Abstract
The areca nut-chewing habit is common in Southeast Asia, India, and Taiwan, and arecoline is the most abundant and potent component in the areca nut. The effects of arecoline on birth defects have been explored in many species, including chicken, mice, and zebrafish. The effects of arecoline on embryos after long-term exposure are well established; however, the effects of short-term embryo exposure to arecoline are not understood. Using zebrafish, we study the effects of short-term exposure of arecoline on embryos to model the human habit of areca nut-chewing during early pregnancy. Arecoline, at concentrations from 0.001% to 0.04%, was administered to zebrafish embryos from 4 to 24 hours post fertilization. The morphological changes, survival rates, body length, and skeletal muscle fiber structure were then investigated by immunohistochemistry, confocal microscopy, and conventional electron microscopy. With exposure of embryos to increasing arecoline concentrations, we observed a significant decline in the hatching and survival rates, general growth retardation, lower locomotor activity, and swimming ability impairment. Immunofluorescent staining demonstrated a loose arrangement of myosin heavy chains, and ultrastructural observations revealed altered myofibril arrangement and swelling of the mitochondria. In addition, the results of flow-cytometry and JC-1 staining to assay mitochondria activity, as well as reverse transcription–polymerase chain reaction analyses of functional gene expression, revealed mitochondrial dysfunctions after exposure to arecoline. We confirmed that short-term arecoline exposure resulted in retarded embryonic development and decreased locomotor activity due to defective somitic skeletal muscle development and mitochondrial dysfunction.
Introduction
Arecoline (methyl 1,2,5,6-tetrahydro-1-methylnicotinate) is the major alkaloid of the areca nut (betel nut or betel quid, the fruit of Areca catechu, L.) and constitutes 85%–95% of the total alkaloid content (2%–4%) of the nut.1 It is released mainly from chewing areca nut,2 and ∼10% of the global population have the habit of chewing areca nut, mainly in India, Taiwan, Southeast Asia, and the South Pacific islands.3,4 The areca nut has been listed as one of the world's four most addictive substances for years, on a similar level with tobacco, alcohol, and caffeine. As early as 1985, the International Agency for Research on Cancer (IARC), Department of International Cancer Research, World Health Organization, proposed that “chewing tobacco-containing areca nut” or “both smoking and chewing areca nut” can cause cancer in humans and that the carcinogenic sites are mainly the oral cavity, pharynx, and esophagus. In 2004, the IARC concluded that there is sufficient evidence to demonstrate that the habit of chewing betel quid, with or without tobacco is carcinogenic to humans. Beyond the carcinogenicity of areca nuts, it has been reported that the ingredients of the nut have deleterious effects on embryos and that the teratogenic effects of areca nuts were similar to those reported for mothers who consumed alcohol or tobacco during pregnancy. It has been observed that areca nut-chewing during pregnancy causes decreased fetal weight and preterm birth,5–7 incidences of lower birth weight, reduced birth length, and early term.6,7 Among ethnic groups who have the habit of areca nut-chewing, metabolic disease symptoms are observed in neonates.8
In recent years, a variety of animal models have been used to validate arecoline's impact on the embryo.9–11 The zebrafish has emerged as an important model organism for vertebrate development due to its easy maintenance, rapid extracorporeal development, transparent embryo, and availability of gene markers.12 To the best of our knowledge, only one study has confirmed that zebrafish embryos displayed developmental retardation after arecoline treatment, exhibiting a slow heart rate and decreased survival rate.13 Apart from the effects mentioned earlier, we found a remarkable effect of arecoline on the locomotor activity in our pilot study; moreover, little is known about the toxicity of arecoline on the embryonic somitic skeletal muscle fibers. Therefore, we designed experiments to expose zebrafish embryo with arecoline from the gastrula sphere stage (4 hours post fertilization [hpf]) to the segmentation stage (24 hpf), at which time somite formation is completed. This treatment paradigm is intended to model a human female who has the betel chewing habit but stops chewing immediately after her awareness of pregnancy. Moreover, in this study, we choose to emphasize how arecoline-affected somite formation influences the growth, external morphology, and locomotion of the zebrafish embryo, with particular regard to immunohistochemical and ultrastructural changes of somitic muscle fibers. In addition, we examined the expression of mitochondrial functional genes, including sdhb, mt_cyb, cox 1, cox 4i1, atp5a1, and atp5f1, which are closely related to the skeletal muscle motor function and motor capability. These experiments were performed to explore the possible causes underlying the developmental toxicity of arecoline on skeletal muscle fibers.
Materials and Methods
Fish maintenance, collection of embryos, developmental staging, and arecoline treatment
Zebrafish (Danio rerio) were obtained from Dr. Shyh-Jye Lee, Institute of Zoology, College of Life Sciences, National Taiwan University (Taipei, Taiwan). The fish were raised under standard conditions14 and were maintained in 20-L aquariums that were supplied with filtered fresh water and aeration at 28.5°C under a 14 h light/10 h dark photoperiod. The descriptions of the developmental stages are according to Kimmel et al.12 and information from the Zebrafish Information Network (2003). All of the experimental protocols were approved by the Laboratory Animals Committee, College of Medicine, National Taiwan University.
Fertilized eggs were collected after natural spawning on the onset of light by crossing adult fish in a grid net. The same batch of eggs was rinsed with tank water, which was placed in Petri dishes containing 30% Danieau buffer as an incubation medium, and left to develop for 4 h at 28.5°C (until the blastula period, sphere stage) before arecoline treatment.
Arecoline hydrobromide (Sigma) was dissolved in 30% Danieau buffer to a concentration of 1% (w/v) and then to final arecoline concentrations of 0.001%, 0.01%, 0.02%, and 0.04% with 30% Danieau buffer. The incubation medium of embryos at gastrula sphere stage was replaced with the arecoline solutions for a period of 20 h (from 4 to 20 hpf) until the first heartbeat, just before the pharyngula stage, at 28.5°C without removing the chorion. At the completion of arecoline treatment, the treated embryos were transferred to a fresh arecoline-free incubation medium and collected at 24-h intervals (24, 48, and 72, 96 hpf) until the larval stage (120 hpf) for analysis of mortality, deformities, and locomotor activity. Dead embryos were discarded immediately whenever detected. Morphological deformities in embryos/larvae were carefully examined under a stereomicroscope, and images were captured with a digital camera.
Toxicity test of arecoline
Normal sphere-stage embryos were randomly distributed into five beakers containing 50 mL of 30% Danieau buffer (control) or with different concentrations of arecoline (0.001%, 0.01%, 0.02%, and 0.04%) and a total of more than 100 embryos for each concentration. The mortality after exposure to arecoline (at the end of segmentation stage, 24 hpf) and the developmental progression at specified time points (48, 72, 96, and 120 hpf) of live embryos/larvae was assessed. Developmental toxicity, including survival rate and hatching rate, was appraised according to Wang et al.15 Malformations (e.g., pericardial edema and tissue ulceration) were also recorded among the embryos and larvae from both control and arecoline-treated groups.
Morphology deformity assessment
Live embryos from 48 to 120 hpf were observed and photographed through a stereomicroscope, and their body length and trunk-tail angle were measured and analyzed with Image J software (NIH). The body length of the zebrafish embryo is defined as the distance from the center of the retina to the end of the tail,12 and the trunk–tail angle is the angle between a line drawn through the notochord in the mid-trunk region intersecting a second line parallel from the end in the mid-tail region. The morphological data were recorded with a charge-coupled device camera fitted on a stereoscopic zoom microscope (Leica Microsystems), and images were processed with Adobe Photoshop CS3.
Video analysis of swimming performance
Surviving larvae at 120 hpf from each group were used for swimming behavior analysis. Briefly, 10 sibling larvae of a given group were transferred to a Petri dish (inner diameter: 54 mm, water temperature: 28.5°C). Groups of zebrafish were placed in the Petri dish, and the swimming movement of zebrafish larvae was recorded with a digital imaging system at 30-s intervals for 10 min. The stored images were analyzed with the Image Tool program (Tracker Software, http://cabrillo.edu/∼dbrown/tracker/webstart) to determine the swimming distance. The total distance moved was defined as the distance the fish moved, in centimeters, over 10 min.
