Abstract
Background
Fusarium graminearum, one of the causal agents of Fusarium Head Blight (FHB, scab), leads to severe losses in grain yield and quality due to the production of mycotoxins which are harmful to human and livestock. Different traits for FHB resistance in wheat were identified for common wheat (Triticum aestivum L.) while the sources of FHB resistance in durum wheat (Triticum turgidum ssp. Durum), one of the cereals most susceptible to F. graminearum infection, have not been found. New lines of evidence indicate that content and composition of cell wall polymers affect the susceptibility of the wall to degrading enzymes produced by pathogens during infection and can play a role in the outcome of host-pathogen interactions. The objective of our research is to identify potential cell wall biochemical traits linked to Fusariosis resistance to be transferred from a resistant common wheat to a susceptible durum wheat line.
Results
A detailed analysis of cell wall composition in spikes isolated from a highly resistant common wheat accession “02-5B-318”, a breeding line derived from the FHB-resistant Chinese cv. Sumai-3 and a high susceptible durum wheat cv. Saragolla was performed. Significant differences in lignin monolignols composition, arabinoxylan (AX) substitutions and pectin methylesterification were found between resistant and susceptible plants. We isolated and characterized a pectin methylesterase gene WheatPME1, which we found being down regulated in the FHB-resistant line and induced by fungal infection in the susceptible wheat.
Conclusions
Our results indicate cell wall traits differing between the FHB sensitive and resistant wheat genotypes, possibly related to FHB-resistance, and identify the line 02-5B-318R as a potential resource of such traits. Evidence suggests that WheatPME1 is involved in wheat response to F. graminearum.
Electronic supplementary material
The online version of this article (doi:10.1186/s12870-014-0369-1) contains supplementary material, which is available to authorized users.
Keywords: Fusarium Head Blight resistance, Wheat, Pectin methylesterase, Cell wall, Fusarium graminearum
Background
Durum wheat (Triticum turgidum ssp. durum) and common wheat (Triticum aestivum L.) are largely cultivated in European countries and the grain used for the human alimentation (http://www.FAO.org) and animal feeds. Common wheat allows producing wheat flour and bread, while durum wheat is primarily processed into semolina to produce pasta and couscous and some specialty breads. Fusarium graminearum, one of the major global pathogens of cereals, is considered the main causal agent of Fusarium head blight (FHB) disease in wheat [1]. F. graminearum infection causes a significant grain yield and quality loss by producing trichothecene mycotoxins that make harvest unsuitable for human and animal consumption [2]. Host resistance is the primary trait used as a control measure, and its manipulation is the best economic and ecological strategy to reduce damage caused by FHB disease. However, the molecular bases of wheat resistance and susceptibility to F.graminerum are scarcely known [3]. Resistance to FHB is a complex and quantitative trait controlled by multiple genes and characterized by large genetic variation in wheat gene pool [4]. Several studies aimed to identify traits involved in FHB resistance were carried out using common wheat (Triticum aestivum L.) while limited information is available for durum wheat (Triticum turgidum ssp. Durum), which is currently one of the cereals most susceptible to F.graminearum infection [4]. Even though in the last decade different studies were focused on the identification of candidate genes involved in F.graminerum resistance in cultivated or wild durum germoplasm, to date the sources of FHB resistance in durum wheat have not been fully identified [4-7].
F. graminearum preferentially infects wheat spikelets at the stage of anthesis, performs inter and intra-cellular growth and spreads systemically along the rachis [2]. During infection, F. graminearum produces cell wall degrading enzymes (CWDEs), such as pectinases, xylanases and cellulases, to degrade cell wall polysaccharides to penetrate and colonize the host tissues [8-10]. The role of cell wall components in plant resistance to disease has been scarcely studied in grasses. New lines of evidence indicate that content and composition of cell wall polymers affect the susceptibility of cell wall (CW) to CWDEs and can play a role in the outcome of host-pathogen interactions [11-14]. Notably, the extent of CW degradation is often associated with severity of disease [15] Cell wall polysaccharides of the graminaceous monocots (Type II cell wall), consist of a network of cellulose fibers embedded in a matrix of hemicelluloses, such as arabinoxylan (AX) and mixed linkage glucans (MLG), with a minor amount of xyloglucan and pectins [16]. AX (20-40% of CW dry weight) is composed of a β1,4-linked xylose backbone substituted by different monosaccharides, such as arabinose, glucuronic acid and, to lesser extent, galactose [17]. The degree of arabinose substitutions are thought to affect the AX degradability by fungal xylanases [18]. MLGs (10-30%) is an unbranched polysaccharide consisting of blocks of (1,4)-β-linked D-glucose residues interrupted by single (1,3)-β-linkages [16,19]. Pectins (5-10%) are complex polymers with different structural domains including homogalacturonan (HG), rhamnogalacturonan I (RG-I), rhamnogalacturonan II (RG-II) and xylogalacturonan (XG). Galacturonosyl residues of pectin backbones are methylesterified in Golgi apparatus and secreted into the cell wall in a highly methylesterified form. In the apoplasm, pectins are de-methylesterified by pectin methyl esterases (PMEs), which modulate the degree and patterns of methylesterification [20]. The de-methylesterification of pectin affects its interaction with cellulose [21,22] and the formation of crosslinks between pectin chains and xyloglucan or lignin [23,24]. The methylesterification makes pectin less susceptible to degradation by pectin degrading enzymes produced by fungal pathogens [5,25-28]. Pectin content and methylesterification in grasses has been associated with plant resistance to pathogens [5,11,20,29,30]. Lignin is a complex aromatic heteropolymer comprising a substantial portion (20%) of the grasses cell wall. Lignin of monocotyledonous species includes three types of monomers such as p-hydroxyphenyl (H), guaiacyl (G), and syringyl (S) phenylpropanoid monolignols [31,32]. Lignin is an important structural component involved in defense against invasive pathogens, making the cell wall more resistant to CWDEs and also preventing the diffusion of the pathogen-produced toxins [33].
The objective of our research is to identify cell wall biochemical traits useful to improve FHB resistance in durum wheat. To that end, detailed comparative analyses of cell wall composition in spikes isolated from a highly resistant common wheat accession “02-5B-318”, a breeding line derived from the FHB-resistant Chinese cv. Sumai-3 and a highly susceptible durum wheat cv. Saragolla were performed. Significant differences in lignin composition, AX substitution and pectin methylesterification were found between resistant and susceptible plants. The genomic sequence and the chromosome location of WheatPME1 gene, differently expressed in resistant and susceptible lines during F. graminearum infection and possibly involved in susceptibility to Fusarium graminearum, was identified and characterized.
Results and discussion
Assessment of Fusarium symptoms on wheat spikes
In the present study, the resistance to FHB was analyzed in common wheat accession line 02-5B-318 and in Saragolla, known as one of the most susceptible durum wheat cultivar [34]. Spikes at anthesis were inoculated with fungal spores and disease symptoms were recorded 4, 10 and 20 days post-infection. Symptoms were evaluated as FHB incidence, expressed as percentage of infected spikes per genotype and FHB severity, expressed as percentage of spikelets showing symptoms on the total number of spikelets per spike [35]. Significantly higher FHB incidence and severity were observed in Saragolla (henceforth SaragollaS) in comparison with line 02-5B-318 (henceforth 02-5B-318R) (Figure 1a and b) indicating that the two genotypes exhibited quite extreme phenotypes for FHB resistance/tolerance.
Figure 1.
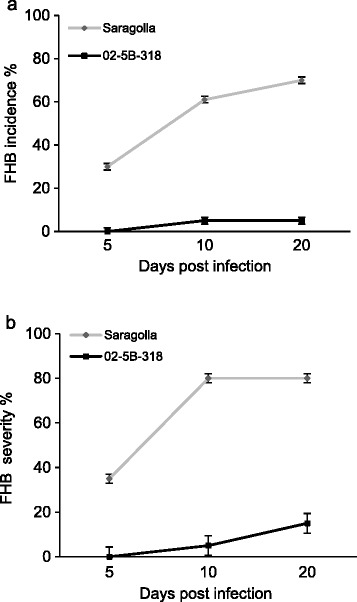
Time-course analysis of FHB symptoms development following F. graminearum infection. (a) FHB incidence and (b) FHB severity of SaragollaS and 02-5B-318R were evaluated. Data are the average ± standard deviation of two independent experiments (n ≥ 20). The average values of SaragollaS and 02-5B-318R lines are significantly different according to Student’s t test (p < 0.001).
The cell wall of 02-5B-318R spikes contain higher content of S lignin with respect to SaragollaS
A detailed analysis of the main structural cell wall components was performed in spikes of 02-5B-318R and SaragollaS plants, at anthesis. The characterization of lignin content and composition demonstrated that, while the two genotypes did not differ in the content of lignin, they showed significant differences in monolignols (Table 1). In particular, lignin of 02-5B-318R spikes contained a significant higher percentage of syringyl (S) and p-hydroxyphenyl (H) monolignols and a lower amount of guaiacyl (G) monolignols, hence having a higher S/G ratio in comparison with SaragollaS genotype. Recent studies aimed to elucidate the effects of lignin composition on the resistance of cell wall to degradation by decay fungi demonstrated that poplar lines extremely rich in syringyl lignin were recalcitrant to fungal degradation [36]. The transcript level of the cinnamoyl-CoA reductase CsCCR4 in the oilseed crop Camelina sativa was observed to be more than 10 times higher in the lines with the higher resistance to Sclerotinia sclerotiorum than in susceptible lines, and this correlated with an high level of constitutive S-lignin [37]. Suppression of F5H (ferulate/coniferaldehyde 5-hydroxylase) or CAOMT (caffeic acid O-methyltransferase), which reside on a branch pathway converting G to S monolignols, greatly reduced the S/G ratio [38]. In addition, the silencing of CAOMT in Triticum monococcum enhanced powdery mildew penetration [39]. Also, the synapyl alcohol-specific peroxidases involved in polymerization of monolignols can be regulated during Fusarium infection. Overall these results suggest that a higher S lignin content is a possible cell wall biochemical trait related to Fusarium resistance and also propose that genes favoring S-type lignin accumulation might potentially be involved in the resistance to the pathogen.
Table 1.
Lignin content and monolignol composition in cell walls from spikes of 02-5B-318 R and Saragolla S plants
| 02-5B-318 R | Saragolla S | |
|---|---|---|
| Lignin (%) | 10.65 ± 1.52 | 11.23 ± 2.27 |
| S (%) | 7.28 ± 0.91 | 2.36 ± 1.00 |
| H (%) | 30.65 ± 1.71 | 20.86 ± 2.68 |
| G (%) | 60.24 ± 4.33 | 76.68 ± 2.11 |
| S/G ratio | 0.121 ± 0.02 | 0.031 ± 0.01 |
Numbers in bold indicate statistically significant differences in each monolignol between the two genotypes, according to Student’s t-test (p <0.05).
