal mucosa after acute ischemia-reperfusion injury. Dig Dis Sci 47: 2704–2710, 2002. [DOI] [PubMed] [Google Scholar]
- 89.Jacobi SK, Moeser AJ, Corl BA, Harrell RJ, Blikslager AT, Odle J. Dietary long-chain PUFA enhance acute repair of ischemia-injured intestine of suckling pigs. J Nutr 142: 1266–1271, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kanwar S, Kubes P. Ischemia/reperfusion-induced granulocyte influx is a multistep process mediated by mast cells. Microcirculation 1: 175–182, 1994. [DOI] [PubMed] [Google Scholar]
- 91.Kanwar S, Tepperman BL, Payne D, Sutherland LR, Kubes P. Time course of nitric oxide production and epithelial dysfunction during ischemia/reperfusion of the feline small intestine. Circ Shock 42: 135–140, 1994. [PubMed] [Google Scholar]
- 92.Kararli TT. Comparison of the gastrointestinal anatomy, physiology, and biochemistry of humans and commonly used laboratory animals. Biopharm Drug Dispos 16: 351–380, 1995. [DOI] [PubMed] [Google Scholar]
- 93.Kaser A, Tomczak M, Blumberg RS. “ER stress(ed out)!”: Paneth cells and ischemia-reperfusion injury of the small intestine. Gastroenterology 140: 393–396, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kawata K, Takeyoshi I, Iwanami K, Sunose Y, Tsutsumi H, Ohwada S, Matsumoto K, Morishita Y. The effects of a selective cyclooxygenase-2 inhibitor on small bowel ischemia-reperfusion injury. Hepatogastroenterology 50: 1970–1974, 2003. [PubMed] [Google Scholar]
- 95.Kim MY, Suh CH, Kim ST, Lee JH, Kong K, Lim TH, Suh JS. Magnetic resonance imaging of bowel ischemia induced by ligation of superior mesenteric artery and vein in a cat model. J Comput Assist Tomogr 28: 187–192, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Klein HM, Klosterhalfen B, Kinzel S, Jansen A, Seggewiss C, Weghaus P, Kamp M, Tons C, Gunther RW. CT and MRI of experimentally induced mesenteric ischemia in a porcine model. J Comput Assist Tomogr 20: 254–261, 1996. [DOI] [PubMed] [Google Scholar]
- 97.Kubes P. Ischemia-reperfusion in feline small intestine: a role for nitric oxide. Am J Physiol Gastrointest Liver Physiol 264: G143–G149, 1993. [DOI] [PubMed] [Google Scholar]
- 98.Kubes P, Hunter J, Granger DN. Ischemia/reperfusion-induced feline intestinal dysfunction: importance of granulocyte recruitment. Gastroenterology 103: 807–812, 1992. [DOI] [PubMed] [Google Scholar]
- 99.Kubes P, Hunter J, Granger DN. Effects of cyclosporin A and FK506 on ischemia/reperfusion-induced neutrophil infiltration in the cat. Dig Dis Sci 36: 1469–1472, 1991. [DOI] [PubMed] [Google Scholar]
- 100.Kubes P, Ibbotson G, Russell J, Wallace JL, Granger DN. Role of platelet-activating factor in ischemia/reperfusion-induced leukocyte adherence. Am J Physiol Gastrointest Liver Physiol 259: G300–G305, 1990. [DOI] [PubMed] [Google Scholar]
- 101.Kurtel H, Tso P, Granger DN. Granulocyte accumulation in postischemic intestine: role of leukocyte adhesion glycoprotein CD11/CD18. Am J Physiol Gastrointest Liver Physiol 262: G878–G882, 1992. [DOI] [PubMed] [Google Scholar]
- 102.Lauronen J, Pakarinen MP, Pirinen P, Kuusanmaki P, Raivio P, Savilahti E, Paavonen T, Halttunen J. Effects of extrinsic denervation with or without ischemia-reperfusion injury on constitutional mucosal characteristics in porcine jejunoileum. Dig Dis Sci 46: 476–485, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Lee H, Ko EH, Lai M, Wei N, Balroop J, Kashem Z, Zhang M. Delineating the relationships among the formation of reactive oxygen species, cell membrane instability and innate autoimmunity in intestinal reperfusion injury. Mol Immunol 58: 151–159, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee HT, Kim M, Kim JY, Brown KM, Ham A, D'Agati VD, Mori-Akiyama Y. Critical role of interleukin-17A in murine intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest Liver Physiol 304: G12–G25, 2013. [DOI] [PubMed] [Google Scholar]