Immunohistochemistry and confocal microscopic analysis
Whole-mount immunohistochemistry was carried out as previously described.16 The embryos were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS), blocked in 5% normal goat serum and 0.1% Tween in PBS, and incubated overnight at 4°C with the primary antibody (1:500, mouse anti-myosin heavy chain antibody F59; Hybridoma Bank). After rinsing with blocking buffer, the specimens were incubated overnight with F-411-conjugated rabbit anti-mouse IgG secondary antibody (Invitrogen). The yolk was removed, and the embryos were mounted in fluorescence-free mounting medium (Vector Laboratories) and observed using a Leica TCS SP5 confocal microscope (Leica Microsystems). The images were captured on the assumption of a few pictures that were 1 μm apart from top to bottom.
Transmission electron microscopy
The embryos and larvae were anesthetized on ice at 4°C in accordance with Standards for Animal Care approved by Animal Center, College of Medicine, National Taiwan University and then fixed by immersion in 4% paraformaldehyde in 0.05 M phosphate buffer (pH 7.2) for 12 h. After rinsing in phosphate buffer, the specimens were post-fixed with 1% aqueous osmium tetroxide for 1 h. The fixed specimens were dehydrated in a graded ethanol series and embedded in a Polybed 812-Araldite mixture (Polysciences, Inc.). Semi-thin sections were stained with toluidine blue for correlative light microscopy. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a Hitachi H-7100 electron microscope equipped with a Gatan 832 digital camera (Gatan, Inc.).
RNA extraction and reverse transcription–polymerase chain reaction assays
Total RNA was extracted from zebrafish embryos and larvae muscle (tail region) with Xprep Tissue RNA Mini Kit (X-PREP; Mundipharma) according to the manufacturer's instructions. The quality of all of the RNAs produced was evaluated by electrophoresis on a 1% agarose-formaldehyde gel, and the concentration was determined by spectrophotometry.
For cDNA synthesis, 1 μg of total RNA was reverse transcribed with oligo(dT)20 primer (Table 1) and Superscript III reverse transcriptase (Invitrogen). The resulting cDNA (0.5 μL) was used for polymerase chain reaction (PCR) amplification in a 100-μL reaction mixture containing 50 mM PCR buffer, 0.2 mM deoxynucleoside triphosphates, 1.5 mM MgCl2, 0.5 μM (each) primers, and 0.5 U of Taq DNA polymerase (Invitrogen). A GeneAmp PCR System 2700 Thermal Cycler (Applied Biosystems) was used for PCR amplification, which consisted of an initial denaturation at 96°C for 5 min; 35 cycles of denaturation at 94°C for 20 s, annealing at 55°C for 30 s, and extension at 72°C for 60 s; and a final extension at 72°C for 5 min.
Table 1.
Primers Used for Reverse Transcription-Polymerase Chain Reaction Analysis
| Gene | Accession number | Forward primer | Reverse primer |
|---|---|---|---|
| Sdhb | NM_001098740 | 5′-TTCTGGACGCACTCATCAAG-3′ | 5′-GATCTCAGCGATGGCTTTTC-3′ |
| my_cyb | AJ388456 | 5′-ATGGGGCTTCCTTCTTCTTC-3′ | 5′-TCCTCATGGAAGGACGTAGC-3′ |
| cox1 | NC_002333 | 5′-AACCCAAGACCTCACACCAG-3′ | 5′-CGGGTACCGCGTCTATTTTA-3′ |
| cox4i1 | NM_214701 | 5′-TACGGCATTTCGTCTTGTTG-3′ | 5′-CCCAGGATCCCTTCTCTTTC-3′ |
| atp5a1 | NM_001077355 | 5′-GCTTTCGCTCAGTTTGGTTC-3′ | 5′-AAGGCCTTCTCGAATTTGGT-3′ |
| atp5f1 | NM_001005960 | 5′-TGCACCAGTACCCCCTCTAC-3′ | 5′-TGATGACTGCACCAATGGAT-3′ |
| GAPDH | AY818346 | 5′-CGACCTCACCTGCCGCCTTACA-3′ | 5′-GTCATTGAGGGAGATGCCAGCG-3′ |
sdhb, succinate dehydrogenase complex, subunit B; mt-cyb, cytochrome b, mitochondrial; coxI, cytochrome c oxydase subunit I; cox4i1, cytochrome c oxydase subunit IV; atp5a1, ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit 1; atp5f1, ATP synthase, H+ transporting, mitochondrial F0 complex, subunit b, isoform 1.
The PCR products were separated on a 1.5% agarose gel and visualized by staining the gels in tris-borate-ethylenediaminetetraaceticacid buffer containing 100 ng/mL ethidium bromide (Sigma-Aldrich). GAPDH was used for the reverse transcription–polymerase chain reaction (RT-PCR) control and for semi-quantitative comparison of sdhb, my_cyb, cox1, cox4i1, atp5a1, and atp5f1 mRNA expression levels.
Isolation of mitochondria
Mitochondria were isolated from the skeletal muscle tissue in the tail region of zebra fish embryo using a Mitoiso 1 isolation kit (Sigma-Aldrich). Skeletal muscle tissue from 80 to 100 zebrafish embryos was collected and dechorionated. All of the tails were collected and homogenized in the supplied extraction buffer (10 mM HEPES buffer, pH 7.5, containing 200 mM mannitol, 70 mM sucrose, 1 mM EGTA, and 2 mg/mL bovine serum albumin). The homogenates were centrifuged at 600 g at 4°C, and supernatants were then centrifuged at 11,000 g for 10 min at 4°C. The resulting pellet was resuspended in extraction buffer, and the centrifugation steps were repeated. The final pellets representing the mitochondrial fraction were resuspended in the storage buffer supplied by the manufacturer.
Assessment of mitochondrial transmembrane potential by flow cytometry
The integrity of the mitochondrial membrane potential was assessed by measuring the intensity of fluorescent dye 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). The mitochondria fractions were incubated with the supplied JC-1 Assay buffer (Sigma) and JC-1 for 10 min at room temperature, following the manufacturer's instructions. Carbonylcyanide m-chlorophenylhydrazone at 50 μM was applied to disrupt mitochondrial transmembrane potential, and the data from these experiments served as a positive control. The samples were measured at 488 and 575 nm with flow cytometry and analyzed with Cell Quest Alias software.
Statistical analysis
All experiments were repeated at least thrice independently. All of the data are presented as the mean±standard error of the mean. One-way analysis of variance and paired Student's t-tests were performed to exam the difference between groups. A p-value <0.05 was considered statistically significant.
