Abstract
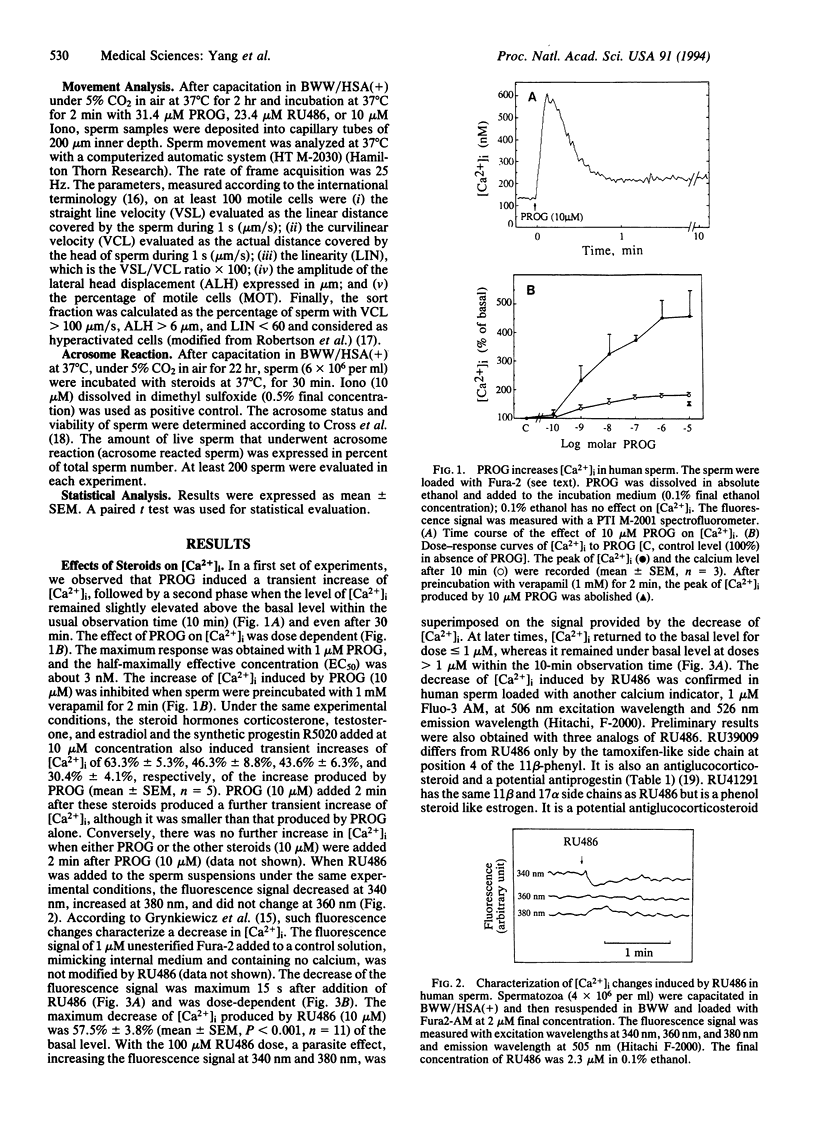
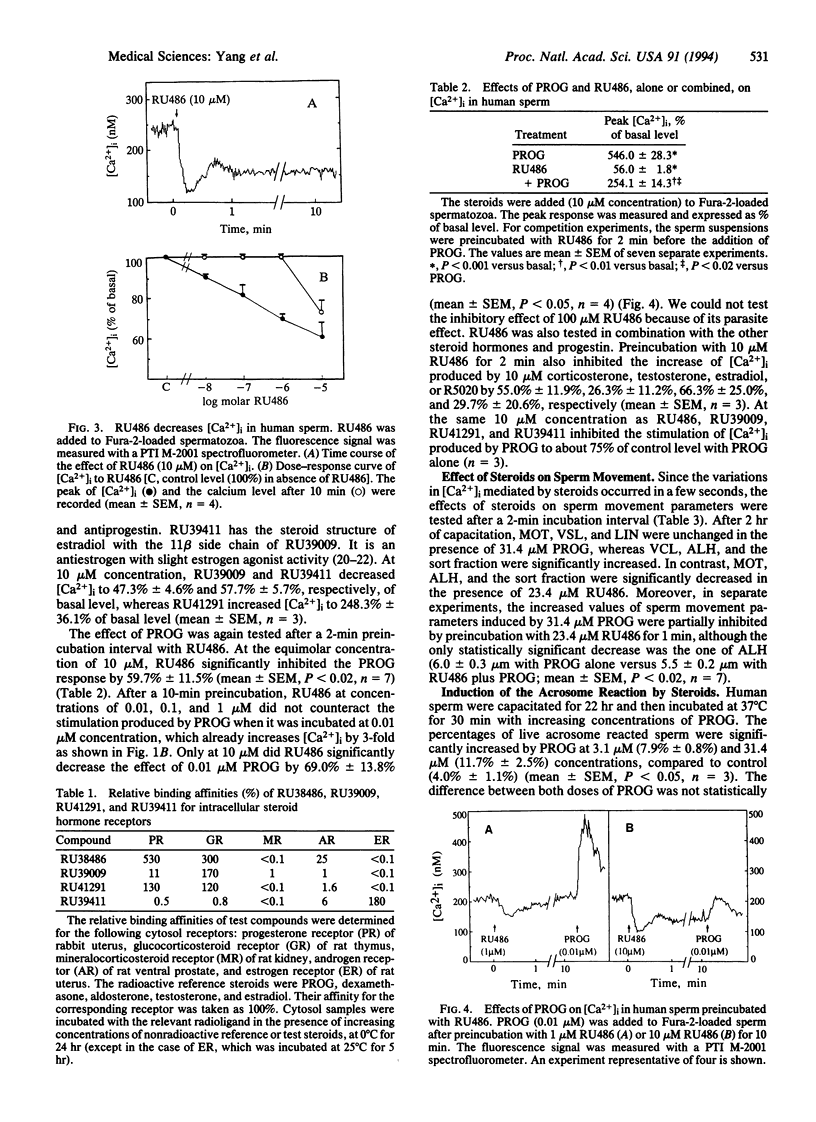
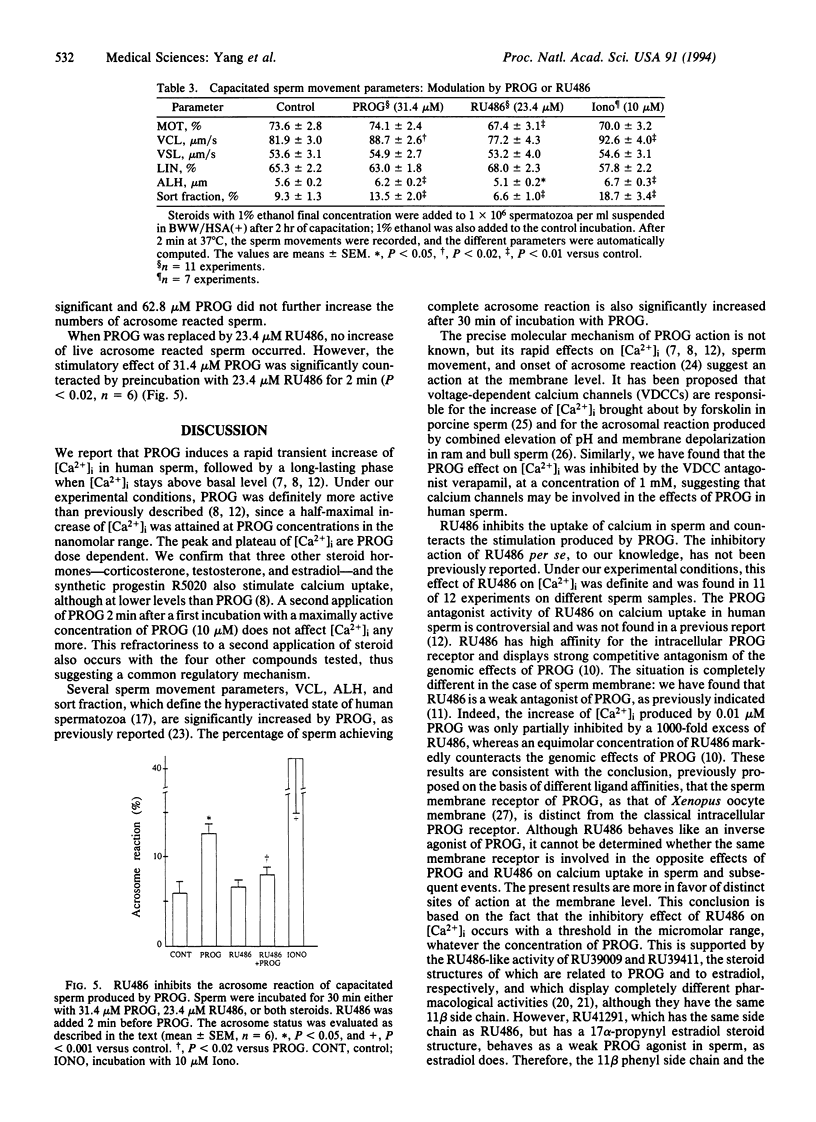
Progesterone induced a rapid influx of calcium in capacitated human sperm, followed by a long-lasting, dose-dependent increase of intracellular free calcium. Thereafter, progesterone increased the fraction of hyperactivated sperm and the acrosome reaction. On the contrary, the progesterone antagonist RU486 (mifepristone) induced an immediate and transient, dose-dependent decrease of intracellular free calcium and a drop in the values of sperm movement parameters related to hyperactivation. Moreover, RU486 counteracted the effects of progesterone on calcium influx, lateral sperm head displacement, and the acrosome reaction. Therefore, RU486 effects were opposite to those of progesterone. The nature of the membrane receptor(s) involved is unknown. Several steroids bearing 11 beta-phenyl substitutions, with different pharmacological profiles, were also investigated. It was concluded that the steroid structure and chemical groups added to the 11 beta-phenyl influence effects on calcium influx.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUSTIN C. R. The capacitation of the mammalian sperm. Nature. 1952 Aug 23;170(4321):326–326. doi: 10.1038/170326a0. [DOI] [PubMed] [Google Scholar]
- Baldi E., Casano R., Falsetti C., Krausz C., Maggi M., Forti G. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J Androl. 1991 Sep-Oct;12(5):323–330. [PubMed] [Google Scholar]