Xylans in cell wall of 02-5B-318R spikes present a higher degree of arabinosylation with respect to SaragollaS
We performed a comparative analysis of CW polysaccharides of 02-5B-318R and SaragollaS wheat plants. The cell walls were extracted from spikes and the cellulose content as well as monosaccharide composition of the non-cellulosic polysaccharides were determined (Figure 2). The amount of the cellulose-derived glucose was not significantly different between the two genotypes indicating that cellulose content is not related to their different FHB resistance/susceptibility (Figure 2a). Monosaccharide composition of non-cellulosic polysaccharides was determined by HPAEC-PAD (high performance anion exchange chromatography–Pulsed Amperometric Detection) after acid hydrolysis of alcohol insoluble solid (AIS) (Figure 2b). As reported for other wheat tissues [40], monosaccharide composition of spike cell walls showed xylose as the main non-cellulosic constituent comprising 70–75 mol% of the total sugars, followed by arabinose (about 15%), glucose and galacturonic acid (about 5%), galactose (about 2.5%), and small contents of fucose, rhamnose and glucuronic acid (less than 1%). The comparison of the composition in monosaccharides between 02-5B-318R and SaragollaS spikes indicated a significantly higher percentage of arabinose, galactose and glucose as well as a lower percentage of xylose in the resistant line as compared to the susceptible one (Figure 2b). The arabinose/xylose ratio (Ara/Xyl), was significantly higher in spikes of 02-5B-318R respect to SaragollaS (Figure 2c). To identify the nature of cell wall polysaccharide differing in the two genotypes, AIS was sequentially fractionated by using solutions with increasingly harsh extraction conditions. Chelating Agent Soluble Solid (ChASS) fractions, mainly containing pectic polysaccharides, and 1 M KOH and 4 M KOH fractions, mainly containing hemicelluloses weakly and strongly bound to the cell wall, respectively, were isolated and analyzed for the monosaccharide composition (Table 2). Pectin fractions were not significantly different between the two genotypes. The hemicellulose-enriched fractions from the 02-5B-318R plants contained a significantly higher amount of arabinose, galactose and glucose, a lower amount of xylose and showed a higher Ara/Xyl ratio in comparison with spikes from SaragollaS. In grasses, xylose and arabinose mainly constitute arabinoxylans (AX) and the combined levels of arabinose and xylose provide a good estimate of arabinoxylan content [16,41]. The percentage of arabinoxylans, calculated as sum of arabinose and xylose, was significantly lower in spikes of 02-5B-318R respect to SaragollaS (Table 2). These results therefore indicate a significantly lower amount of arabinoxylans and higher degree of arbinoxylation in the hemicellulose of the 02-5B-318R plants in comparison with SaragollaS and that the differences previously observed between the two genotypes (Figure 2b) can be mainly attributed to the hemicellulose polymers. Monoclonal antibodies can be used to define structural features of polysaccharides in isolated cell wall fractions. In particular LM11 monoclonal antibody is specific to xylan domains enriched in arabinose substitutions [42]. 1 M KOH fractions extracted from spikes of 02-5B-318R and SaragollaS were analyzed with LM11 antibodies using immunodot assay. A higher level of LM11-binding epitopes was detected in 02-5B-318R spikes in comparison with the FHB susceptible wheat genotype (Figure 2d) confirming the higher degree of xylan arabinosylation of 2-5B-318R spikes in comparison with SaragollaS. A negative correlation between the Ara/Xyl ratio and wheat bran digestibility by fungal xylanases have been previously demonstrated [43]. In grasses, arabinose residues of xylans can form ferulic acid-mediated crosslinks between xylan chains and lignin components that limit the enzymatic digestibility of cell walls and improve Fusarium resistance [44-48]. The greater arabinosylation of xylans observed in 02-5B-318R spikes could contribute to a lower degradability of these polymers during Fusarium infection and could consequently represent a potential cell wall trait contributing to FHB resistance. Recently, glycosyltransferases of family 61 were found to be arabinosyltransferases (XATs) in grasses [49]. Interestingly, arabinoxylan also influences desease resistance of barley against the powdery mildew fungus Blumeria graminis f. sp. hordei indicating that in monocot this hemicellulose is important in response to fungal infection [50]. The higher amount of glucose observed in 02-5B-318R in comparison with SaragollaS (Figure 2b and Table 2) indicate a different amount of (1,3;1,4)-β-D-glucan (Mixed linkage glucans; MLG) in their cell walls. Also in this case CslF and CslH glycosyltransferases implicated in MLG biosynthesis have been identified in grasses [51,52] Consistently, a decreased β-D-glucan content was observed in susceptible but not in resistant genotypes after inoculation of wheat spikes with Fusarium culmorum [53].
Figure 2.
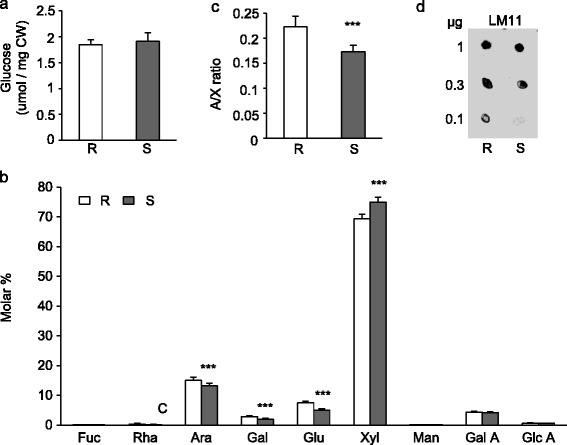
Monosaccharide compositions and immunodot analysis of cell wall polysaccharides in spikes of 02-5B-318 R and Saragolla S plants. (a) Cellulose-derived glucose, (b) Fucose (Fuc), rhamnose (Rha), arabinose (Ara), galactose (Gal), glucose (Glc), xylose (Xyl), galacturonic acid (Gal A) and glucuronic acid (Glu A) released after 2 M TFA hydrolysis were determined by using a high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) system, (c) Arabinose/Xylose ratio in spikes of 02-5B-318R and SaragollaS. Results represent the mean ± SD of three replicates (n = 6). Asterisks indicate data sets significantly different between 02-5B-318R and SaragollaS according to Student’s t-test (p < 0.001). (d) Immunodot analysis for xylan substitution using LM11 antibody. The micrograms of in KOH 1 M hemicellulose fraction from the two genotypes were applied to the nitrocellulose membrane were indicated. The experiments were repeated three times with similar results. R = 02-5B-318R; S = SaragollaS.
Table 2.
Monosaccharide composition of the ChASS, KOH 1 M and KOH 4 M fractions and Residues
| ChASS | KOH 1 M | KOH 4 M | Residue | |||||
|---|---|---|---|---|---|---|---|---|
| R | S | R | S | R | S | R | S | |
| Fuc | 1.4 ± 0.2 | 1.4 ± 0.2 | nd | nd | nd | nd | nd | nd |
| Rha | 4.5 ± 0.5 | 3.9 ± 0.4 | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.26 ± 0.01 | 0.23 ± 0.03 | 0.38 ± 0.04 | 0.36 ± 0.07 |
| Ara | 22.8 ± 1.2 | 22.8 ± 1.9 | 14.4 ± 0.8 | 11.3 ± 0.1 | 12.9 ± 0.3 | 11.4 ± 0.6 | 16.5 ± 1.6 | 16.3 ± 0.6 |
| Gal | 21.1 ± 1.5 | 21.8 ± 0.8 | 2.2 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.2 | 1.2 ± 0.1 | 3.1 ± 0.8 | 2.5 ± 0.3 |
| Glu | 7.9 ± 0.6 | 8.8 ± 0.9 | 10.4 ± 1.1 | 6.6 ± 0.5 | 9.5 ± 0.9 | 5.9 ± 0.1 | 11.7 ± 1.7 | 12.5 ± 2 |
| Xyl | 8.6 ± 0.5 | 8.7 ± 0.9 | 69.8 ± 1.2 | 77.2 ± 0.6 | 73.0 ± 1.5 | 78.7 ± 0.7 | 63.0 ± 2.2 | 62.6 ± 1.4 |
| Man | 10.2 ± 1.2 | 9.7 ± 0.9 | nd | nd | nd | nd | nd | nd |
| GalA | 21.9 ± 0.4 | 21.2 ± 0.7 | 2.8 ± 0.1 | 2.8 ± 0.2 | 2.4 ± 0.1 | 2.4 ± 0.1 | 5.1 ± 0.7 | 5.5 ± 1.3 |
| GlcA | 1.5 ± 0.1 | 1.5 ± 0.4 | 0.26 ± 0.02 | 0.27 ± 0.02 | 0.18 ± 0.02 | 0.18 ± 0.05 | 0.18 ± 0.01 | 0.21 ± 0.05 |
| Ara + Xyl | --- | --- | 83.2 ± 1.2 | 86 ± 0.6 | 84.9 ± 1.2 | 89.1 ± 0.2 | 75.6 ± 2.1 | 74.8 ± 2.6 |
| Ara/Xyl | --- | --- | 0.207 ± 0.014 | 0.147 ± 0.002 | 0.177 ± 0.007 | 0.144 ± 0.009 | 0.262 ± 0.032 | 0.259 ± 0.006 |
Monosaccharide composition of cell walls from spike of 02-5B-318R and SaragollaS wheat plants was determined by HPAEC-PAD. Values are expressed in mol% for each monosaccharide in each fraction. Value represent means ± SD (n = 4). Number in bold indicate statistically significant differences in each monosaccharides between the two genotypes, according to according to Student’s t-test (p < 0.05). ChASS, chelating agent-soluble solids; R = 02-5B-318R; S = SaragollaS.