Results
Effect of arecoline on the survival and hatching rates of zebrafish embryos
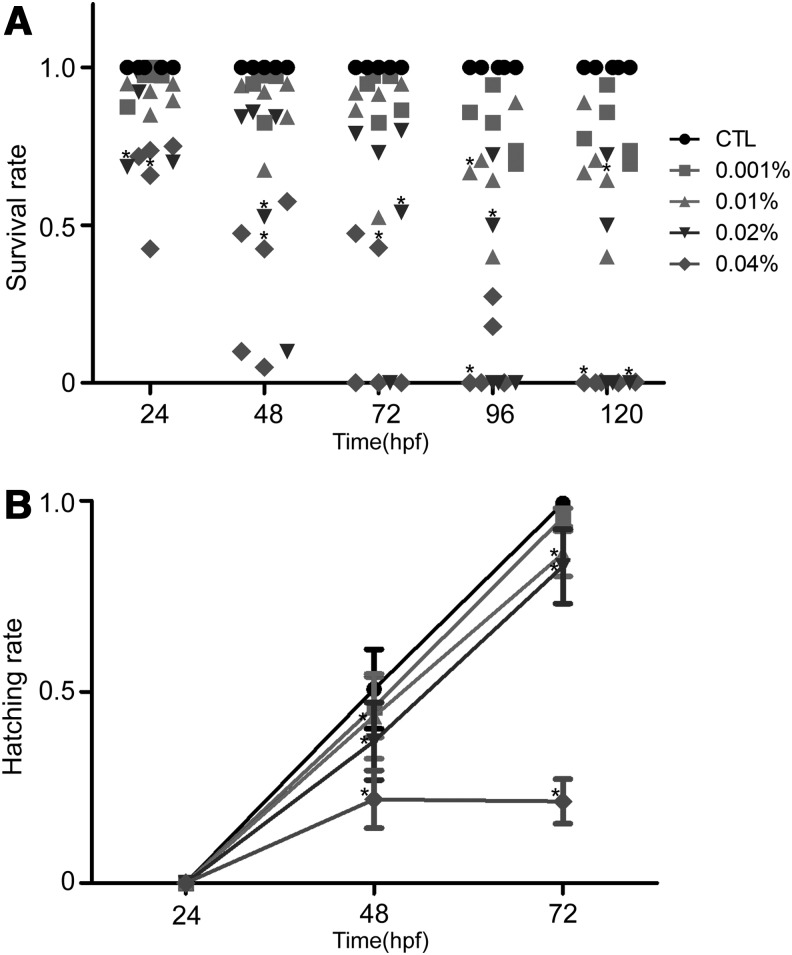
The survival and hatching rates of zebrafish embryos from the control and arecoline-exposed (0.001%, 0.01%, 0.02%, and 0.04%, from 4 to 24 hpf) groups are shown in Figure 1. From the data in Figure 1A, it is clear that the survival rate of the control group was high and that only 3% of the embryos failed to survive beyond 48 hpf. This is the typical survival rate for zebrafish under normal conditions in our laboratory. A slight decline in the survival rate was found in the group of embryos exposed to 0.001% arecoline (red square), and no significant difference in the survival rates was found between controls and 0.001% arecoline-treated group at 24 hpf. However, within 120 hpf, ∼91% and 86% of embryos survived in the presence of 0.01% and 0.02% arecoline treatment, respectively. In the 0.04% arecoline-treated group, <18% and 1% of embryos survived over 72 and 120 hpf, respectively.
FIG. 1.
Effects of arecoline treatment on embryo survival and hatching rates. The effects of arecoline on the embryo survival (A) and hatching (B) rates. The embryos were left to develop for 4 h and were then incubated in arecoline solution at different concentrations (0% [control], 0.001%, 0.01%, 0.02%, and 0.04% [w/v]) for 20 h. The embryos were then examined every 24 h. Compared with the control embryos, the survival and hatching rates of the embryos significantly decreased as the concentration of arecoline increased. (n=100, 20 embryos in five replicates each.*p<0.05).
Figure 1B showed that the hatching success rate of zebrafish embryos decreased with increasing exposure time (from 24 to 72 hpf), as well as with increasing concentrations of arecoline (from 0.01% to 0.04%) between 48 and 72 hpf. These results clearly indicated that the survival and the hatching rate of arecoline-exposed zebrafish embryos decreased with arecoline exposure.
Effect of arecoline on the external appearance of developing zebrafish embryos
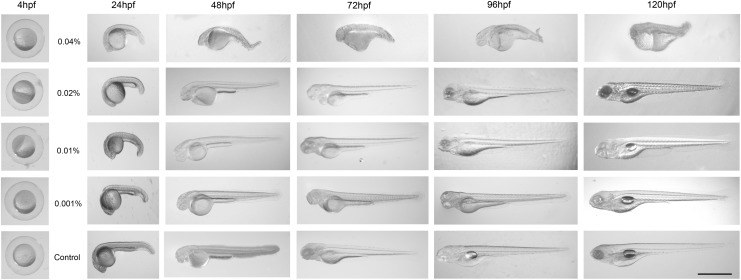
External morphological changes of developing zebrafish embryo after arecoline treatment were observed at 24, 48, 72, 96, and 120 hpf (Fig. 2). These observations revealed that the hatched larvae developed normally in the control group (Fig. 2, lowest panel). At 0.001% or higher concentrations of arecoline exposure, zebrafish embryos were smaller than those of the control group, exhibited a high incidence of pericardial edema, and displayed significant axial-tail curvature and external morphological malformations (Fig. 2). Our study demonstrated that zebrafish embryos exposed to low arecoline concentrations (i.e., 0.001% and 0.01%) showed low malformation rates (0.001%: 6/95, 0.01%: 8/82). However, when treating with higher concentrations of arecoline (0.02% and 0.04%), more than 50% (0.002%: 36/66) and 99.9% (0.04%: 45/45) of zebrafish embryos exhibited an abnormal external appearance. We also noted that these defects increased in severity and frequency as the concentration of arecoline increased.
FIG. 2.
Gross morphological changes after arecoline treatment. The embryos were exposed to 0%–0.04% arecoline from 4 to 24 hours post fertilization (hpf) and then allowed to develop in normal arecoline-free egg water. Images of living zebrafish were acquired at four time points, every 24 h: 1 day post treatment (d.p.t.) (48 hpf), 2 d.p.t. (72 hpf), 3 d.p.t. (96 hpf), and 4 d.p.t. (120 hpf). Embryos that were exposed to higher arecoline concentrations exhibited altered tail length, curved spines, and pericardial edema. Scale bar: 1mm.
Arecoline toxicity on body length, growth pattern, and locomotion activity of zebrafish embryos
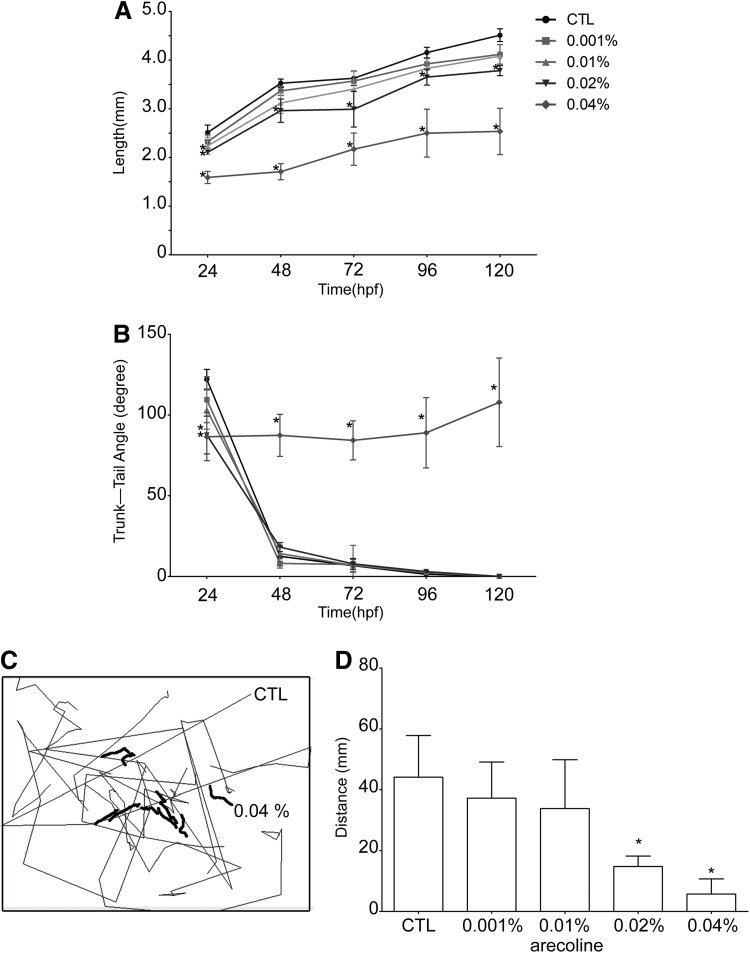
Two developmental parameters, the total body length and trunk–tail angle, were measured to investigate the influence of hatching delay on the arecoline-exposed zebrafish embryos. The effects of arecoline on total body length and the trunk–tail angle of zebrafish embryo are shown in Figure 3A and B, respectively. The data in Figure 3A indicated that body length was markedly decreased after arecoline exposure. At 24 hpf, the average body length of control embryos was 2.51±0.16 mm, but that of arecoline-treated embryos decreased with increasing concentrations of arecoline (0.001%: 2.32±0.14 mm; 0.01%: 2.24±0.18 mm; 0.02%: 2.11±0.01 mm; and 0.04%: 1.59±0.12 mm). Furthermore, the average body length of hatched larvae at 120 hpf (0.02%: 3.97±0.24 mm, 0.04%: 3.12±0.32 mm) was significantly shorter than that of the controls (4.51±0.30 mm, p<0.05).