- 105.Lehr HA, Menger MD, Granger DN. Ischemia-reperfusion injury: enthusiasm in laboratory research but dilemma in clinical trials? Circulation 90: 1580, 1994. [PubMed] [Google Scholar]
- 106.Lenaerts K, Ceulemans LJ, Hundscheid IH, Grootjans J, Dejong CH, Olde Damink SS. New insights in intestinal ischemia-reperfusion injury: implications for intestinal transplantation. Curr Opin Organ Transplant 18: 298–303, 2013. [DOI] [PubMed] [Google Scholar]
- 107.Leung FW, Su KC, Passaro E Jr, Guth PH. Regional differences in gut blood flow and mucosal damage in response to ischemia and reperfusion. Am J Physiol Gastrointest Liver Physiol 263: G301–G305, 1992. [DOI] [PubMed] [Google Scholar]
- 108.Linfert D, Chowdhry T, Rabb H. Lymphocytes and ischemia-reperfusion injury. Transplant Rev (Orlando) 23: 1–10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Linkermann A, Hackl MJ, Kunzendorf U, Walczak H, Krautwald S, Jevnikar AM. Necroptosis in immunity and ischemia-reperfusion injury. Am J Transplant 13: 2797–2804, 2013. [DOI] [PubMed] [Google Scholar]
- 110.Little D, Dean RA, Young KM, McKane SA, Martin LD, Jones SL, Blikslager AT. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol 284: G46–G56, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Louis K, Netea MG, Carrer DP, Kotsaki A, Mylona V, Pistiki A, Savva A, Roditis K, Alexis A, Van der Meer JW, Giamarellos-Bourboulis EJ. Bacterial translocation in an experimental model of multiple organ dysfunctions. J Surg Res 183: 686–694, 2013. [DOI] [PubMed] [Google Scholar]
- 112.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci 49: 1359–1377, 2004. [DOI] [PubMed] [Google Scholar]
- 113.Markel TA, Crisostomo PR, Lahm T, Novotny NM, Rescorla FJ, Tector J, Meldrum DR. Stem cells as a potential future treatment of pediatric intestinal disorders. J Pediatr Surg 43: 1953–1963, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martin AE, Luquette MH, Besner GE. Timing, route, and dose of administration of heparin-binding epidermal growth factor-like growth factor in protection against intestinal ischemia-reperfusion injury. J Pediatr Surg 40: 1741–1747, 2005. [DOI] [PubMed] [Google Scholar]
- 115.Matthijsen RA, Derikx JP, Kuipers D, van Dam RM, Dejong CH, Buurman WA. Enterocyte shedding and epithelial lining repair following ischemia of the human small intestine attenuate inflammation. PLoS One 4: e7045, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Megison SM, Horton JW, Chao H, Walker PB. A new model for intestinal ischemia in the rat. J Surg Res 49: 168–173, 1990. [DOI] [PubMed] [Google Scholar]
- 117.Moeser AJ, Haskell MM, Shifflett DE, Little D, Schultz BD, Blikslager AT. ClC-2 chloride secretion mediates prostaglandin-induced recovery of barrier function in ischemia-injured porcine ileum. Gastroenterology 127: 802–815, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Moeser AJ, Nighot PK, Engelke KJ, Ueno R, Blikslager AT. Recovery of mucosal barrier function in ischemic porcine ileum and colon is stimulated by a novel agonist of the ClC-2 chloride channel, lubiprostone. Am J Physiol Gastrointest Liver Physiol 292: G647–G656, 2007. [DOI] [PubMed] [Google Scholar]