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Beebe S. J., Danforth D. R., Alexander N. Progesterone and 17 alpha-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem. 1990 Jan 25;265(3):1376–1380. [PubMed] [Google Scholar]
- Blackmore P. F., Lattanzio F. A. Cell surface localization of a novel non-genomic progesterone receptor on the head of human sperm. Biochem Biophys Res Commun. 1991 Nov 27;181(1):331–336. doi: 10.1016/s0006-291x(05)81422-6. [DOI] [PubMed] [Google Scholar]
- Blackmore P. F., Neulen J., Lattanzio F., Beebe S. J. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991 Oct 5;266(28):18655–18659. [PubMed] [Google Scholar]
- Blondeau J. P., Baulieu E. E. Progesterone receptor characterized by photoaffinity labelling in the plasma membrane of Xenopus laevis oocytes. Biochem J. 1984 May 1;219(3):785–792. doi: 10.1042/bj2190785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M. C. The meaning of sperm capacitation. A historical perspective. J Androl. 1984 Mar-Apr;5(2):45–50. doi: 10.1002/j.1939-4640.1984.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Claussner A., Nédélec L., Nique F., Philibert D., Teutsch G., Van de Velde P. 11 beta-amidoalkyl estradiols, a new series of pure antiestrogens. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):609–614. doi: 10.1016/0960-0760(92)90392-v. [DOI] [PubMed] [Google Scholar]