FIG. 3.
The effects of arecoline on locomotor activity. The effects of different concentrations of arecoline on the development (A, B) and locomotor activity (C, D) of zebrafish embryos from 24 to 120 hpf. (A) Mean body length in mm, and (B) trunk–tail angle in degrees, of control (n=50) and arecoline-treated (n=50) zebrafish embryos examined at 24, 48, 72, 96, and 120 hpf. Zebrafish swimming patterns (C) and their swimming distance (D) in mm of the control (n=10) and arecoline-treated larvae (n=10) at 120 hpf. (*p<0.05).
During zebrafish development, the body axis straightens, and the trunk–tail angle decreases between 24 and 72 hpf. In this study, the measured trunk–tail angle is defined as a function of hours of zebrafish development,17 and Figure 3B demonstrates that the trunk–tail angle was significantly altered in arecoline-treated embryos. At 24 hpf, when somite formations are complete, the trunk–tail angle of each arecoline-treated embryo was significantly smaller than that of the controls (data in degrees, CTL: 122.1±6.1; 0.001%: 109.5±14.3; 0.01%: 103.4±12.1; 0.02%: 87.6±11.7; and 0.04%: 86.6±14.9, p<0.05). This result may imply retarded growth of zebrafish embryo after arecoline exposure. At 48 hpf and thereafter, zebrafish embryos treated with low concentrations of arecoline (0.001%, 0.01%, and 0.02%) showed no obvious changes and maintained their straight morphology (data in degrees: CTL: 12.5±5.9; 0.001%: 8.1±2.9; 0.01%: 14.2±2.8; and 0.02%: 18.2±2.8). However, as the concentrations of arecoline increased to 0.04%, the mean head–trunk angle of the embryos was significantly greater than in the control group and the other arecoline-exposure groups (data in degrees: 0.04%: 87.4±13.1, p<0.05). We conclude that for both body length and trunk–tail angle measurements, low concentrations of arecoline exposure retard the development of the zebrafish embryos more severely and conspicuously than treatments with higher concentrations of arecoline.
In this study, we did not observe any erratic movement from the control group; however, alterations of swimming activity were observed in arecoline-treated zebrafish embryos. Larvae from 0.02% to 0.04% displayed balance-defective swimming behavior at ∼120 hpf. At this stage, wild-type larvae were active; they were able to change swimming directions spontaneously and direct their swimming toward targets. Compared with the control group, the 0.02% and 0.04% arecoline exposure larvae were not active, and they rested either on their side or on their back. Some of the 0.02% and most of the 0.04% arecoline exposure larvae followed a corkscrew-like path while swimming. The frequency and locomotive activity of tail beats in the groups treated with arecoline were significantly decreased, and these effects were more significant with increasing concentrations of arecoline (Supplementary Video S1; Supplementary Data are available online at www.liebertpub.com/zeb). Figure 3C illustrates traces of swimming movement of zebrafish larvae for 10 min at 120 hpf from a control (black line) and a surviving larvae exposed to 0.04% arecoline (red line). Figure 3D shows the total distance moved of the zebrafish larvae for 10 min at 120 hpf after arecoline exposure. We observed that zebrafish larvae hatched from embryos exposed to 0.02% and 0.04% arecoline displayed significant impairment of swimming movement and a dramatic decrease in the swimming distance at 120 hpf compared with the control group (control: 44.1±13.7 mm; 0.001%: 37.2±11.9 mm; 0.01%: 33.8±16.1 mm; 0.02%: 14.8±3.4 mm; and 0.04%: 5.72±5.1 mm).
Arecoline decreased myosin protein accumulation in zebrafish embryos
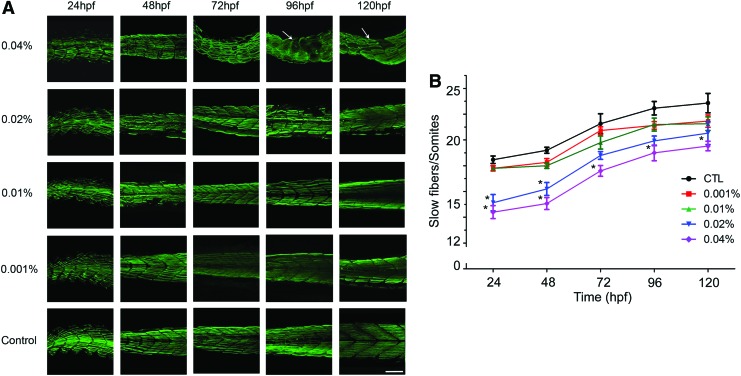
We examined the expression pattern of a sarcomere protein, myosin heavy chain (MYH), in whole-mount zebrafish embryo immunofluorescence preparations labeled with monoclonal antibody F59, which recognizes a sarcomere epitope in adaxial skeletal muscle fibers in the zebrafish tail.18 Figure 4 demonstrates the expression of MYH and the developmental pattern of somites in zebrafish embryos. The lowest panel of Figure 4 illustrates the normal development and regular organization of somite muscle fibers and chevron-shaped somite boundaries of the control group from 24 to 120 hpf. At 24 hpf (Fig. 4, left column), zebrafish embryos displayed disorganized pattern of the tail somite after arecoline exposure, and this disorganization is more severe with increased arecoline concentration (first column of Fig. 4 from bottom). At 48 hpf and thereafter, except the for the 0.04% arecoline-treated group (Fig. 4, upper panel), the muscle fiber alignment appeared rather normal and regular in all other groups. In the 0.04% arecoline-treated group (Fig. 4, upper panel), however, the adaxial muscle fibers were disorganized and randomly arranged, and with the increasing time of arecoline incubation, the muscle fiber arrangement was more disorganized. We quantified the number of slow-muscle fibers per somite in tail somites and found that arecoline significantly reduced the number of fibers at 24–120 hpf (Fig. 4B).
FIG. 4.