- 119.Moeser AJ, Nighot PK, Ryan KA, Wooten JG, Blikslager AT. Prostaglandin-mediated inhibition of Na+/H+ exchanger isoform 2 stimulates recovery of barrier function in ischemia-injured intestine. Am J Physiol Gastrointest Liver Physiol 291: G885–G894, 2006. [DOI] [PubMed] [Google Scholar]
- 120.Moore RM, Bertone AL, Muir WW, Stromberg PC, Beard WL. Histopathological evidence of reperfusion injury in the large colon of horses after low-flow ischemia. Am J Vet Res 55: 1434–1443, 1994. [PubMed] [Google Scholar]
- 121.Moore RM, Muir WW, Granger DN. Mechanisms of gastrointestinal ischemia-reperfusion injury and potential therapeutic interventions: a review and its implications in the horse. J Vet Intern Med 9: 115–132, 1995. [DOI] [PubMed] [Google Scholar]
- 122.Murthy S, Hui-Qi Q, Sakai T, Depace DE, Fondacaro JD. Ischemia/reperfusion injury in the rat colon. Inflammation 21: 173–190, 1997. [DOI] [PubMed] [Google Scholar]
- 123.Myer MS. Paneth cells in the pig-a controversial issue. J S Afr Vet Assoc 53: 69, 1982. [PubMed] [Google Scholar]
- 124.Myer MS. The presence of Paneth cells confirmed in the pig. Onderstepoort J Vet Res 49: 131–132, 1982. [PubMed] [Google Scholar]
- 125.Nankervis CA, Giannone PJ, Reber KM. The neonatal intestinal vasculature: contributing factors to necrotizing enterocolitis. Semin Perinatol 32: 83–91, 2008. [DOI] [PubMed] [Google Scholar]
- 126.Nejdfors P, Ekelund M, Jeppsson B, Westrom BR. Mucosal in vitro permeability in the intestinal tract of the pig, the rat, and man: species- and region-related differences. Scand J Gastroenterol 35: 501–507, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 364: 255–264, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nilsson UA, Aberg J, Aneman A, Lundgren O. Feline intestinal ischemia and reperfusion: relation between radical formation and tissue damage. Eur Surg Res 25: 20–29, 1993. [DOI] [PubMed] [Google Scholar]
- 129.Nilsson UA, Lundgren O, Haglind E, Bylund-Fellenius AC. Radical production during in vivo intestinal ischemia and reperfusion in the cat. Am J Physiol Gastrointest Liver Physiol 257: G409–G414, 1989. [DOI] [PubMed] [Google Scholar]
- 130.Nilsson UA, Schoenberg MH, Aneman A, Poch B, Magadum S, Beger HG, Lundgren O. Free radicals and pathogenesis during ischemia and reperfusion of the cat small intestine. Gastroenterology 106: 629–636, 1994. [DOI] [PubMed] [Google Scholar]
- 131.Osborne DL, Aw TY, Cepinskas G, Kvietys PR. Development of ischemia/reperfusion tolerance in the rat small intestine. An epithelium-independent event. J Clin Invest 94: 1910–1918, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pacher P, Nivorozhkin A, Szabo C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacol Rev 58: 87–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pakarinen MP, Pirinen P, Lauronen J, Raivio P, Kuusanmaki P, Halttunen J. Effects of transection and extrinsic denervation and a model of autotransplantation of the porcine jejunoileum on cholesterol biodynamics. J Pediatr Surg 38: 1585–1590, 2003. [DOI] [PubMed] [Google Scholar]
- 134.Park PO, Haglund U. Regeneration of small bowel mucosa after intestinal ischemia. Crit Care Med 20: 135–139, 1992. [DOI] [PubMed] [Google Scholar]
- 135.Park PO, Haglund U, Bulkley GB, Falt K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 107: 574–580, 1990. [PubMed] [Google Scholar]