- Cross N. L., Morales P., Overstreet J. W., Hanson F. W. Induction of acrosome reactions by the human zona pellucida. Biol Reprod. 1988 Feb;38(1):235–244. doi: 10.1095/biolreprod38.1.235. [DOI] [PubMed] [Google Scholar]
- Florman H. M., Corron M. E., Kim T. D., Babcock D. F. Activation of voltage-dependent calcium channels of mammalian sperm is required for zona pellucida-induced acrosomal exocytosis. Dev Biol. 1992 Aug;152(2):304–314. doi: 10.1016/0012-1606(92)90137-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Juneja S. C., Dodson M. G. In vitro effect of RU 486 on sperm-egg interaction in mice. Am J Obstet Gynecol. 1990 Jul;163(1 Pt 1):216–221. doi: 10.1016/s0002-9378(11)90701-7. [DOI] [PubMed] [Google Scholar]
- Ojasoo T., Raynaud J. P. Unique steroid congeners for receptor studies. Cancer Res. 1978 Nov;38(11 Pt 2):4186–4198. [PubMed] [Google Scholar]
- Okamura N., Tanba M., Fukuda A., Sugita Y., Nagai T. Forskolin stimulates porcine sperm capacitation by increasing calcium uptake. FEBS Lett. 1993 Feb 1;316(3):283–286. doi: 10.1016/0014-5793(93)81309-n. [DOI] [PubMed] [Google Scholar]
- Osman R. A., Andria M. L., Jones A. D., Meizel S. Steroid induced exocytosis: the human sperm acrosome reaction. Biochem Biophys Res Commun. 1989 Apr 28;160(2):828–833. doi: 10.1016/0006-291x(89)92508-4. [DOI] [PubMed] [Google Scholar]