Morphologically abnormal muscle fibers in arecoline-treated zebrafish embryos. (A) Lateral views of whole-mount immunofluorescence staining of slow myosin heavy chain in zebrafish embryos at 24, 48, 72, 96, and 120 hpf. The embryos are oriented in a cranial to caudal direction, from right to left. The whole mounts were viewed by confocal microscopy. Arrows indicate areas where twisted or broken fibers have been induced by the high concentration of arecoline (0.04%). Scale bar: 60 μm. (B) The number of slow muscle fibers per somite in the tail region between 24 and 120 hpf. (n=10). (*p<0.05). Color images available online at www.liebertpub.com/zeb
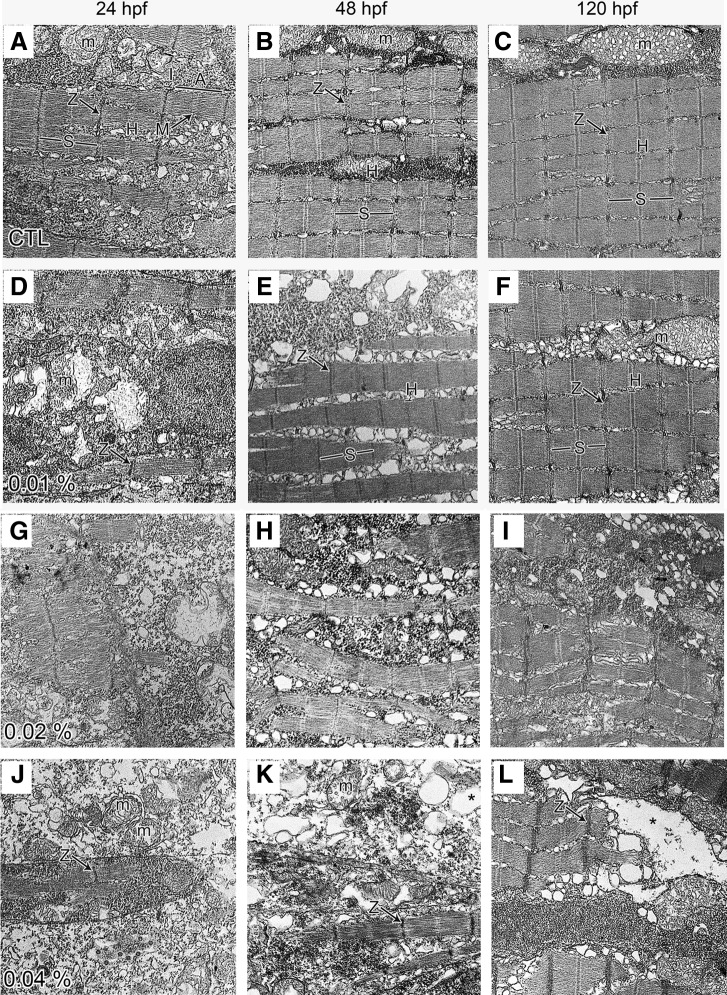
Effect of arecoline on the ultrastructure of the zebrafish tail myofibrils
To understand the effect of arecoline on the skeletal muscle and locomotive activity of developing zebrafish, we examined the ultrastructural changes of embryos with various concentrations of arecoline incubation. In control zebrafish embryos, the ultrastructure of the developing myofibrils appeared normal and well aligned from 24 to 120 hpf. At 24 hpf (Fig. 5A), myofibrils were 0.72±0.04 μm in diameter, and thin and thick myofilaments, as well as Z-discs, A-bands, I-bands, H zones, and M lines were clearly visible. The inter-fibrillar space/sarcoplasm was filled with numerous mitochondria (m), abundant free ribosomes and glycogen particles, short profiles of endoplasmic reticulum, and some vesicular membrane profiles. At 48 hpf, control embryos exhibited a well-defined muscle fiber structure, with highly organized bundles of myofibrils surrounded by mitochondria and intersected with the complex membrane system, mainly sarcoplasmic reticulum, of the contractile apparatus (Fig. 5B). At 120 hpf, normal zebrafish skeletal muscle displayed tightly juxtaposed arrays of myofibrils that were extremely uniform and precisely aligned. The myofibrils were more tightly packed and had a mature morphology, exhibiting discernible and clear substructures, namely, A-, I-, and H-bands and M lines (Fig. 5C). Consistent with previous ultrastructural studies of muscle fiber architecture,19 the mitochondria were typically found in pairs, positioned on either side of the Z-disc in each I band. In addition, there was massive accumulation of ribosomes, glycogen particles, and endoplasmic reticulum profiles that were tightly packed throughout the space between the myofibrils. This finding is consistent with the orientation observed during normal development and revealed their largely mature morphology and phenotype.
FIG. 5.
Ultrastructural analysis of arecoline-induced alterations in zebrafish embryos. Electron micrographs of tail muscle myofibrils from zebrafish embryos treated with different concentrations of arecoline: control [(A) 24 hpf, (B) 48 hpf and (C) 120 hpf], 0.01% arecoline [(D) 24 hpf, (E) 48 hpf and (F) 120 hpf], 0.02% arecoline [(G) 24 hpf, (H) 48 hpf and (I) 120 hpf] or 0.04% arecoline [(J) 24 hpf; (K) 48 hpf and (L) 120 hpf). (A–C) Compact sarcoplasm with mitochondria (m) surround the densely packed and highly organized myofibrils. S, sarcomere; A, A-band; Z, Z-line; H, H-band. (D–F) Well-organized myofibrils were not evident. Small patches of disorganized fibers and mitochondria are scattered throughout the cytoplasm. In (G) and (H), it should be noted that the intercellular spaces (asterisks) are greatly enlarged compared with 0.02% arecoline-incubated embryos. The 0.02% arecoline causes defects in muscle fibers, although defects are not as bad as those in embryos with 0.04% arecoline treatment (J–L). Those defects lead to the shortage of length and decrease of swimming ability of embryos. Scale bars: 1 μm.
In the 0.01% or 0.04% arecoline-treated zebrafish embryos (Fig. 5D–I), the disintegrated and poorly aligned myofibrils were smaller in diameter (0.56±0.08 μm for 0.04%). In addition, the myofibrils contained fewer thick and thin filaments, the A- and I- bands were barely visible, and the Z-line density was obviously decreased. In addition, mitochondria swelling and cytoplasmic vacuoles were frequently observed in the sarcoplasm. There were significantly fewer and shorter myofibrils compared with controls, and disorganization of myofibrils was more severe in embryos treated with 0.04% arecoline (Fig. 5J–L). At 24 hpf (Fig. 5J), myofilaments were widely dispersed throughout the cells, sarcomeric organization was less discernible, more spaces were observed between myofibrils, and the mitochondria were swollen, with a few cristae. At 48 and 120 hpf (Fig. 5K, L), the general disorganization of myofibrils was more severe in embryos incubated with 0.04% arecoline. Moreover, the ultrastructure of the myofibers obtained from embryos treated with 0.04% arecoline showed highly distorted myofibrils, and there were irregular-shaped myoseptal boundaries and sarcomeres in most of the somites. The ultrastructure of embryos treated with 0.01% arecoline was not disrupted to the degree observed in 0.04%-treated embryos; rather, myofibril development was only delayed compared with normal embryos. The structures of myofibrils at 48 hpf (Fig. 5E) or 120 hpf (Fig. 5F) were similar to the structures observed at 24 hpf (Fig. 5A) and 48 hpf (Fig. 5B) in CTL embryos, respectively.
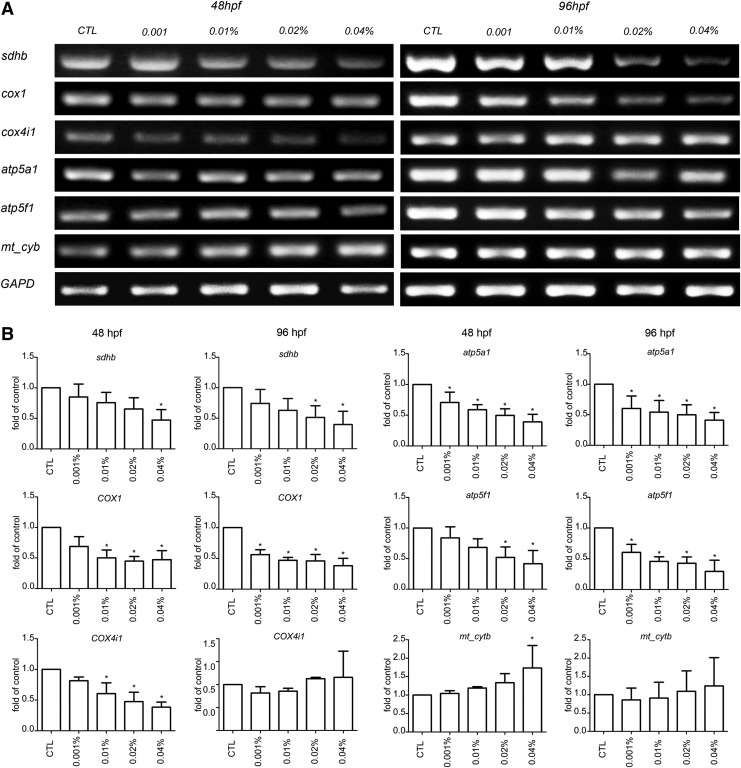
Analysis of mitochondrial functional gene expression
The number of slow muscle fibers continues to increase after 24 hpf (Fig. 4) in normal fish development. However, in this study, significant locomotor activity attenuation (Fig. 3C, D) and sarcomeric mitochondrial alterations (Fig. 5) were observed in arecoline-treated embryos. We, therefore, examined the expression level of mitochondrial functional genes (sdhb, cox1, cox4i1, atp5a1, atp5f1, and mt_cytb) by RT-PCR. GAPDH was used as the internal control in response to arecoline exposure (Fig. 6A, B). Figure 6 showed that the expression levels of the sdhb, cox1, cox4i1, atp5a1, and atp5f1 genes decreased significantly with increasing arecoline concentrations, especially at 0.04%. However, the expression level of mt_cytb was significantly increased with increasing arecoline concentrations, with a notable increase at 0.04%. In addition, a concentration-dependent, but not proportional, effect was observed with regard to the cDNA expression levels of mitochondrial function genes.