A different degree and pattern of methylesterification was observed in 02-5B-318R and SaragollaS spikes
The degree and pattern of pectin methylesterification impact the plant susceptibility to fungal and bacterial pathogens and affect the outcome of disease [20]. The degree of methylesterification (DM) of cell wall isolated from spikes of 02-5B-318R was significantly higher (a about 30%) in comparison with SaragollaS genotype (Figure 3a). In accordance with this, durum wheat plants overexpressing the pectin methylesterase inhibitor from kiwi, AcPMEI, exhibited a costitutive increased degree of methylesterification (DM) and were more resistant to F. graminerum, Bipolaris sorokiniana and Claviceps purpurea in comparison with untransformed plants [5,30]. It was also demonstrated that highly methylesterified pectins were less susceptible to the action of polygalacturonases (PGs) of both B. sorokiniana and F. graminearum and a reduced growth of both fungal pathogens was detected on cell walls isolated from the transgenic plants indicating that the increased resistance of AcPMEI plants was due to the impaired ability of these fungi to colonize the host tissue [5]. Pectin domains with a random pattern of methylesterification, recognized by the monoclonal antibody LM7, have been demonstrated to be more sensitive to fungal PGs and pectate lyases (PLs) [54,55]. Immunodot assay performed with LM7 antibodies on ChASS enriched pectin fraction extracted from spikes of 02-5B-318R and SaragollaS showed a significant lower level of LM7-binding epitopes in the 02-5B-318R plants in comparison with the susceptible genotype (Figure 3b). These results indicate that pectin of 02-5B-318R spikes is enriched in domains less susceptible to PGs of F. graminearum secreted at early stages of infection [8]. Noteworthy, LM7 epitopes were also reduced in wheat plants overexpressing AcPMEI and showing improved resistance to F. graminearum [5].
Figure 3.
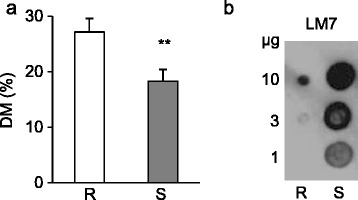
Degree and pattern of pectin methylesterification (DM) in cell wall extracted from spikes of 02-5B-318 R and Saragolla S plants. (a) The DM was quantified and expressed as methanol to uronic acid molecular ratio (%). Data represent the average ± standard deviation (n = 6). Asterisks indicate data sets significantly different between 02-5B-318R and SaragollaS according to Student’s t-test (p < 0.01). (b) Immunodot analysis of pectin extracted from spikes of 02-5B-318R and SaragollaS plants using LM7 antibody. The micrograms of chelating agent soluble solid fractions from the two genotypes applied to the nitrocellulose membrane were indicated. The experiments were repeated three times with similar results. R = 02-5B-318R; S = SaragollaS.
Recent evidence indicates that pectin de-methylesterification is induced at early stages of pathogen infection and favor the outcome of disease [56-58]. To determine whether pectin methylesterification is altered during fungal infection, DM was monitored at different times in uninfected and infected 02-5B-318R and SaragollaS spikes. The level of pectin methylesterification was significantly reduced in both genotypes during the early stages of Fusarium infection (Figure 4a). However, while a significant decrease of DM was observed in SaragollaS spikes 48 h hour post inoculation (hpi), the DM reduction in 02-5B-318R infected spikes was evident only after 72 hpi. Notably at 72 hpi, the reduction of DM in the susceptible SaragollaS genotype was approximately 60% compared to a 25% reduction in the resistant genotype.
Figure 4.
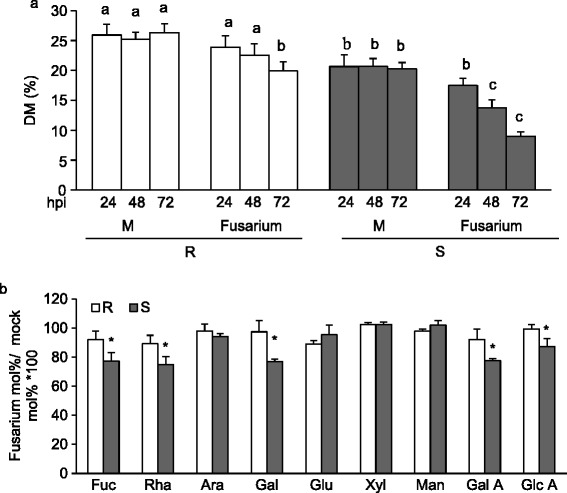
Characterization of cell wall from spikes of 02-5B-318 R and Saragolla S plants during Fusarium infection. (a) Quantification of degree of pectin methylesterification (DM) at early stages of Fusarium infection. The DM was performed at the indicated hours post-inoculation (hpi). (b) Monosaccharide compositions of matricial polysaccharides was analysed at 72hpi; M, mock-inoculated plants; Fusarium, fusarium-inoculated plants. Data represent the average ± standard deviation (n = 6). The experiment was repeated twice with similar results. The different letters indicate datasets significantly different according to analysis of variance (ANOVA) followed by Tukey’s test (p < 0.05). Asterisks indicate data sets significantly different between 02-5B-318R and SaragollaS according to Student’s t-test (p < 0.05). R = 02-5B-318R; S = SaragollaS.
Studies focused on the analysis of modification of CW composition during fungal infection indicate that CW degradation occur in a sequential manner. Pectic enzymes, mainly including PGs and PLs, are the first to be produced by fungal pathogens during the early stages of infection followed by hemicellulases and cellulases [11,59,60] and although wheat contain a low level of pectin, PGs and PLs produced by Fusarium during infection are important determinants of the outcome of disease [8,61-63]. The analysis of the cell wall degradation by F.graminearum was performed by monitoring the monosaccharide composition of AIS isolated from infected spikes at different hpi (Figure 4b). No difference in monosaccharide composition was detected in the cell walls of infected spikes at 24 and 48 hpi (data not shown). At 72 hpi, the level of Fuc, Rha, Gal, GalA and GlcA monosaccharides was significantly reduced in SaragollaS cell walls as compared to 02-5B-318R indicating an higher extent of pectin degradation in the susceptible line. These results suggest that the higher DM and reduced content of pectin domains with random pattern methylesterification in 02-5b-318R spikes as well as the reduced demethylesterification observed during infection can contribute to protect CW by fungal CWDEs degradation. The hemicellulose alteration was not observed at these stages of infection most likely, because the degradation of hemicelluloses occurs at late stages of infection as reported [8].
Isolation and characterization of WheatPME1
The degree and pattern of pectin methylesterification in planta is regulated by PMEs. In addition to their important role in plant development [64,65] more recent evidence indicates that plant PMEs are directly involved in plant response against pathogens [56,57,66]. With the aim to identify wheat PME genes involved in Fusarium resistance, we focused our attention on Brachypodium distachyon, which is considered, in respect to vast majority of traits (i.e. cell wall composition, cell wall biosynthesis and plant-pathogen interactions), a convenient model system for monocots [67]. Among different PME sequences, identified using phytozome web site, we focused our attention on Bradi1g16780.1 gene (hereafter named BdPME1). This gene showed the highest sequence similarity with wheat ESTs corresponding to a PME gene localized on the chromosome 2A, where the major FHB QTLs were found. The BdPME1 complete genomic sequence consists of 1812 bp corresponding to a mRNA of 1728 bp encoding a 576 amino acids protein. BdPME1 belongs to type I PME containing, in addition to the catalytic PME domain, an N-terminal pro region that share homology with PMEIs [64,68]. BdPME1 gene is located on chromosome 1 of Brachypodium genome and composed of two exons: the first at the 5’ end is 498 bp long including the pro region; the second including the PME domain is 1230 bp long. The two exons are separated by a very short intron sequence 84 bp long.
With the aim to isolate the BdPMEI1 orthologous in wheat, the gene sequence was blasted against public databases. Two wheat ESTs, showing a sequence identity higher than 80% with respect BdPME1, were found: the first one (BJ252439) entirely covered the BdPME1 longer exon, while the second one (BJ246509) partially matched to the shorter exon at the 5’end of the gene sequence. The hexaploid wheat cv. Chinese Spring draft genome and the row 454 sequence reads of cv. Chinese Spring annotated at Cereals-DB archive (http://www.cerealsdb.uk.net) were searched to extend both ESTs and three larger consensus contigs were obtained assignable to each of the three A, B and D genomes. The three genes were identified using Softbarry prediction software (http://linux1.softberry.com) and named WheatPME1-A, WheatPME1-B and WheatPME1-D (Additional file 1: Figure S1). They showed a 99% nucleotide sequence identity among each other (Additional file 2: Figure S2) and the same intron/exons structure comprising two exons of 1053 and 555 bp, separated by an intron of 54 bp, corresponding to a mRNA sequence of 1608 bp (Figure 5a). The translation of the three WheatPME1-A, WheatPME1-B and WheatPME1-D sequences resulted in a same 537 amino acid protein, sharing an amino acid identity of 77% with BdPME1 (Additional file 1: Figure S1 and Additional file 3: Figure S3). The Propt.Comp. v.9.0 software indicates WheatPME1 as an “extracellular secreted protein”, conforming with the apoplastic locatization of the enzyme. The genomic sequences of WheatPME1 homoeologous genes were obtained in 02-5B-318R (A, B and D genomes) and SaragollaS (A and B genomes) using genomic specific primers. The nucleotide sequences and intron/exons structures were respectively identical to the corresponding homoeologous WheatPME1 genes in A, B and D genomes of 02-5B-318R and in A and B genomes of SaragollaS indicating that the sequence of this gene is strongly conserved in different wheat genotypes. No polymorphism in the WheatPME1 gene was detected between 02-5B-318R and SaragollaS. A BLAST search for plant sequences related to WheatPME1 mRNA (BlastX, http://blast.ncbi.nlm.nih.gov) revealed a number of genes which predicted amino acid sequences were analyzed using non-redundant protein database. The search for grass sequences related to WheatPME1 in Phytozome database (http://www.phytozome.net) revealed a number highly conserved PMEs genes, which encode proteins with a slightly variable length ranging from 566 aminoacids (in Setaria italica, Panicum virgatum, Oryza sativa) to 576 aminoacids (in B. distachyon) and with an identity level ranging from 63 to 78% (Figure 5b). All the selected PMEs belong to type I PME accounting for a smaller pro region at N-terminus of the PME gene, with length range of 151–153 aa, and a longer PME domain with length range of 297–299 aa; consistently with other evidence, these are highly conserved among the selected species [68]. Among the selected WheatPME1 orthologous the gene structure appeared to be highly conserved (Figure 5b), and always composed by one single exon. The exceptions are rice and Brachypodium distachyon where the sequences are accounted for two gene copies, one is composed by one and another by two exons. Multi-alignment of genomic sequences showed that the different orthologous are characterized by several synthenic regions, particularly one of which showed the same position and orientation in all the selected grasses, likely corresponding to the active site of the enzyme (Figure 5b).
Figure 5.