- 136.Parks DA, Bulkley GB, Granger DN, Hamilton SR, McCord JM. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology 82: 9–15, 1982. [PubMed] [Google Scholar]
- 137.Parks DA, Granger DN. Contributions of ischemia and reperfusion to mucosal lesion formation. Am J Physiol Gastrointest Liver Physiol 250: G749–G753, 1986. [DOI] [PubMed] [Google Scholar]
- 138.Parks DA, Granger DN. Ischemia-induced vascular changes: role of xanthine oxidase and hydroxyl radicals. Am J Physiol Gastrointest Liver Physiol 245: G285–G289, 1983. [DOI] [PubMed] [Google Scholar]
- 139.Perricone AJ, Vander Heide RS. Novel therapeutic strategies for ischemic heart disease. Pharmacol Res 89C: 36–45, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol Gastrointest Liver Physiol 277: G495–G499, 1999. [DOI] [PubMed] [Google Scholar]
- 141.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol Cell Physiol 277: C183–C201, 1999. [DOI] [PubMed] [Google Scholar]
- 142.Premen AJ, Banchs V, Womack WA, Kvietys PR, Granger DN. Importance of collateral circulation in the vascularly occluded feline intestine. Gastroenterology 92: 1215–1219, 1987. [DOI] [PubMed] [Google Scholar]
- 143.Puleo F, Arvanitakis M, Van Gossum A, Preiser JC. Gut failure in the ICU. Semin Respir Crit Care Med 32: 626–638, 2011. [DOI] [PubMed] [Google Scholar]
- 144.Riaz AA, Wan MX, Schafer T, Dawson P, Menger MD, Jeppsson B, Thorlacius H. Allopurinol and superoxide dismutase protect against leucocyte-endothelium interactions in a novel model of colonic ischaemia-reperfusion. Br J Surg 89: 1572–1580, 2002. [DOI] [PubMed] [Google Scholar]
- 145.Rizk P, Barker N. Gut stem cells in tissue renewal and disease: methods, markers, and myths. Wiley Interdiscip Rev Syst Biol Med 4: 475–496, 2012. [DOI] [PubMed] [Google Scholar]
- 146.Robinson A, Keely S, Karhausen J, Gerich ME, Furuta GT, Colgan SP. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology 134: 145–155, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Robinson JW, Mirkovitch V, Winistorfer B, Saegesser F. Response of the intestinal mucosa to ischaemia. Gut 22: 512–527, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rosow DE, Sahani D, Strobel O, Kalva S, Mino-Kenudson M, Holalkere NS, Alsfasser G, Saini S, Lee SI, Mueller PR, Fernandez-del Castillo C, Warshaw AL, Thayer SP. Imaging of acute mesenteric ischemia using multidetector CT and CT angiography in a porcine model. J Gastrointest Surg 9: 1262–1274; discussion 1274–1275, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rothkotter HJ. Anatomical particularities of the porcine immune system—a physician's view. Dev Comp Immunol 33: 267–272, 2009. [DOI] [PubMed] [Google Scholar]
- 150.San Norberto Garcia EM, Taylor JH, Cenizo N, Vaquero C. Beneficial effects of intra-arterial and intravenous prostaglandin E1 in intestinal ischaemia-reperfusion injury. Interact Cardiovasc Thorac Surg 18: 466–474, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sastry P, Hardman G, Page A, Parker R, Goddard M, Large S, Jenkins DP. Mesenteric ischaemia following cardiac surgery: the influence of intraoperative perfusion parameters. Interact Cardiovasc Thorac Surg 19: 419–424, 2014. [DOI] [PubMed] [Google Scholar]
- 152.Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469: 415–418, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schoenberg MH, Poch B, Younes M, Schwarz A, Baczako K, Lundberg C, Haglund U, Beger HG. Involvement of neutrophils in postischaemic damage to the small intestine. Gut 32: 905–912, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwartz CA, Haage P, Hohl C. Experimental early detection of acute mesenteric ischemia with functional MRI (DWI) and parallel imaging. Rofo 184: 520–526, 2012. [DOI] [PubMed] [Google Scholar]