FIG. 6.
The expression of zebrafish mitochondrial-associated genes in the developing embryos and larval muscle, as determined by reverse transcription–polymerase chain reaction (RT-PCR). (A) RT-PCR analysis of the mRNA expression levels of sdhb, cox1, cox4i1, atp5a1, atp5f1, and mtcytb in different developmental stages in zebrafish with or without arecoline treatment. GAPDH was used as an internal control to evaluate the relative transcript levels. (B) mRNA expression levels in different stages of developing zebrafish with or without arecoline treatment. (n=3, mean±standard error of the mean [SEM], *p<0.05).
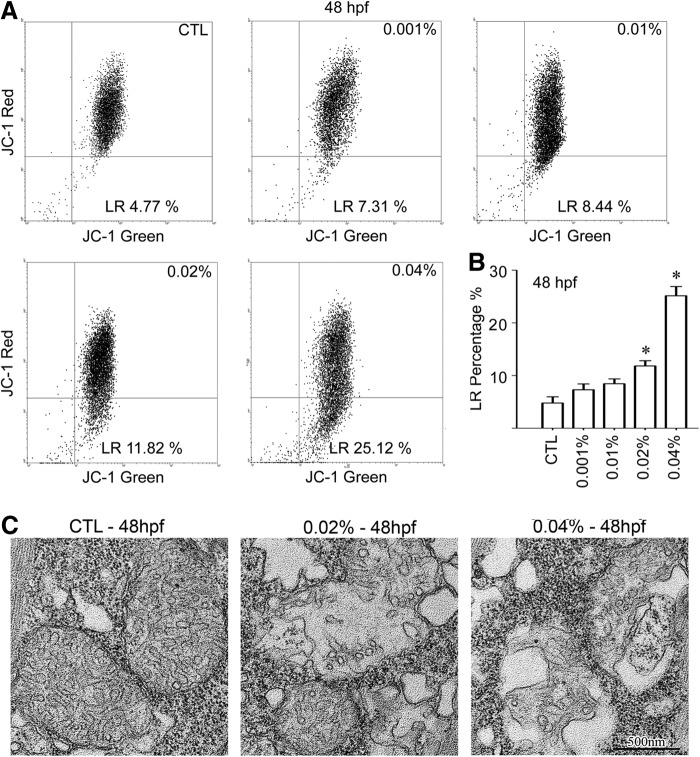
Changes in the mitochondrial membrane potential (Δψm) in response to arecoline treatment in zebrafish
To examine Δψm loss, mitochondria isolated from larvae tail region were stained with JC-1. JC-1 aggregates and exhibited a red fluorescence at high membrane potentials (Δψm) in the mitochondria, dissociating into green-fluorescing monomers when Δψm is lost. As displayed in dot plots (Fig. 7A), 20 hpf arecoline exposure caused a marked decrease of FL2-H fluorescence (red). Decreased potential (i.e., decreased red fluorescence) is represented in the bottom right quadrant, with these cells considered to be those undergoing mitochondrial depolarization at 48 hpf stage. Figure 7B shows the percentage of cells with incomplete mitochondrial membrane depolarization out of the total population. The data were then quantitated from at least three independent experiments. As shown in Figure 7B, treating larvae with different concentrations of arecoline for 20 hpf significantly increased mitochondrial membrane depolarization compared with uninjured controls (4.77±2.66%) at 48 hpf. This result indicates that arecoline treatment significantly reduced the Δψm in a dose-dependent manner, especially at 0.02% and 0.04% (48 hpf, 0.001%: 7.31±2.43, 0.01%: 8.44±2.07, 0.02%: 11.82±2.22, 0.04%: 25.12±3.92). In addition, arecoline modestly increased the mitochondrial membrane depolarization in embryos treated until 96 hpf, and the effects of arecoline were significant (96 hpf, CTL: 0.66±1.57, 0.001%: 3.33±3.36, 0.01%: 4.88±2.54, 0.02%: 6.57±3.99, 0.04%: 7.01±2.92, p=0.045 vs. untreated, respectively). These data suggest that the mitochondrial function was impaired with arecoline treatment, and these changes are clearly linked to mitochondria ultrastructural changes (Fig. 7C).
FIG. 7.
Determination of the effect of arecoline on JC-1 staining patterns. The effect of arecoline on the loss of mitochondrial membrane potential in isolated mitochondria, as determined by JC-1 staining. The mean JC-1 fluorescence intensity was detected using fluorescence-activated cell sorting (FACS) analysis. A loss of mitochondrial membrane potential (ΔΨm) was demonstrated by the change in JC-1 fluorescence from red (JC-1 aggregates) to green (JC-1 monomers). (A) Analysis of mitochondrial membrane potential (ΔΨm) in each treatment group at 48 hpf. (B) The quantification of FACS analysis. The data are shown as the mean±SEM from three independent experiments. *p<0.01 versus control group. (C) The mitochondrial ultrastructure is shown in the tail region at 48 hpf. Representative transmission electron micrographs (×20,000).
Discussion
Effects of arecoline on the survival and growth of zebrafish development
The zebrafish is a good model vertebrate for studying the effects of drug toxicity on development.20 In this study, we used zebrafish embryos to explore the effects of arecoline on growth, motor development, and somitic muscle fibers. These analyses were performed from the blastula sphere stage to segmentation stage. Our results showed that arecoline treatment decreased the survival rate and induced growth retardation in zebrafish embryos. The results also indicated that the toxicity of arecoline becomes more discernible at higher concentrations. This phenomenon is consistent with results of previously published studies in other animal models, such as mouse9 and chick embryos.10,11 In chicken embryos injected with arecoline on day 2 of incubation, the arecoline induced developmental abnormalities, reduced body size, scanty feathering, generalized edema with light body color, and shortened lower beak and clubfoot. The effect of arecoline on mouse and chick embryos also caused decreased viability and a reduction in the body weight of fetuses.10,21
We observed a slow heart rate and decreased viability with arecoline at concentrations of 0.001%, 0.01%, 0.02%, and 0.04% after short-term exposure (20 h, from 4 to 24 hpf). Similar effects were observed by Chang et al.13 using zebrafish embryos incubated with medium containing different concentrations of arecoline (0.01%, 0.02%, and 0.04%) during an experimental period till 72 hpf. These authors also reported that most of the embryos did not survive past 72 hpf. In contrast to their results, we demonstrated a longer survival period of approximately 120 hpf and a higher survival rate (Fig. 1). This result may be due to the short period of exposure and lower concentration of arecoline treatment in this study. In addition, we demonstrated that viability was lower and growth retardation was more pronounced with increasing concentrations of arecoline. Compared with a previous study by Chang et al.,13 a relatively lower concentration of arecoline (0.001%–0.04%) and a shorter exposure period, from the blastula sphere stage to the segmentation stage (20 h, from 4 to 24 hpf), were applied to zebrafish embryos in this study. These conditions model a human female who has the betel nut-chewing habit and is not aware of her pregnancy in the early period. In this case, the embryos may be exposed to arecoline for only a short period.
Locomotion and locomotive activity defects in arecoline-exposed zebrafish
Behavioral changes can affect how an animal senses and responds to environment; therefore, behavioral changes are sensitive biomarkers of environmental stresses in zebrafish.22,23 Zebrafish embryos exhibit three locomotive behaviors: spontaneous, alternating contractions of the trunk, vigorous coiling of the body and swimming.24–26 The swimming activity test of zebrafish embryos in a small well was easily performed and allowed for the determination of neuromuscular behavioral effects for a wide variety of toxicants over a large dose range. Previous studies have investigated the swimming behavior of zebrafish after various toxin exposures, such as chronic sublethal dietary selenomethionine,26 domoic acid,27 perfluorooctanesulfonate,28 alcohol,29 and sodium hypochlorite.30 All of these chemicals can alter both behavioral and physiological responses in adult zebrafish. This study examined the behavioral effects of arecoline on zebrafish 120 hpf larvae and showed significant differences in swimming patterns, speed, and distance after arecoline treatment (Fig. 3C, D). It is concluded that zebrafish embryos exposed to arecoline exhibit impeded locomotive activity.