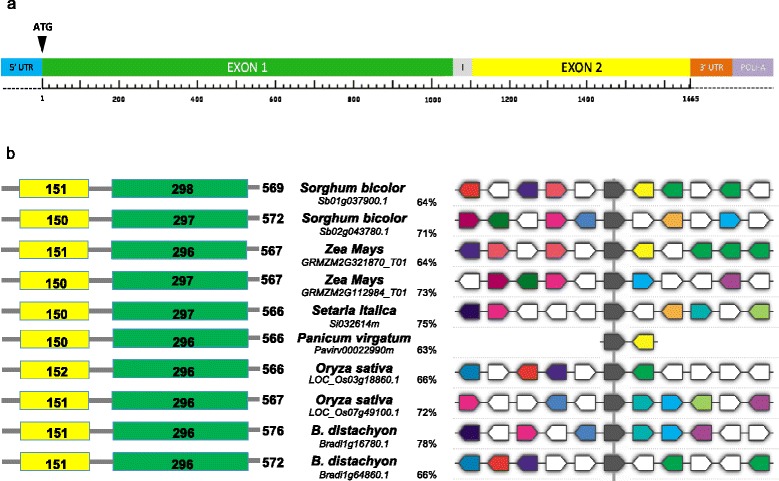
Protein and gene structure of grasses PMEs. (a) Schematic representation of WheatPME1 structure in Triticum aestivum cv. Chinese Spring as predicted by FGENESH (http://linux1.softberry.com). In color the different gene regions. I = intron sequence of 84 bp; Exon 1 = 1053 bp; Exon 2 = 555 bp. (b) Protein and gene structures of grasses PME sequences related to WheatPME1. Left: graphic representation of PMEs; in yellow is indicated the pro region and in green the PME domain. Numbers inside the blocks indicate the lenght of aminoacid sequences. Right: Syntenic relationships among the PME genes; the black block indicates the most conserved nucleotide stretch showing the same position and orientation in all the grasses domain. For each PME, the plant origin, accession number and % of aa identity with respect to WheatPME1 are indicated.
WheatPME1 gene chromosomal position and gene expression in 02-5B-318R and SaragollaS during F.graminearum infection
The chromosome position of the homoeologous WheatPME1 genes was obtained using genetic stocks including nulli-tetrasomic, di-telosomic and a set of wheat deletion bin lines. The homeologous genes were physically located on the short arm of chromosome group 2 in 2BS1-0.53-0.75 and C-2AS5-0.78 bins, respectively. This chromosome position supports a role of WheatPME1 gene in the control of Fusarium resistance since several major QTLs for FHB resistance have been found located in the same bin position with a R2 ranging from 3% to 27% [4].
To evaluate whether the expression of WheatPME1 is modulated during Fusarium infection in 02-5B-318R and SaragollaS, suitable primers were designed in a conserved region of the gene sequences in the three genomes and used for qRT-PCR analysis of transcripts from infected and mock-inoculated spikes. WheatPME1 expression level was measured at 0, 24, 48 and 72 hours post inoculation (hpi). In both wheat lines, the WheatPME1 expression level at 24 hpi did not show significant difference in comparison with the mock-inoculated controls (Figure 6). In 02-5B-318R, the level of WheatPME1 expression tends to decrease showing a 1-fold lower expression at 72 hpi. It is possible that during Fusarium infection, plants down regulate WheatPME1 to ensure a higher degree of CW methylesterification which would protect the CW against Fusarium pectic enzymes. On the contrary, in susceptible SaragollaS spikes the expression level of WheatPME1 showed a 2-fold increase at 48hpi in comparison with the non-infected control, and then dropped back to the basal expression level. Consistently with this observation, the analysis of Wheat 61 k GeneChip annotated at PLEXdb database (http://www.plexdb.org) indicated that the expression of WheatPME1 is only induced by Fusarium in the susceptible hexaploid wheat cv. Chinese spring but not in a line carrying a resistance locus from the wild Thinopyrum elongatum chromosome 7E [69] which supports the involvement of this specific PME isoform in wheat response to FHB. The induced expression of WheatPME1 in the susceptible SaragollaS line at 48 hpi likely contributes to the observed greater reduction of pectin methylesterification and increased pectin degradation in comparison with 02-5B-318R, making SaragollaS CWs likely more susceptible to fungal CWDEs action and tissue more accessible to fungal colonization. Fusarium growth was assessed by measuring the expression of beta-tubulin 2 gene (βTUB2; FJ526863.1) in spikes from infected and mock-inoculated 02-5B-318R and SaragollaS plants (Figure 6). The βTUB2 expression showed increased levels at 24, 48 and 72 hpi in both inoculated lines, however, to a higher extent in the susceptible SaragollaS reflecting an increased fungal growth in these plants. This result also indicates that the repression of WheatPME1 observed in 02-5B-318R was most likely, due to a negative regulation of the gene.
Figure 6.
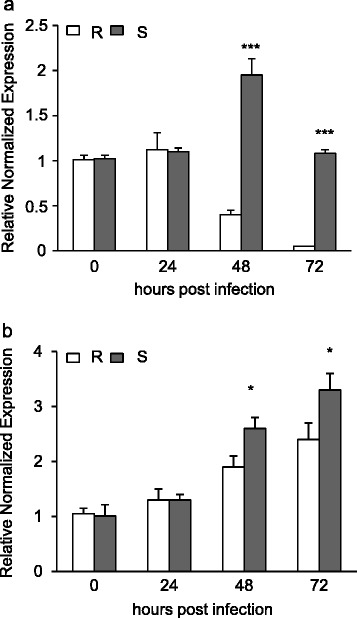
WheatPME1 and F. graminearum βTUB2 expression in spikes of resistant 02-5B-318 R and susceptible Saragoll S wheat lines during infection. a) WheatPME1 expression was normalized to the average of four different internal references (Actin, CDC, ADP-RF and RLI) reported as fold-change with respect to the mock-inoculated control. b) βTUB2 expression. The expression level was determined at 24, 48 and 72 hpi. Asterisks indicate data sets significantly different according to Student’s t-test (***p < 0.001; *p < 0.05). R = 02-5B-318R; S = SaragollaS.
Conclusions
Different mechanisms of disease resistance of wheat against F. graminearum have been elucidated, mainly in common wheat. These include the specific activation of defense signaling pathways, detoxification/ tolerance and resistance to fungal toxins, and the induction of plant defense secondary metabolites [70,71]. Durum wheat is one of the most susceptible cereals to F.graminearum infection and breeding for the FHB resistance is complicated by the lack of resistance sources. It was speculated that durum wheat either lacks resistance genes or carries effective susceptibility factors and/or suppressor genes that compromise FHB resistance [72,73].
Emerging evidence indicates that content and composition of cell wall polymers affect the susceptibility of cell wall to CWDEs and can play a role in the outcome of host-pathogen interactions [11-14]. In this study we provide a comprehensive overview of cell wall composition of spikes at anthesis, a key developmental stage particularly susceptible to Fusarium infection, from a resistant common wheat and a susceptible durum wheat genotypes. The comparative CW analysis revealed constitutive differences in monolignol composition of lignin, with a higher amount of S-type lignin present in the resistant 02-5B-318R wheat as compared to the SaragollaS susceptible plants. We also detected differences in hemicellulose and pectic polymers of the cell wall in spikes of the two genotypes. In particular, resistant line was enriched in AXs with a higher degree of arabinose substitution. The CW of resistant line contained a higher amount of methylesterified pectin with a less random distribution of methylated GalA.
The analysis of degree of methylesterification and monosaccharide composition of the cell wall of spikes at early stages of Fusarium infection indicated an higher demethylesterification and an higher extent of pectin degradation in the susceptible line as compared to 02-5B-318R. We propose that cell wall differences between the susceptible and resistant genotype could contribute to the different polysaccharide degradation we observed at early stage of F.graminearum infection as well as could influence the outcome of the disease. Cell wall genes which regulating the cell wall traits identified could be involved in FHB resistance. Among these genes, WheatPME1 was identified, characterized and proposed to participate in the control of pectin methylesterification during the interaction of wheat with F. graminearum. In addition to the cell wall components here identified, other cell wall traits are known to be involved in monocot resistance to Fusarium [70]. Examples are the cell wall‐bound thionins, having growth inhibition activity toward pathogens as well as callose and structural hydroxyproline‐rich glycoproteins, both involved in cell wall reinforcement at the site of pathogen infection [14,74,75]. Moreover, inhibitors of CWDEs such as polygalacturonase inhibiting proteins (PGIPs), PMEIs, Triticum aestivum xylanase inhibitors (TAXIs) and xylanase inhibitor proteins (XIPs), influencing cell wall degradability during infection, have been associated to wheat resistance against Fusarium [6,11,76]. All these cell wall traits are potential molecular markers useful in plant breeding programs targeted to the selection of wheat varieties with a durable resistance to Fusariosis.
Methods
Growing condition of wheat and pathogenicity tests
Wheat seeds were surface-sterilized in Sodium hypochlorite and transferred on petri dishes containing 3MM paper soaked with water. Plates were stored at 4°C in the dark for 24-48 h and transferred in a growth chamber at 23°C in the dark for 15 days. Plants grown in a controlled environmental chamber maintained at 22°C, 70% humidity with a 16 hours photoperiod (300μE m-2 s-1).
Pathogenicity tests were conducted using the Fusarium resistant common wheat line, accession n. 02-5B-318 (a breeding line derived from cv. Sumai3, kindly provided by dott. Stefano Ravaglia, S.I.S., Bologna, Italy) and on the susceptible durum wheat cv. Saragolla. Uniform inoculum pressure was applied during flowering by using the Fusarium graminearum PH 1 isolate (kindly provided by prof. Quirico Migheli, University of Study of Sassari, Italy). Plants were artificially inoculated by spraying on each plants 100 mL of a suspension containing a mixture of conidia of F. graminearum (about 1.0 × 105 conidia per mL). Fusarium strain was grown for one week on PDA (Potato Dextrose Agar) and conidia were isolated by growing pieces of mycelium in shaking cultures in 2 L PIREX flasks containing 1 L sterile CMC (Carboxyl-methyl-cellulose) medium (15gr CMC, 1gr NH4NO3; 1gr KH2PO4; 0.5 gr MgSO4*7H20; 1gr yeast extract; 50 ug/mL chloramphenicol). After 5-day incubation in the dark at 25°C shaking at 150 rpm, flasks content was filtered through two layers of cheesecloth by centrifugation at 3,000 rpm for 10 min; pellet was re-suspended in sterile water and centrifuged again. Filtered conidia were finally re-suspended in 10 mL of sterile water. The concentration of the inoculum was measured with a Burker camera (HBG Henneberg-Sander GmbH, Lutzellinden, Germany) using a light-microscope.