- Robertson L., Wolf D. P., Tash J. S. Temporal changes in motility parameters related to acrosomal status: identification and characterization of populations of hyperactivated human sperm. Biol Reprod. 1988 Nov;39(4):797–805. doi: 10.1095/biolreprod39.4.797. [DOI] [PubMed] [Google Scholar]
- Tash J. S., Means A. R. Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod. 1983 Feb;28(1):75–104. doi: 10.1095/biolreprod28.1.75. [DOI] [PubMed] [Google Scholar]
- Tesarik J., Mendoza C., Moos J., Carreras A. Selective expression of a progesterone receptor on the human sperm surface. Fertil Steril. 1992 Oct;58(4):784–792. doi: 10.1016/s0015-0282(16)55328-x. [DOI] [PubMed] [Google Scholar]
- Tesarík J. Comparison of acrosome reaction-inducing activities of human cumulus oophorus, follicular fluid and ionophore A23187 in human sperm populations of proven fertilizing ability in vitro. J Reprod Fertil. 1985 Jul;74(2):383–388. doi: 10.1530/jrf.0.0740383. [DOI] [PubMed] [Google Scholar]
- Teutsch G., Gaillard-Moguilewsky M., Lemoine G., Nique F., Philibert D. Design of ligands for the glucocorticoid and progestin receptors. Biochem Soc Trans. 1991 Nov;19(4):901–908. doi: 10.1042/bst0190901. [DOI] [PubMed] [Google Scholar]
- Thomas P., Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989 Dec 1;264(2):539–546. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhler M. L., Leung A., Chan S. Y., Wang C. Direct effects of progesterone and antiprogesterone on human sperm hyperactivated motility and acrosome reaction. Fertil Steril. 1992 Dec;58(6):1191–1198. [PubMed] [Google Scholar]