Muscle morphology
The zebrafish embryo is an important model for the study of vertebrate muscle development.31 Since locomotor activity was significantly reduced after arecoline treatment, we examined the somitic muscle morphology of zebrafish embryos exposed to different concentrations of arecoline. We found marked changes in muscle fiber arrangement (Fig. 4) and ultrastructural morphology with the increasing arecoline concentration (Fig. 5). Moreover, cytoplasmic vacuoles; swollen mitochondria; and significantly fewer, shorter, and less-oriented myofibrils in the cytoplasm were noted in the somitic muscle fibers of arecoline-treated embryos compared with the controls (Fig. 5). This result suggested that arecoline affected either the arrangement or the integrity of muscle fibers and thus locomotor activity.
Previous in vivo studies showed that the knockout of myogenesis-associated genes or toxin exposure leads to disordered muscle development and significantly reduced embryo survival.32–34 Our result also shows that the survival rate decreased as the arecoline concentration increased (Fig. 1). Accordingly, we speculate that arecoline inhibited myogenesis process and suggest that myogenesis plays a key role in zebrafish survival. However, future experiments should be performed to determine whether arecoline affects the expression of myogenesis-associate genes. Moreover, it is worth noting that the morphology of zebrafish embryos exposed to different concentrations arecoline for 48 hpf exhibited more severe changes than those treated for 120 hpf based on electron micrograph analysis. We can only speculate that the larvae that survived for more than 120 hpf in arecoline, especially in high concentrations (0.02% or 0.04%), may have been strong enough to tolerate the extremely toxic environment, thereby exhibiting less serious muscle structure and motor activity impairment.
Recent studies demonstrated that arecoline is cytotoxic to mouse myoblast C2C12 cells. Arecoline inhibits myotube formation by reducing the number of nuclei in each myotube and suppresses the expression of MYH and myogenin during myogenic differentiation of C2C12 cells.35 Impaired sarcomere development with greatly reduced myofibril numbers was also noted in zebrafish models of arthrogryposis with MYBPC1 mutations.36 Similar observations were found in this study in arecoline-treated zebrafish. To the best of our knowledge, no in vivo study has shown that arecoline suppresses the expression of MYH during myogenesis in zebrafish. This is the first report to demonstrate that short-term arecoline treatment (from 4 to 24 hpf) is capable of altering the ultrastructural morphology of differentiating somite muscle fibers and impairing the locomotive function of zebrafish in vivo. These findings may help explain why areca nut-chewing during pregnancy often results in lower birth weight and growth-retarded infants.
Mitochondria
Skeletal muscle is a tissue with a high mitochondria content and is strongly reliant on oxidative phosphorylation for energy production. Mitochondria also play an important role in maintaining a low sarcoplasmic calcium concentration.37 The functional activities of skeletal muscle have been associated with the capacity of mitochondria. Furthermore, several studies have highlighted that mitochondria play a role in regulating myogenic differentiation.38,39 Therefore, it is possible that arecoline may lead to the inhibition of myogenic differentiation by affecting mitochondria.
Mitochondria are vital and highly sensitive organelles, responding rapidly to cellular activity changes. Ultrastructural studies demonstrated that muscle inactivity is intimately associated with significant changes in mitochondrial morphology, including swelling and disruption of the cristae, reductions in mitochondrial number, irregular mitochondrial shapes, and disrupted myofibril organization.40,41 Mitochondrial respiratory dysfunction, decreased expression levels of mitochondrial proteins (functional enzymes), and increased mitochondrial reactive oxygen species production have also been observed in defective muscles.42–45 All of these studies confirmed that prolonged skeletal muscle inactivity results not only in morphological alterations but also in functional changes in mitochondria. In this study, we observed that zebrafish embryos exposed to arecoline exhibited abnormal mitochondrial morphology and swelling and disappearance of mitochondrial cristae at the ultrastructural level. These results indicate altered mitochondria function in terms of activity and enzyme activity. Therefore, it is reasonable to speculate that the expression levels of mitochondrial functional enzymes (or electron transport chain complex) would be altered in the muscle fibers after arecoline treatment.
Many functional enzymes, such as NADH dehydrogenase, succinate dehydrogenase, ubiquinol-cytochrome c oxidoreductase, cytochrome c, cytochrome c oxidase, and ATP synthase,46 are localized in the inner mitochondria membrane and constitute a multi-enzyme energy-generating machinery. These enzymes are closely related to the functional activity of mitochondria; therefore, the gene expression levels of these enzymes were detected by RT-PCR after arecoline treatment in this study. We observed that the expression levels of mitochondrial respiratory chain-associated genes were altered after arecoline exposure (Fig. 6). The carbocyanine dye (JC-1) assay, which is used to reflect mitochondrial membrane potential, was also performed. We observed that arecoline caused a marked decrease in the fluorescence of FL2-H (red) (Fig. 7A), indicating lower mitochondria function.
These results were indicative of a loss of mitochondrial potential and an alteration in the activity of mitochondrial metabolic genes in zebrafish embryos/larvae after arecoline exposure. We postulate that mitochondrial dysfunction and pro-oxidative effects could be involved in the disrupted myogenesis and somite myofilament disorganization after arecoline exposure in zebrafish embryos. Thus, this exposure impaired muscular contraction and the swimming performance of zebrafish embryos.
In recent years, a variety of animal models were used to validate arecoline impact on the embryo and it is know that arecoline is a toxic chemical for development. Here, we provide the evidence that arecoline may induce mitochondria of skeletal muscle dysfunction, alter the muscle of embryo development, and impair swimming activity. Although there is no evidence that arecoline could hurt development of other tissues, from the retardation of embryo development, we expect there may be also effects on the central nervous system or cardiovascular development and this issue is under further investigation.
In summary, our study established that a relatively short early exposure of zebrafish embryos to arecoline (from 4 to 24 hpf) leads to an increased frequency of malformations and other defects in the fry, including a reduced hatching rate, decreased survival, changes in body length, trunk-tail angle malformation, alterations of the somite muscle fiber arrays, and the ultrastructure of myofibrils. These malformations might subsequently modify the basal swimming pattern and swimming distance of zebrafish larvae. Furthermore, we found using genetic, functional, and morphological assays that arecoline treatment resulted in larvae mitochondrial dysfunction in the muscle. Furthermore, these alterations are intimately related to the function and motor activity of somitic muscle fibers. Here, we confirm the developmental toxicity of arecoline and conclude that arecoline is detrimental and damaging to exposed organisms.
Supplementary Material
Acknowledgment
Funding provided by National Science Council (NSC 101-2320-B-002-008), (NSC 101-2320-B-002-020-MY3).
Disclosure Statement
No competing financial interests exist.