Twenty plants for 02-5B-318 and Saragolla line were artificially spray-inoculated during anthesis with a 106/mL distilled-water macroconidia suspension, for each plant 5 spikes were chosen for a total of 100 spikes per lines. Fusarium head blight (FHB) incidence and severity were recorded five, ten and twenty days after inoculation on both infected and mock-inoculated (controls) wheat plants: FHB severity was averaged as the percentage of infected spikelets per plant, while FHB incidence was averaged as the number of infected spikes per plant; a mean value of at least 20 plants per genotype was assessed. Infection experiments were statistically evaluated by performing analysis of variance followed by the Student’s t test.
Alcohol-insoluble solids (AIS) extraction
Wheat spikes were collected at anthesis stage and infected spikes were collected after 24, 48 and 72 hours post Fusarium inoculation. Tissues excised from the central part of each spike, including rachis and spikelets were ground to a fine powder with a mortar and pestle in presence of liquid nitrogen. Milled tissue (200 mg) was washed twice in a pre-warmed (70°C) 70% ethanol, vortexed, and pelleted by centrifugation at 25,000 g for 10 min. The pellet was suspended with a chloroform:methanol mixture (1:1, v/v) and shaked for 30 min at room temperature. Samples were pelleted by centrifugation at 25,000 g for 10 min. Pellets were re-suspended in 1 ml 80% acetone and spin at 25,000 g for 5 min. Supernatants were discarded and pellets were dried at room temperature over-night.. Starch was removed by treating the AIS with the porcine Type I-A α-amylase (100 U g-1 AIS; product number A4268; Sigma-Aldrich) in a 100 mM potassium phosphate buffer pH 7.5 mM NaCl and 0.02% (w/v) NaN3 for 24 hours at 37°C. The suspension was centrifuged at 25,000 × g for 20 minutes, and pellet was then washed with distilled water and 80% acetone.
Lignin content and monolignol composition
Acetyl bromide lignin in de-strached AIS from the spikes of both wheat varieties was determined according to [77] with some modifications. Briefly, 3 mg of AIS were placed in glass vials, and then 200 μl 25% acetyl bromide in acetic acid and 600 μl of acetic acid (glacial) were added. Mixtures were incubated at 50°C for 2 h, with occasional shaking. 15 μl of reaction mixture after cooling was transferred to 96-well plate (UV transparent), and 15 μl 0.3 M NaOH, 5 μl 0.5 M hydroxylamine hydrochloride and 65 μl acetic acid (glacial) were added. After shaking, optical density at 280 nm against blanks (all reagents without AIS samples) was measured using plate reader. Lignin concentration was determined using the following equation: % lignin content = (absorbance × 100)/SAC × AIS concentration (g−1) where SAC is the specific absorption coefficient of lignin [78]. Specific monolignol composition was determined using Pyrolysis-GC-MS. De-starched AIS (3 mg) were single-shot pyrolized at 500°C and the volatile compounds were separated on HP-5 MS column (30 m × 0.25 mm, Agilent Technologies Inc, USA) using GC system (6890 N GC-system interfaced to 5975B inert MSD, Agilent Tech., USA). Oven temperature was initially set at 50°C and ramped to 280°C over a period of 53 min. Helium was the carrier gas for the volatile compounds and the split ratio was set at 50:1. Peak identification was performed by comparison of sample spectra with those published by [79]. The monolignol composition was calculated as %, combining the peak areas of similar type of lignin.
Determination of the degree of methylesterification
De-starched AIS (4 mg) were saponified by suspending them in 60 μl H2O up and 20 μl of 1 M NaOH. The solution was incubated at room temperature for 1 h and afterward neutralized with HCl. After centrifugation at 25,000 × g, aliquots of the supernatant (50 μl) of 02-5B-318R and SaragollaS were loaded in microtiter plates (96-well cod.9018 from Costar, Cambridge, MA, U.S.A.). Alcohol oxidase (50 μl) was added to each well (0.03 units in 0.1 M sodium phosphate, pH 7.5) (Sigma, St. Louis), and this mixture was incubated at room temperature for 15 min on a shaker. Thereafter, 100 μl of a mixture containing 0.02 M 2,4-pentanedione in 2 M ammonium acetate and 0.05 M acetic acid was added to each well. After 10 min of incubation at 68°C, samples were cooled on ice and absorbance was measured at 412 nm in a microplate reader (ETI-System reader; Sorin Biomedica Cardio S.p.A., Saluggia, Italy. The amount of methanol was estimated as described [80]. For uronic acid quantification, 4 mg saponified AIS samples were incubated in 200 μl of 2 M Trifluoracetic acid (TFA) at 121°C. After 1.5 hours, 200 μl of isopropanol was added and the mixtures evaporated at 40°C with a stream of N2 gas. This step was repeated twice and samples were dried at room temperature overnight. The TFA hydrolyzed monosaccharides were suspended in 200 μl of water and the Uronic acid content in the supernatant was quantified colorimetrically using the automated sulfamate/m-hydroxy diphenyl assay [81] and galacturonic acid (Fluka 48280) as standard. The degree of methylesterification was expressed as methanol to uronic acid molar ratio (%).
Cell wall fractionation and monosaccharides composition
To isolate fractions enriched in various cell wall components, AIS were subjected to sequential extraction buffers (at final concentration of 30 mg/ml) in constant mixing for 24 hours at room temperature. The following order was followed: 50 mM ammonium oxalate (Chelating Agent Soluble Solid, ChASS) pH 5.2 with 0.02% sodium azide; 1 M KOH, 1% (w/v) of sodium borohydride with 0.02% sodium azide and 4 M KOH with 1% (w/v) of sodium borohydride with 0.02% sodium azide. The 1MKOH and 4 M KOH fractions were neutralized using glacial acetic acid. All of the extracts were dialyzed against four changes of 4 L of deionized water and then lyophilized. For each genotype six independent replicates were analyzed. The monosaccharide composition of destarched AIS, the ChASS, 1 M KOH, 4 M KOH fractions and of residue, all hydrolysed with TFA was determined by HPAEC-PAD using a PA20 column (Dionex, CA, USA). Peaks were identified and quantified by comparison to a standard mixture of rhamnose (Rha), arabinose (Ara), fucose (Fuc), galactose (Gal), glucose (Glc), xylose (Xyl), mannose (Man), galacturonic Acid (GalUA),and glucuronic acid (GlcUA) (Sigma-Aldrich).
The crystalline cellulose was determined as previously described [82]. The cellulose derived glucose content in destarched AIS was determined by an anthrone colorimetric assay [83] with glucose (Sigma G8270) as a standard.
Immunodot assay
For each experiment, ChASS and KOH 1 M fractions were applied as 1 μL aliquots to nitrocellulose membrane (0.45 μm pore size; Bio-Rad, Hercules, CA, USA) in a threefold dilution series. Arrays were incubated for 1 hour in 5% (w/v) milk protein (MP; Bio-Rad) in PBS pH 7.8 (MP-PBS), and probed for 1.5 hours with primary LM7 and LM11 monoclonal antibodies (purchased from PlantProbes, Paul Knox Cell Wall Lab, University of Leeds,Leeds, UK) diluted 1:20 in 3% MP-PBS. After extensive washes in PBS, arrays were incubated with anti-rat conjugated to horseradish peroxidase (A7058; Sigma-Aldrich) diluted 1:1000 in MP-PBS buffer. After washing in PBS, LM11 arrays was developed using 4-chloro-1-naphthol [84] and, due to a weak signal, LM7 was developed using ECL detection reagent (Amersham).
Bioinformatic analysis
In order to identify homologous proteins to wheat methylesterase enzyme, a bioinformatic analysis was carried out on grass species (Sorghum bicolor, Zea mays, Setaria italica, Panicum virgatum, Oryza sativa and Brachypodium distachyon) annotated in Phytozome v.9.1 database (http://www.phytozome.net). Brachypodium BdPME1 complete genomic sequence was used as the initial query in a BLAST-search against wheat EST (Expressed Sequence Tags) database at NCBI (http://blast.ncbi.nlm.nih.gov), with the aim to retrieve sequences with a high similarity score (>80%). Each suitable EST was finally searched for similarity in the Chinese Spring database at Cereal DB (http://www.cerealsdb.uk.net/search_reads.htm), to extract 454 reads and obtain larger consensus contigs of the hexaploid reference cultivar using an e-value cut-off of e−5.
Isolation and characterization of WheatPME1 sequence in wheat lines
WheatPME1 gene isolation was conducted in the 02-5B-318 accession of T. aestivum and in the durum wheat cv. Saragolla, respectively FHB-resistant and susceptible. Genomic DNA was isolated from the two wheat lines according to the extraction protocol by [85] starting from 0.1 gr of fresh leaves, then checked for quality and concentration at a Nanodrop device (Thermo Scientific, Walthman, MA, USA). Purity of extracted DNA was assessed by measuring 260 nm/280 nm ratio, with a value of approximately 1.8-2 indicating a good quality.
Genomic DNA was PCR-amplified with several primer pairs opportunely designed by OligoExplorer software on Brachypodium genomic sequence, Chinese Spring ESTs and consensus contigs, in order to cover the entire gene sequence. All the amplification reactions were initially carried out in a gradient of annealing temperature in order to check for primer specificity and identify the optimal annealing conditions for each primer combination. PCR reactions were conducted in a total volume of 25 μl containing 100 ng of template gDNA, 250 nmol/L of each primer, 1X reaction Buffer (10 mmol/L Tris–HCl, pH 8.3; 10 mmol/L KCl), 200 μmol/L of each dNTP, 2.5 mmol/L of MgCl2, and 1 unit of Taq DNA polimerase (EuroTaq, Euroclone®). Amplifications were run in a MyCycler™ Personal Thermal Cycler (Bio-Rad®) according to the following protocol: 5 min at 95°C, followed by 32 cycles of: 1 min at 95°C, 1 min at the given annealing temperature, and 2 min at 72°C, followed by a final extension step of 15 min at 72°C. Finally, PCR products were checked for the expected molecular size by visualization on 1.5-2% agarose gel stained with Gel-Red® dying solution (Biotium, Inc., Hayward, CA).
For the chromosomal localization of WheatPME1 genes, nulli-tetrasomic lines (NTs) of Triticum aestivum cv. Chinese Spring [86,87] were used to physically localize PME markers to chromosomes. Chinese Spring di-telosomic lines [88] were used for the assignment of markers to each chromosomal arm. Physical location on chromosome bins of each PCR fragment was obtained using a set of common wheat deletions lines dividing genome chromosomes into bins (kindly provided by B. S. Gill, USDA-ARS, Kansas State University) [89]. Single-band PCR products were directly purified from a volume of about 100 μl using the EuroGold Cycle Pure Kit (Euroclone®) following the manufacturer instructions, with the only exception of using sterile deionized water rather than the supplied elution buffer, to increase the efficiency of following sequencing reactions. Purified DNA fragments were checked on 1.5-2% agarose gel stained with Gel-Red® dye solution, then evaluated for concentration by detecting absorbance at a 260 nm wave length at a Nano Drop device (Thermo Scientific®). Sequencing analyses were performed for each fragment in both strands by BMR Genomics S.r.l (Padova). Sequence assembly was obtained with Codone Code Aligner and Geneious softwares. Multi-alignments of gene sequences between 02-5B-318 and Saragolla were carried out by ClustalW (http://www.ebi.ac.uk) and BLAST (http://blast.ncbi.nlm.nih.gov). Gene structure prediction was performed by the FGENESH on-line tool (http://linux1.softberry.com/cgi-bin/programs/gfind/bestorf.pl).