- 155.Schwartz MZ. Novel therapies for the management of short bowel syndrome in children. Pediatr Surg Int 29: 967–974, 2013. [DOI] [PubMed] [Google Scholar]
- 156.Semenza GL. Series introduction: tissue ischemia: pathophysiology and therapeutics. J Clin Invest 106: 613–614, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Shafik AN. Febuxostat improves the local and remote organ changes induced by intestinal ischemia/reperfusion in rats. Dig Dis Sci 58: 650–659, 2013. [DOI] [PubMed] [Google Scholar]
- 158.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-alpha-regulated mechanism. World J Gastroenterol 19: 3583–3595, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shepherd AP. Local control of intestinal oxygenation and blood flow. Annu Rev Physiol 44: 13–27, 1982. [DOI] [PubMed] [Google Scholar]
- 160.Shifflett DE, Bottone FG Jr, Young KM, Moeser AJ, Jones SL, Blikslager AT. Neutrophils augment recovery of porcine ischemia-injured ileal mucosa by an IL-1β- and COX-2-dependent mechanism. Am J Physiol Gastrointest Liver Physiol 287: G50–G57, 2004. [DOI] [PubMed] [Google Scholar]
- 161.Spurrier RG, Grikscheit TC. Tissue engineering the small intestine. Clin Gastroenterol Hepatol 11: 354–358, 2013. [DOI] [PubMed] [Google Scholar]
- 162.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol 162: 449–455, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Suto Y, Oshima K, Arakawa K, Sato H, Yamazaki H, Matsumoto K, Takeyoshi I. The effect of nicorandil on small intestinal ischemia-reperfusion injury in a canine model. Dig Dis Sci 56: 2276–2282, 2011. [DOI] [PubMed] [Google Scholar]
- 164.Suzuki M, Grisham MB, Granger DN. Leukocyte-endothelial cell adhesive interactions: role of xanthine oxidase-derived oxidants. J Leukoc Biol 50: 488–494, 1991. [DOI] [PubMed] [Google Scholar]
- 165.Suzuki M, Inauen W, Kvietys PR, Grisham MB, Meininger C, Schelling ME, Granger HJ, Granger DN. Superoxide mediates reperfusion-induced leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol 257: H1740–H1745, 1989. [DOI] [PubMed] [Google Scholar]
- 166.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg 20: 411–417, 1996. [DOI] [PubMed] [Google Scholar]
- 167.Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science 334: 1420–1424, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takizawa Y, Kishimoto H, Kitazato T, Tomita M, Hayashi M. Changes in protein and mRNA expression levels of claudin family after mucosal lesion by intestinal ischemia/reperfusion. Int J Pharm 426: 82–89, 2012. [DOI] [PubMed] [Google Scholar]
- 169.Takizawa Y, Kishimoto H, Tomita M, Hayashi M. Changes in the expression levels of tight junction components during reconstruction of tight junction from mucosal lesion by intestinal ischemia/reperfusion. Eur J Drug Metab Pharmacokinet 39: 211–220, 2013. [DOI] [PubMed] [Google Scholar]
- 170.Tang ZH, Qiang JW, Feng XY, Li RK, Sun RX, Ye XG. Acute mesenteric ischemia induced by ligation of porcine superior mesenteric vein: multidetector CT evaluations. Acad Radiol 17: 1146–1152, 2010. [DOI] [PubMed] [Google Scholar]
- 171.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med 85: 1295–1300, 2007. [DOI] [PubMed] [Google Scholar]