References
- 1.Arjungi KN. Areca nut: a review. Arzneimittelforschung 1976;26:951–956 [PubMed] [Google Scholar]
- 2.Nery R. The metabolic interconversion of arecoline and arecoline 1-oxide in the rat. Biochem J 1971;122:503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boucher BJ, Mannan N. Metabolic effects of the consumption of Areca catechu. Addict Biol 2002;7:103–110 [DOI] [PubMed] [Google Scholar]
- 4.Winstock AR, Trivedy CR, Warnakulasuriya KA, Peters TJ. A dependency syndrome related to areca nut use: some medical and psychological aspects among areca nut users in the Gujarat community in the UK. Addict Biol 2000;5:173–179 [DOI] [PubMed] [Google Scholar]
- 5.de Costa C, Griew AR. Effects of betel chewing on pregnancy outcome. Aust N Z J Obstet Gynaecol 1982;22:22–24 [DOI] [PubMed] [Google Scholar]
- 6.Yang MS, Lee CH, Chang SJ, Chung TC, Tsai EM, Ko AM, et al. . The effect of maternal betel quid exposure during pregnancy on adverse birth outcomes among aborigines in Taiwan. Drug Alcohol Depend 2008;95:134–139 [DOI] [PubMed] [Google Scholar]
- 7.Senn M, Baiwog F, Winmai J, Mueller I, Rogerson S, Senn N. Betel nut chewing during pregnancy, Madang province, Papua New Guinea. Drug Alcohol Depend 2009;105:126–131 [DOI] [PubMed] [Google Scholar]
- 8.Guh JY, Chuang LY, Chen HC. Betel-quid use is associated with the risk of the metabolic syndrome in adults. Am J Clin Nutr 2006;83:1313–1320 [DOI] [PubMed] [Google Scholar]
- 9.Liu ST, Young GC, Lee YC, Chang YF. A preliminary report on the toxicity of arecoline on early pregnancy in mice. Food Chem Toxicol 2011;49:144–148 [DOI] [PubMed] [Google Scholar]
- 10.Paul K, Moitra PK, Mukherjee I, Maity C, Ghosal SK. Teratogenicity of arecoline hydrobromide on developing chick embryos: a preliminary report. Bull Environ Contam Toxicol 1999;62:356–362 [DOI] [PubMed] [Google Scholar]
- 11.Bogdanov OV. The effect of arecoline on the cardiac activity of the chick of the chick embryo at various stages of its development. Bull Exp Biol Med 1961;50:1153–1157 [Google Scholar]
- 12.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn 1995;203:253–310 [DOI] [PubMed] [Google Scholar]
- 13.Chang BE, Liao MH, Kuo MY, Chen CH. Developmental toxicity of arecoline, the major alkaloid in betel nuts, in zebrafish embryos. Birth Defects Res A Clin Mol Teratol 2004;70:28–36 [DOI] [PubMed] [Google Scholar]
- 14.Westerfield M, Wegner J, Jegalian BG, DeRobertis EM, Puschel AW. Specific activation of mammalian Hox promoters in mosaic transgenic zebrafish. Genes Dev 1992;6:591–598 [DOI] [PubMed] [Google Scholar]
- 15.Wang K, Ma J, He M, Gao G, Xu H, Sang J, et al. . Toxicity assessments of near-infrared upconversion luminescent LaF3:Yb,Er in early development of zebrafish embryos. Theranostics 2013;3:258–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development 1989;105:61–74 [DOI] [PubMed] [Google Scholar]
- 17.Kashyap B, Frederickson LC, Stenkamp DL. Mechanisms for persistent microphthalmia following ethanol exposure during retinal neurogenesis in zebrafish embryos. Vis Neurosci 2007;24:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devoto SH, Melançon E, Eisen JS, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development 1996;122:3371–3380 [DOI] [PubMed] [Google Scholar]
- 19.Steffen LS, Guyon JR, Vogel ED, Howell MH, Zhou Y, Weber GJ, et al. . The zebrafish runzel muscular dystrophy is linked to the titin gene. Dev Biol 2007;309:180–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci 2005;86:6–19 [DOI] [PubMed] [Google Scholar]
- 21.Sinha A, Rao AR. Embryotoxicity of betel nuts in mice. Toxicology 1985;37:315–326 [DOI] [PubMed] [Google Scholar]
- 22.Bégout Anras Marie-Laure, Paul LJ. Measuring cultured fish swimming behaviour: first results on rainbow trout using acoustic telemetry in tanks. Aquaculture 2004;240:175–186 [Google Scholar]
- 23.Cazenave J, Bistoni Mde L, Zwirnmann E, Wunderlin DA, Wiegand C. Attenuating effects of natural organic matter on microcystin toxicity in zebra fish (Danio rerio) embryos—benefits and costs of microcystin detoxication. Environ Toxicol 2006;21:22–32 [DOI] [PubMed] [Google Scholar]
- 24.Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol 1998;37:622–632 [DOI] [PubMed] [Google Scholar]
- 25.Liu DW, Westerfield M. Function of identified motoneurones and co-ordination of primary and secondary motor systems during zebra fish swimming. J Physiol 1988;403:73–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomas JK, Janz DM. Dietary selenomethionine exposure in adult zebrafish alters swimming performance, energetics and the physiological stress response. Aquat Toxicol 2011;102:79–86 [DOI] [PubMed] [Google Scholar]
- 27.Tiedeken JA, Ramsdell JS, Ramsdell AF. Developmental toxicity of domoic acid in zebrafish (Danio rerio). Neurotoxicol Teratol 2005;27:711–717 [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Huang C, Wang L, Ye X, Bai C, Simonich MT, et al. . Toxicity, uptake kinetics and behavior assessment in zebrafish embryos following exposure to perfluorooctanesulphonicacid (PFOS). Aquat Toxicol 2010;98:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sylvain NJ, Brewster DL, Ali DW. Zebrafish embryos exposed to alcohol undergo abnormal development of motor neurons and muscle fibers. Neurotoxicol Teratol 2010;32:472–480 [DOI] [PubMed] [Google Scholar]
- 30.Nimkerdphol K, Nakagawa M. Effect of sodium hypochlorite on zebrafish swimming behavior estimated by fractal dimension analysis. J Biosci Bioeng 2008;105:486–492 [DOI] [PubMed] [Google Scholar]
- 31.Stickney HL, Barresi MJ, Devoto SH. Somite development in zebrafish. Dev Dyn 2000;219:287–303 [DOI] [PubMed] [Google Scholar]
- 32.Chen YH, Huang YH, Wen CC, Wang YH, Chen WL, Chen LC, et al. . Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicol Teratol 2008;30:440–447 [DOI] [PubMed] [Google Scholar]
- 33.Hassoun E, Kariya C, Williams FE. Dichloroacetate-induced developmental toxicity and production of reactive oxygen species in zebrafish embryos. J Biochem Mol Toxicol 2005;19:52–58 [DOI] [PubMed] [Google Scholar]
- 34.Drachman DB. Atrophy of skeletal muscle in chick embryos treated with botulinum toxin. Science 1964;145:719–721 [DOI] [PubMed] [Google Scholar]
- 35.Chang YF, Liu TY, Liu ST, Tseng CN. Arecoline inhibits myogenic differentiation of C2C12 myoblasts by reducing STAT3 phosphorylation. Food Chem Toxicol 2012;50:3433–3439 [DOI] [PubMed] [Google Scholar]
- 36.Ha K, Buchan JG, Alvarado DM, McCall K, Vydyanath A, Luther PK, et al. . MYBPC1 mutations impair skeletal muscle function in zebrafish models of arthrogryposis. Hum Mol Genet 2013;22:4967–4977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gopalakrishnakone P, Dempster DW, Hawgood BJ, Elder HY. Cellular and mitochondrial changes induced in the structure of murine skeletal muscle by crotoxin, a neurotoxic phospholipase A2 complex. Toxicon 1984;22:85–98 [DOI] [PubMed] [Google Scholar]
- 38.Seyer P, Grandemange S, Rochard P, Busson M, Pessemesse L, Casas F, et al. . P43-dependent mitochondrial activity regulates myoblast differentiation and slow myosin isoform expression by control of Calcineurin expression. Exp Cell Res 2011;317:2059–2071 [DOI] [PubMed] [Google Scholar]
- 39.Seyer P, Grandemange S, Busson M, Carazo A, Gamaleri F, Pessemesse L, et al. . Mitochondrial activity regulates myoblast differentiation by control of c-Myc expression. J Cell Physiol 2006;207:75–86 [DOI] [PubMed] [Google Scholar]
- 40.Muscatello U, Margreth A, Aloisi M. On the differential response of sarcoplasm and myoplasm to denervation in frog muscle. J Cell Biol 1965;27:1–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muscatello U, Patriarca PL. Denervation and disuse atrophy in pigeon breast muscle. An electron microscopic and biochemical study. Am J Pathol 1968;52:1169–1189 [PMC free article] [PubMed] [Google Scholar]
- 42.Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol 2007;102:1143–1151 [DOI] [PubMed] [Google Scholar]
- 43.Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, et al. . Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One 2009;4:e6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, et al. . Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 2011;39:1749–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh K, Hood DA. Effect of denervation-induced muscle disuse on mitochondrial protein import. Am J Physiol Cell Physiol 2011;300:138–145 [DOI] [PubMed] [Google Scholar]
- 46.Kim KB, Lee JW, Lee CS, Kim BW, Choo HJ, Jung SY, et al. . Oxidation-reduction respiratory chains and ATP synthase complex are localized in detergent-resistant lipid rafts. Proteomics 2006;6:2444–2453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.