Gene expression analysis
Total RNA was isolated from spikes of infected and mock-inoculated (control) plants of both resistant 02-5B-318 and susceptible Saragolla at 24, 48 and 72 hours post inoculation. For each sample three biological replicates were collected from different plants. Tissues were harvested in each phase, immediately frozen in liquid nitrogen and stored at −80°C until RNA extraction. Total RNA was extracted using the RNeasy Plant Mini Kit (Qiagen®) and checked on 1.5% denaturing agarose gel; amount and purity were determined with a Nano-Drop spectrophotometer. All RNA samples were led to the same concentration (1 μg/μl) and reverse-transcribed into double stranded cDNA by using the Quanti-Tect Reverse Transcription Kit (Qiagen®) following the manufacturer instructions, after a prior treatment with a DNA Wipeout Buffer for the removal of gDNA contamination.
Primer pairs were designed by using OligoExplorer software on a conserved pme nucleotide region between the three wheat genomes, in order to determine the total pectin methyl-esterase gene expression in the two wheat lines. As shorter amplicons work more efficiently, primers were designed to amplify small DNA fragments in the range of 50–200 bp. Actin, CDC (Cell Division Control), ADP-RF (ADP-Ribosilation Factor) and RLI (RNase L Inhibitor-like protein) genes were used as internal references to normalize PME expression data. Specific primers for Fusarium β-tubulin 2 (βTUB2) gene were used to assay fungal infection in both inoculated and non-inoculated wheat samples (Additional file 4: Table S1).
In order to identify the best temperature to ensure primer specificity, standard PCR on cDNA were performed with a gradient of annealing temperatures (ranging between 55°C and 65°C) for both target and reference primer pairs, by using high fidelity MyTaq DNA polymerase (BioLine). Amplicon specificity was confirmed for each primer pair by checking the presence of single PCR products of expected molecular size on 2% (w/v) agarose gel stained with Gel Red® dying solution, and by direct sequencing of the amplified fragments (BMR Genomics, Padova, Italy).
Primer concentration was optimized for each gene in preliminary Real-Time amplification experiments by running reactions with different combinations of forward and reverse primers in the final mix (100, 300, 500 and 900 nM), then choosing those giving the highest endpoint fluorescence and a low Cq value. Primer specificity was also checked by performing melting curves of PCR products following Real Time amplifications.
qRT-PCR reactions were performed using EvaGreen® chemistry in the CFX96™ Real-time PCR System (Bio-Rad) following these conditions: 95°C for 3 min, followed by 40 cycles of: 95°C for 10 sec and 60°C for 30 sec. In each qPCR experiment 1 μl of a 1:10 dilution of cDNA was used in a final volume of 10 μl containing 5 μl of SsoFast EvaGreen® SuperMix 10X (Bio-Rad) and a primer concentration of 500 nM for WheatPME1, and 100 nM for Actin, CDC, ADP-RF and RLI. Three independent amplification reactions (technical replicates) were carried out for each biological replicate.
PCR reaction efficiency was calculated for both target and reference genes by generating six-point standard curves of three-fold serial dilutions of cDNA. Standards were run in the same amplification plate of the unknown samples. All experiments were performed in Hard-Shell 96-well skirted PCR plates (HSP9601) with Microseal® ‘B’ Adhesive Seals (MSB-1001) from Bio-Rad®.
Data analyses were performed with the CFX Manager™ 3.1 software, using the Normalized Expression mode (ΔΔCq) which calculated the relative quantity of target (WheatPME1) normalized to the relative quantity of internal references (geometric mean of multiple reference genes). For both target and reference genes, relative expression was calculated as fold-change respect to the mock-inoculated controls at each harvesting stage, and determining the standard deviation (SD) for the relative quantity. All the results were analyzed by ANOVA.
Availability of supporting data
All the supporting data are included as additional files in this manuscript.
Acknowledgements
The work in the labs of DB and AGa was supported by Ministero dell’Istruzione, dell’Universita’ e della Ricerca; PRIN Grant n. 2010T7247Z. The work in the lab of OAZ was supported by National Science Foundation; grant n.1121163, 2011–2015).
Abbreviations
- FHB
Fusarium Head Blight
- CW
Cell wall
- CWDEs
Cell wall degrading Enzymes
- PME
Pectin Methylesterase
- PMEI
Pectin Methylesterase inhibitor
- XIP
Xylanase inhibitor protein
- PGIP
Polygalacturonase inhibiting protein
- TAXI
Triticum aestivum xylanase inhibitor
- QTL
Quantitative trait Loci
- EST
Expressed sequence tags
- CDC
Cell division control
- ADP-RF
ADP-ribosilation factor
- RLI
RNase L inhibitor-like protein
- βTUB2
β-tubulin 2
- SD
Standard deviation
- Cq
Quantification cycle
- qRT-PCR
Quantitative reverse-transcription PCR
Additional files
WheatPME1genes and protein sequences. Fasta sequences of hexaploid cv Chinese Spring WheatPME1-A, WheatPME1-B and WheatPME1-D genes and encoded polypeptides.
Multiple alignment of WheatPME1 genes identified in Triticum aestivum, cv. Chinese Spring in the corresponding A, B and D genomes. In yellow are highlighted the SNPs between the A/D and B genomes.
Multiple alignment of WheatPME1 from A, B and D genomes of Triticum aestivum cv. Chinese Spring and from Brachypodium distachyon (BdPME1). The yellow box indicates the pro region, whereas the green box corresponds to the PME domain. The protein is reported in C terminus-N terminus orientation.
Primer pairs sequences for housekeeping and target genes.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LV, AGa, and DB designed experiments. LV and EF perform the characterization of cell wall polysaccharide composition and structure. NR and OAZ perform the characterization of lignin composition. SLG performed FHB disease symptoms assessment and RNA extraction. AGi performed qRT-PCR experiments, characterization and isolation of WheatPME1. AB contributed to data interpretation and assisted in drafting the manuscript. LV, AGi, AGa, OAZ, and DB wrote the paper. All authors read and approved the final manuscript.
Contributor Information
Vincenzo Lionetti, Email: vincenzo.lionetti@uniroma1.it.
Angelica Giancaspro, Email: angelica.giancaspro@libero.it.
Eleonora Fabri, Email: eleonora.fabri@uniroma1.it.
Stefania L Giove, Email: s.giove1@inwind.it.
Nathan Reem, Email: nreem@iastate.edu.
Olga A Zabotina, Email: zabotina@iastate.edu.
Antonio Blanco, Email: antonio.blanco@uniba.it.
Agata Gadaleta, Email: agata.gadaleta@uniba.it.
Daniela Bellincampi, Email: daniela.bellincampi@uniroma1.it.
References
- 1.Kazan K, Gardiner DM, Manners JM. On the trail of a cereal killer: recent advances in Fusarium graminearum pathogenomics and host resistance. Mol Plant Pathol. 2012;13:399–413. doi: 10.1111/j.1364-3703.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown NA, Urban M, Van De Meene AML, Hammond-Kosack KE. The infection biology of Fusarium graminearum: Defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biol. 2010;114:555–571. doi: 10.1016/j.funbio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Bischof M, Eichmann R, Huckelhoven R. Pathogenesis-associated transcriptional patterns in Triticeae. J Plant Physiol. 2011;168:9–19. doi: 10.1016/j.jplph.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Buerstmayr H, Ban T, Anderson JA. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed. 2009;128:1–26. doi: 10.1111/j.1439-0523.2008.01550.x. [DOI] [Google Scholar]
- 5.Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D’Ovidio R. The Ectopic Expression of a Pectin Methyl Esterase Inhibitor Increases Pectin Methyl Esterification and Limits Fungal Diseases in Wheat. Mol Plant-Microbe Interact. 2011;24:1012–1019. doi: 10.1094/MPMI-01-11-0021. [DOI] [PubMed] [Google Scholar]
- 6.Moscetti I, Tundo S, Janni M, Sella L, Gazzetti K, Tauzin A, Giardina T, Masci S, Favaron F, D’Ovidio R. Constitutive Expression of the Xylanase Inhibitor TAXI-III Delays Fusarium Head Blight Symptoms in Durum Wheat Transgenic Plants. Mol Plant-Microbe Interact. 2013;26:1464–1472. doi: 10.1094/MPMI-04-13-0121-R. [DOI] [PubMed] [Google Scholar]
- 7.Miedaner T, Longin CFH. Genetic variation for resistance to Fusarium head blight in winter durum material. Crop Pasture Sci. 2014;65:46–51. [Google Scholar]
- 8.Tomassini A, Sella L, Raiola A, D’Ovidio R, Favaron F. Characterization and expression of Fusarium graminearum endo-polygalacturonases in vitro and during wheat infection. Plant Pathol. 2009;58:556–564. doi: 10.1111/j.1365-3059.2008.02019.x. [DOI] [Google Scholar]
- 9.Wanyoike MW, Kang Z, Heinrich B. Importance of Cell Wall Degrading Enzymes Produced by Fusarium graminearum during Infection of Wheat Heads. Eur J Pl Pathol. 2002;108:803–810. doi: 10.1023/A:1020847216155. [DOI] [Google Scholar]
- 10.Yang F, Jensen JD, Svensson B, Jorgensen HJL, Collinge DB, Finnie C. Secretomics identifies Fusarium graminearum proteins involved in the interaction with barley and wheat. Mol Plant Pathol. 2012;13:445–453. doi: 10.1111/j.1364-3703.2011.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellincampi D, Cervone F, Lionetti V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front Plant Sci. 2014;5:228. doi: 10.3389/fpls.2014.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008;13:610–617. doi: 10.1016/j.tplants.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Pogorelko G, Lionetti V, Bellincampi D, Zabotina O. Cell wall integrity: Targeted post-synthetic modifications to reveal its role in plant growth and defense against pathogens. Plant Signal Behav. 2013;8:e25435. doi: 10.4161/psb.25435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blümke A, Falter C, Herrfurth C, Sode B, Bode R, Schäfer W, Feussner I, Voigt CA. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol. 2014;165:346–358. doi: 10.1104/pp.114.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King BC, Waxman KD, Nenni NV, Walker LP, Bergstrom GC, Gibson DM. Arsenal of plant cell wall degrading enzymes reflects host preference among plant pathogenic fungi. Biotechnol Biofuels. 2011;4:4. doi: 10.1186/1754-6834-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogel J. Unique aspects of the grass cell wall. Curr Opin Plant Biol. 2008;11:301–307. doi: 10.1016/j.pbi.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Rennie EA, Scheller HV. Xylan biosynthesis. Curr Opin Biotechnol. 2014;26:100–107. doi: 10.1016/j.copbio.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Berrin JG, Juge N. Factors affecting xylanase functionality in the degradation of arabinoxylans. Biotechnol Lett. 2008;30:1139–1150. doi: 10.1007/s10529-008-9669-6. [DOI] [PubMed] [Google Scholar]
- 19.Fincher GB. Revolutionary Times in Our Understanding of Cell Wall Biosynthesis and Remodeling in the Grasses. Plant Physiol. 2009;149:27–37. doi: 10.1104/pp.108.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lionetti V, Cervone F, Bellincampi D. Methyl esterification of pectin plays a role during plant-pathogen interactions and affects plant resistance to diseases. J Plant Physiol. 2012;169:1623–1630. doi: 10.1016/j.jplph.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Chanliaud E, Gidley MJ. In vitro synthesis and properties of pectin/Acetobacter xylinus cellulose composites. Plant J. 1999;20:25–35. doi: 10.1046/j.1365-313X.1999.00571.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda A, Ito T, Higaki T, Kutsuna N, Saito T, Ishimizu T, Osada H, Hasezawa S, Matsui M, Demura T. Cobtorin target analysis reveals that pectin functions in the deposition of cellulose microfibrils in parallel with cortical microtubules. Plant J. 2010;64:657–667. doi: 10.1111/j.1365-313X.2010.04356.x. [DOI] [PubMed] [Google Scholar]
- 23.Koshijima T, Watanabe T: Association between lignin and carbohydrates in wood and other plant tissues. Springer-Verlag; Springer, Berlin; 2003.