- 172.Trompeter M, Brazda T, Remy CT, Vestring T, Reimer P. Non-occlusive mesenteric ischemia: etiology, diagnosis, and interventional therapy. Eur Radiol 12: 1179–1187, 2002. [DOI] [PubMed] [Google Scholar]
- 173.Udassin R, Vromen A, Haskel Y. The time sequence of injury and recovery following transient reversible intestinal ischemia. J Surg Res 56: 221–225, 1994. [DOI] [PubMed] [Google Scholar]
- 174.Vachon PH, Harnois C, Grenier A, Dufour G, Bouchard V, Han J, Landry J, Beaulieu JF, Vezina A, Dydensborg AB, Gauthier R, Cote A, Drolet JF, Lareau F. Differentiation state-selective roles of p38 isoforms in human intestinal epithelial cell anoikis. Gastroenterology 123: 1980–1991, 2002. [DOI] [PubMed] [Google Scholar]
- 175.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology 137: 15–17, 2009. [DOI] [PubMed] [Google Scholar]
- 176.van der Flier LG, van Gijn ME, Hatzis P, Kujala P, Haegebarth A, Stange DE, Begthel H, van den Born M, Guryev V, Oving I, van Es JH, Barker N, Peters PJ, van de Wetering M, Clevers H. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell 136: 903–912, 2009. [DOI] [PubMed] [Google Scholar]
- 177.Van Landeghem L, Santoro MA, Krebs AE, Mah AT, Dehmer JJ, Gracz AD, Scull BP, McNaughton K, Magness ST, Lund PK. Activation of two distinct Sox9-EGFP-expressing intestinal stem cell populations during crypt regeneration after irradiation. Am J Physiol Gastrointest Liver Physiol 302: G1111–G1132, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wada K, Montalto MC, Stahl GL. Inhibition of complement C5 reduces local and remote organ injury after intestinal ischemia/reperfusion in the rat. Gastroenterology 120: 126–133, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Wang F, Li Q, Wang C, Tang C, Li J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS One 7: e42027, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Weixiong H, Aneman A, Nilsson U, Lundgren O. Quantification of tissue damage in the feline small intestine during ischaemia-reperfusion: the importance of free radicals. Acta Physiol Scand 150: 241–250, 1994. [DOI] [PubMed] [Google Scholar]
- 181.Wu R, Dong W, Wang Z, Jacob A, Cui T, Wang P. Enhancing apoptotic cell clearance mitigates bacterial translocation and promotes tissue repair after gut ischemia-reperfusion injury. Int J Mol Med 30: 593–598, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yandza T, Tauc M, Saint-Paul MC, Ouaissi M, Gugenheim J, Hebuterne X. The pig as a preclinical model for intestinal ischemia-reperfusion and transplantation studies. J Surg Res 178: 807–819, 2012. [DOI] [PubMed] [Google Scholar]
- 183.Yasuhara H. Acute mesenteric ischemia: the challenge of gastroenterology. Surg Today 35: 185–195, 2005. [DOI] [PubMed] [Google Scholar]
- 184.Younes M, Schoenberg MH, Jung H, Fredholm BB, Haglund U, Schildberg FW. Oxidative tissue damage following regional intestinal ischemia and reperfusion in the cat. Res Exp Med (Berl) 184: 259–264, 1984. [DOI] [PubMed] [Google Scholar]
- 185.Zhao H, Montalto MC, Pfeiffer KJ, Hao L, Stahl GL. Murine model of gastrointestinal ischemia associated with complement-dependent injury. J Appl Physiol 93: 338–345, 2002. [DOI] [PubMed] [Google Scholar]
- 186.Zimmerman BJ, Granger DN. Reperfusion-induced leukocyte infiltration: role of elastase. Am J Physiol Heart Circ Physiol 259: H390–H394, 1990. [DOI] [PubMed] [Google Scholar]
- 187.Zimmerman BJ, Grisham MB, Granger DN. Role of oxidants in ischemia/reperfusion-induced granulocyte infiltration. Am J Physiol Gastrointest Liver Physiol 258: G185–G190, 1990. [DOI] [PubMed] [Google Scholar]