- 24.Caffall KH, Mohnen D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res. 2009;344:1879–1900. doi: 10.1016/j.carres.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Lionetti V, Francocci F, Ferrari S, Volpi C, Bellincampi D, Galletti R, D’Ovidio R, De Lorenzo G, Cervone F. Engineering the cell wall by reducing de-methyl-esterified homogalacturonan improves saccharification of plant tissues for bioconversion. Proc Natl Acad Sci U S A. 2010;107:616–621. doi: 10.1073/pnas.0907549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willats WG, McCartney L, Mackie W, Knox JP. Pectin: cell biology and prospects for functional analysis. Plant Mol Biol. 2001;47:9–27. doi: 10.1023/A:1010662911148. [DOI] [PubMed] [Google Scholar]
- 27.Bonnin E, Le Goff A, Korner R, Vigouroux J, Roepstorff P, Thibault JF. Hydrolysis of pectins with different degrees and patterns of methylation by the endopolygalacturonase of Fusarium moniliforme. Biochim Biophys Acta. 2002;1596:83–94. doi: 10.1016/S0167-4838(02)00207-8. [DOI] [PubMed] [Google Scholar]
- 28.Limberg G, Korner R, Buchholt HC, Christensen TM, Roepstorff P, Mikkelsen JD. Analysis of different de-esterification mechanisms for pectin by enzymatic fingerprinting using endopectin lyase and endopolygalacturonase II from A. niger. Carbohydr Res. 2000;327:293–307. doi: 10.1016/S0008-6215(00)00067-7. [DOI] [PubMed] [Google Scholar]
- 29.Wietholter N, Graessner B, Mierau M, Mort AJ, Moerschbacher BM. Differences in the methyl ester distribution of homogalacturonans from near-isogenic wheat lines resistant and susceptible to the wheat stem rust fungus. Mol Plant Microbe Interact. 2003;16:945–952. doi: 10.1094/MPMI.2003.16.10.945. [DOI] [PubMed] [Google Scholar]
- 30.Volpi C, Raiola A, Janni M, Gordon A, O’Sullivan DM, Favaron F, D’Ovidio R. Claviceps purpurea expressing polygalacturonases escaping PGIP inhibition fully infects PvPGIP2 wheat transgenic plants but its infection is delayed in wheat transgenic plants with increased level of pectin methyl esterification. Plant Physiol Biochem. 2013;73:294–301. doi: 10.1016/j.plaphy.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Baucher M, Monties B, Van Montagu M, Boerjan W. Biosynthesis and genetic engineering of lignin. Crit Rev Plant Sci. 1998;17:125–197. doi: 10.1016/S0735-2689(98)00360-8. [DOI] [Google Scholar]
- 32.Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W. Lignin Biosynthesis and Structure. Plant Physiol. 2010;153:895–905. doi: 10.1104/pp.110.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sattler SE, Funnell-Harris DL. Modifying lignin to improve bioenergy feedstocks: strengthening the barrier against pathogens? Front Plant Sci. 2013;4:70. doi: 10.3389/fpls.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haidukowski M, Visconti A, Perrone G, Vanadia S, Pancaldi D, Covarelli L, Balestrazzi R, Pascale M. Effect of prothioconazole-based fungicides on Fusarium head blight, grain yield and deoxynivalenol accumulation in wheat under field conditions. Phytopathol Mediterr. 2012;51:236–246. [Google Scholar]
- 35.Schroeder HW, Christensen JJ. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology. 1963;53:831–838. [Google Scholar]
- 36.Skyba O, Douglas CJ, Mansfield SD. Syringyl-Rich Lignin Renders Poplars More Resistant to Degradation by Wood Decay Fungi. Appl Environ Microbiol. 2013;79:2560–2571. doi: 10.1128/AEM.03182-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eynck C, Seguin-Swartz G, Clarke WE, Parkin IAP. Monolignol biosynthesis is associated with resistance to Sclerotinia sclerotiorum in Camelina sativa. Mol Plant Pathol. 2012;13:887–899. doi: 10.1111/j.1364-3703.2012.00798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen F, Reddy MSS, Temple S, Jackson L, Shadle G, Dixon RA. Multi-site genetic modulation of monolignol biosynthesis suggests new routes for formation of syringyl lignin and wall-bound ferulic acid in alfalfa (Medicago sativa L.) Plant J. 2006;48:113–124. doi: 10.1111/j.1365-313X.2006.02857.x. [DOI] [PubMed] [Google Scholar]
- 39.Bhuiyan NH, Selvaraj G, Wei YD, King J. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion. J Exp Bot. 2009;60:509–521. doi: 10.1093/jxb/ern290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez LD, Bristow JK, Statham ER, McQueen-Mason SJ. Analysis of saccharification in Brachypodium distachyon stems under mild conditions of hydrolysis. Biotechnol Biofuels. 2008;1:15. doi: 10.1186/1754-6834-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carpita NC, Defernez M, Findlay K, Wells B, Shoue DA, Catchpole G, Wilson RH, McCann MC. Cell wall architecture of the elongating maize coleoptile. Plant Physiol. 2001;127:551–565. doi: 10.1104/pp.010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCartney L, Marcus SE, Knox JP. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem. 2005;53:543–546. doi: 10.1369/jhc.4B6578.2005. [DOI] [PubMed] [Google Scholar]
- 43.Beaugrand J, Croner D, Debeire P, Chabbert B. Arabinoxylan and hydroxycinnamate content of wheat bran in relation to endoxylanase susceptibility. J Cereal Sci. 2004;40:223–230. doi: 10.1016/j.jcs.2004.05.003. [DOI] [Google Scholar]
- 44.Bily AC, Reid LM, Taylor JH, Johnston D, Malouin C, Burt AJ, Bakan B, Regnault-Roger C, Pauls KP, Arnason JT, Philogène BJ. Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: Resistance factors to Fusarium graminearum. Phytopathol. 2003;93:712–719. doi: 10.1094/PHYTO.2003.93.6.712. [DOI] [PubMed] [Google Scholar]
- 45.Santiago R, Malvar RA. Role of Dehydrodiferulates in Maize Resistance to Pests and Diseases. Int J Mol Sci. 2010;11:691–703. doi: 10.3390/ijms11020691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ralph J, Guillaumie S, Grabber JH, Lapierre C, Barriere Y. Genetic and molecular basis of grass cell-wall biosynthesis and degradability. III. Towards a forage grass ideotype. C R Biol. 2004;327:467–479. doi: 10.1016/j.crvi.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 47.Molinari HBC, Pellny TK, Freeman J, Shewry PR, Mitchell RAC. Grass cell wall feruloylation: distribution of bound ferulate and candidate gene expression in Brachypodium distachyon. Front Plant Sci. 2013;4:50. doi: 10.3389/fpls.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishii T. Isolation and characterization of a diferuloyl arabinoxylan hexasaccharide from bamboo shoot cell-walls. Carbohydr Res. 1991;219:15–22. doi: 10.1016/0008-6215(91)89039-I. [DOI] [PubMed] [Google Scholar]
- 49.Anders N, Wilkinson MD, Lovegrove A, Freeman J, Tryfona T, Pellny TK, Weimar T, Mortimer JC, Stott K, Baker JM, Defoin-Platel M, Shewry PR, Dupree P, Mitchell RA. Glycosyl transferases in family 61 mediate arabinofuranosyl transfer onto xylan in grasses. Proc Natl Acad Sci U S A. 2012;109:989–993. doi: 10.1073/pnas.1115858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chowdhury J, Henderson M, Schweizer P, Burton RA, Fincher GB, Little A. Differential accumulation of callose, arabinoxylan and cellulose in nonpenetrated versus penetrated papillae on leaves of barley infected with Blumeria graminis f. sp. hordei. New Phytol. 2014;204:650–660. doi: 10.1111/nph.12974. [DOI] [PubMed] [Google Scholar]
- 51.Burton RA, Wilson SM, Hrmova M, Harvey AJ, Shirley NJ, Stone BA, Newbigin EJ, Bacic A, Fincher GB. Cellulose synthase-like CslF genes mediate the synthesis of cell wall (1,3;1,4)-beta-D-glucans. Science. 2006;311:1940–1942. doi: 10.1126/science.1122975. [DOI] [PubMed] [Google Scholar]
- 52.Doblin MS, Pettolino FA, Wilson SM, Campbell R, Burton RA, Fincher GB, Newbigin E, Bacic A. A barley cellulose synthase-like CSLH gene mediates (1,3;1,4)-beta-D-glucan synthesis in transgenic Arabidopsis. Proc Natl Acad Sci U S A. 2009;106:5996–6001. doi: 10.1073/pnas.0902019106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slikova S, Havrlentova M, Sudyova V, Mihalik D, Gregova E. Cell wall beta-D-glucan during disease progress (Fusarium head blight) in wheat spikes. Cereal Res Commun. 2008;36:167–169. doi: 10.1556/CRC.36.2008.2.8. [DOI] [Google Scholar]
- 54.Clausen MH, Willats WGT, Knox JP. Synthetic methyl hexagalacturonate hapten inhibitors of antihomogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr Res. 2003;338:1797–1800. doi: 10.1016/S0008-6215(03)00272-6. [DOI] [PubMed] [Google Scholar]
- 55.Willats WG, Orfila C, Limberg G, Buchholt HC, van Alebeek GJ, Voragen AG, Marcus SE, Christensen TM, Mikkelsen JD, Murray BS, Knox JP: Modulation of the degree and pattern of methyl esterification of pectic homogalacturonan in plant cell walls: implications for pectin methyl esterase action, matrix properties and cell adhesion.J Biol Chem 2001, 276:19404-19413. [DOI] [PubMed]
- 56.Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Mol Plant-Microbe Interact. 2011;24:432–440. doi: 10.1094/MPMI-07-10-0157. [DOI] [PubMed] [Google Scholar]
- 57.Bethke G, Grundman RE, Sreekanta S, Truman W, Katagiri F, Glazebrook J. Arabidopsis PECTIN METHYLESTERASES Contribute to Immunity Against Pseudomonas syringae. Plant Physiol. 2014;164:1093–1107. doi: 10.1104/pp.113.227637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lionetti V, Raiola A, Cervone F, Bellincampi D. Transgenic expression of pectin methylesterase inhibitors limits tobamovirus spread in tobacco and Arabidopsis. Mol Plant Pathol. 2014;15:265–274. doi: 10.1111/mpp.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phalip V, Goubet F, Carapito R, Jeltsch JM. Plant Cell Wall Degradation with a Powerful Fusarium graminearum Enzymatic Arsenal. J Microbiol Biotechnol. 2009;19:573–581. doi: 10.4014/jmb.0807.459. [DOI] [PubMed] [Google Scholar]
- 60.De Lorenzo G, Castoria R, Bellincampi D, Cervone F. Fungal invasion enzymes and their inhibition. In: Carroll GC, Tudzynski P, editors. The Mycota. V. Plant Relationships, Part B. Berlin: Springer-Verlag; 1997. pp. 61–83. [Google Scholar]
- 61.Aleandri MP, Magro P, Chilosi G. Modulation of host pH during the wheat-Fusarium culmorum interaction and its influence on the production and activity of pectolytic enzymes. Plant Pathol. 2007;56:517–525. doi: 10.1111/j.1365-3059.2007.01574.x. [DOI] [Google Scholar]
- 62.Kikot GE, Hours RA, Alconada TM. Contribution of cell wall degrading enzymes to pathogenesis of Fusarium graminearum: a review. J Basic Microbiol. 2009;49:231–241. doi: 10.1002/jobm.200800231. [DOI] [PubMed] [Google Scholar]
- 63.Ferrari S, Sella L, Janni M, De Lorenzo G, Favaron F, D’Ovidio R. Transgenic expression of polygalacturonase-inhibiting proteins in Arabidopsis and wheat increases resistance to the flower pathogen Fusarium graminearum. Plant Biol. 2011;31:8. doi: 10.1111/j.1438-8677.2011.00449.x. [DOI] [PubMed] [Google Scholar]
- 64.Jolie RP, Duvetter T, Van Loey AM, Hendrickx ME. Pectin methylesterase and its proteinaceous inhibitor: a review. Carbohydr Res. 2010;345:2583–2595. doi: 10.1016/j.carres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Reca IB, Lionetti V, Camardella L, D’Avino R, Giardina T, Cervone F, Bellincampi D. A functional pectin methylesterase inhibitor protein (SolyPMEI) is expressed during tomato fruit ripening and interacts with PME-1. Plant Mol Biol. 2012;79:429–442. doi: 10.1007/s11103-012-9921-2. [DOI] [PubMed] [Google Scholar]
- 66.Ma L, Jiang S, Lin GM, Cai JH, Ye XX, Chen HB, Li MH, Li HP, Takac T, Samaj J, Xu C. Wound-induced pectin methylesterases enhance banana (Musa spp. AAA) susceptibility to Fusarium oxysporum f. sp cubense. J Exp Bot. 2013;64:2219–2229. doi: 10.1093/jxb/ert088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brkljacic J, Grotewold E, Scholl R, Mockler T, Garvin DF, Vain P, Brutnell T, Sibout R, Bevan M, Budak H, Caicedo AL, Gao C, Gu Y, Hazen SP, Holt BF, 3rd, Hong SY, Jordan M, Manzaneda AJ, Mitchell-Olds T, Mochida K, Mur LA, Park CM, Sedbrook J, Watt M, Zheng SJ, Vogel JP. Brachypodium as a Model for the Grasses: Today and the Future. Plant Physiol. 2011;157:3–13. doi: 10.1104/pp.111.179531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang MJ, Yuan DJ, Gao WH, Li Y, Tan JF, Zhang XL. A Comparative Genome Analysis of PME and PMEI Families Reveals the Evolution of Pectin Metabolism in Plant Cell Walls. PLoS One. 2013;8:e72082. doi: 10.1371/journal.pone.0072082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen SQ, Huang ZF, Dai Y, Qin SW, Gao YY, Zhang LL, Gao Y, Chen JM. The Development of 7E Chromosome-Specific Molecular Markers for Thinopyrum elongatum Based on SLAF-seq Technology. PLoS One. 2013;8:e65122. doi: 10.1371/journal.pone.0065122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walter S, Nicholson P, Doohan FM. Action and reaction of host and pathogen during Fusarium head blight disease. New Phytol. 2010;185:54–66. doi: 10.1111/j.1469-8137.2009.03041.x. [DOI] [PubMed] [Google Scholar]
- 71.Jansen C, von Wettstein D, Schafer W, Kogel KH, Felk A, Maier FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci U S A. 2005;102:16892–16897. doi: 10.1073/pnas.0508467102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buerstmayr M, Huber K, Heckmann J, Steiner B, Nelson JC, Buerstmayr H. Mapping of QTL for Fusarium head blight resistance and morphological and developmental traits in three backcross populations derived from Triticum dicoccum x Triticum durum. Theor Appl Genet. 2012;125:1751–1765. doi: 10.1007/s00122-012-1951-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ban T, Watanabe N. The effects of chromosomes 3A and 3B on resistance to Fusarium head blight in tetraploid wheat. Hereditas. 2001;135:95–99. doi: 10.1111/j.1601-5223.2001.00095.x. [DOI] [PubMed] [Google Scholar]
- 74.Pelegrini PB, Franco OL. Plant gamma-thionins: Novel insights on the mechanism of action of a multi-functional class of defense proteins. Int J Biochem Cell Biol. 2005;37:2239–2253. doi: 10.1016/j.biocel.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 75.Deepak S, Shailasree S, Kini RK, Muck A, Mithofer A, Shetty SH. Hydroxyproline-rich Glycoproteins and Plant Defence. J Phytopathol. 2010;158:585–593. [Google Scholar]
- 76.Janni M, Sella L, Favaron F, Blechl AE, De Lorenzo G, D’Ovidio R. The expression of a bean polygalacturonase-inhibiting proteins in transgenic wheat confers increased resistance to the fungal pathogen Bipolaris sorokiniana. Mol Plant-Microbe Interact. 2008;21:171–177. doi: 10.1094/MPMI-21-2-0171. [DOI] [PubMed] [Google Scholar]
- 77.Fukushima RS, Hatfield RD. Comparison of the acetyl bromide spectrophotometric method with other analytical lignin methods for determining lignin concentration in forage samples. J Agric Food Chem. 2004;52:3713–3720. doi: 10.1021/jf035497l. [DOI] [PubMed] [Google Scholar]
- 78.Sasaki M, Yamamoto Y, Matsumoto H. Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiol Plant. 1996;96:193–198. doi: 10.1111/j.1399-3054.1996.tb00201.x. [DOI] [Google Scholar]
- 79.Ralph J, Hatfield RD. Pyrolysis-Gc-Ms Characterization of Forage Materials. J Agric Food Chem. 1991;39:1426–1437. doi: 10.1021/jf00008a014. [DOI] [Google Scholar]
- 80.Klavons JA, Bennett RD. Determination of methanol using alcohol oxidase and its application to methyl ester content of pectins. J Agric Food Chem. 1986;34:597–599. doi: 10.1021/jf00070a004. [DOI] [Google Scholar]
- 81.Filisetti-Cozzi TMCC, Carpita NC. Measurement of uronic acids without interference from neutral sugars. Anal Biochem. 1991;197:157–162. doi: 10.1016/0003-2697(91)90372-Z. [DOI] [PubMed] [Google Scholar]
- 82.Updegraff DM. Semimicro determination of cellulose in biological material. Anal Biochem. 1969;32:420–424. doi: 10.1016/S0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- 83.Scott TA, Jr, Melvin EH. Determination of dextran with anthrone. Anal Chem. 1953;25:1656–1661. doi: 10.1021/ac60083a023. [DOI] [Google Scholar]
- 84.Willats WG, Limberg G, Buchholt HC, van Alebeek GJ, Benen J, Christensen TM, Visser J, Voragen A, Mikkelsen JD, Knox JP. Analysis of pectic epitopes recognised by hybridoma and phage display monoclonal antibodies using defined oligosaccharides, polysaccharides, and enzymatic degradation. Carbohydr Res. 2000;327:309–320. doi: 10.1016/S0008-6215(00)00039-2. [DOI] [PubMed] [Google Scholar]
- 85.Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Rep. 1983;1:19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- 86.Sears ER: The aneuploids of common wheat.Mo Agric Exp Stn Res Bull 1954, 572:1-58.
- 87.Sears ER: Nullisomic-tetrasomic combinations in hexaploid wheat. In: Chromosome manipulations and plant genetics. Riley R. and Lewis K.R. edition. Oliver & Boyd, Edinburgh; 1966:29–45.
- 88.Sears ER, Sears LMS: The telocentric chromosomes of common wheat. Edited by Ramanujam S. Proc. 5th Int Wheat Genetics Symp New Dehli, Indian Agricultural Research Institute 1978:389–407.
- 89.Endo TR, Gill BS. The deletion stocks of common wheat. J Hered. 1996;87:295–307. doi: 10.1093/oxfordjournals.jhered.a023003. [DOI] [Google Scholar]


