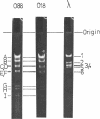
Abstract
The persistence of an O18 Escherichia coli strain resistant to tetracycline, streptomycin, and sulfonamide has been followed in the fecal flora of a single individual over a period of 9 months. The strain in question carrying an R plasmid was detectable from the beginning of the survey, but it was only after a 10-day period of tetracycline therapy that it reached an all but permanent dominance in the fecal flora. No transfer of the R plasmid carried by the strain to any other coliform could be detected for 202 days after the end of tetracycline treatment. At this point, however, an O88 E. coli carrying the same plasmid as the O18 strain appeared briefly as a predominant component of the flora. The two plasmids isolated from the O18 and the O88 E. coli strains have been characterized in molecular terms and found to be similar. This suggests that R-plasmid transfer between two E. coli strains occurred in an individual who was living a normal daily life and who was not receiving antibiotics.
Full text
PDF

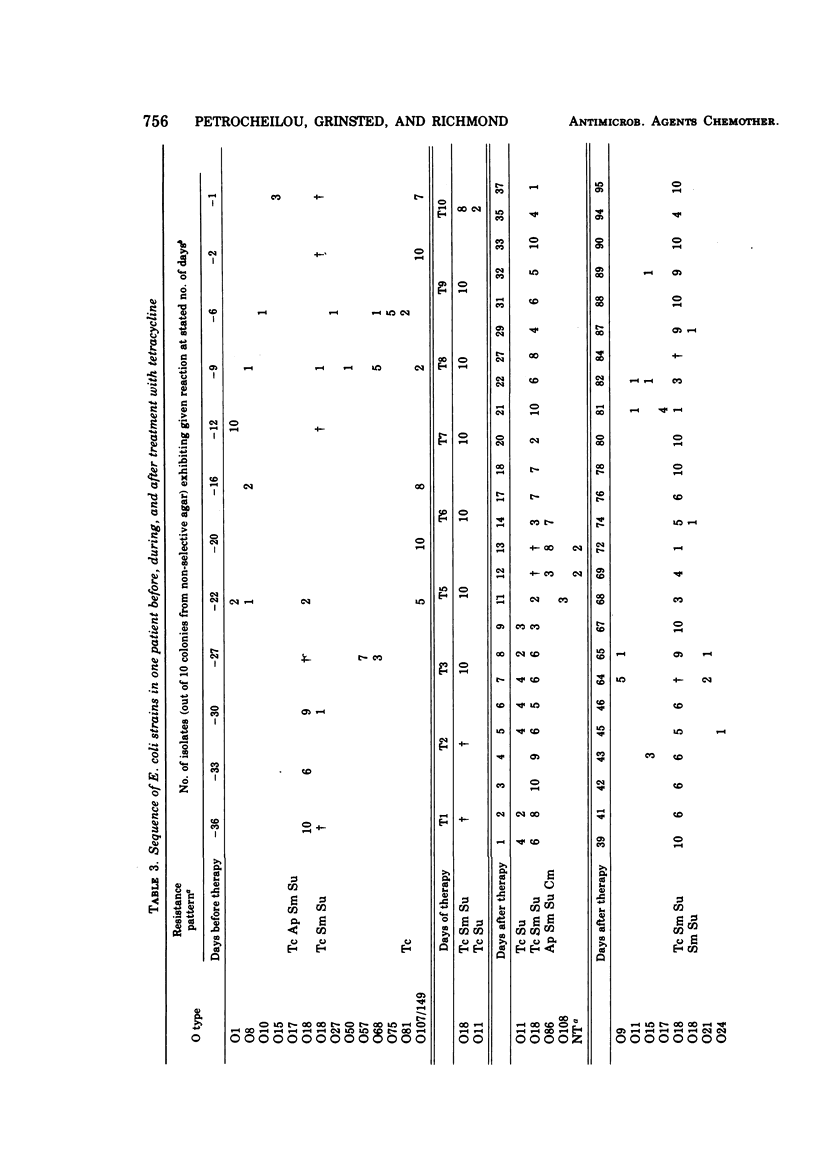
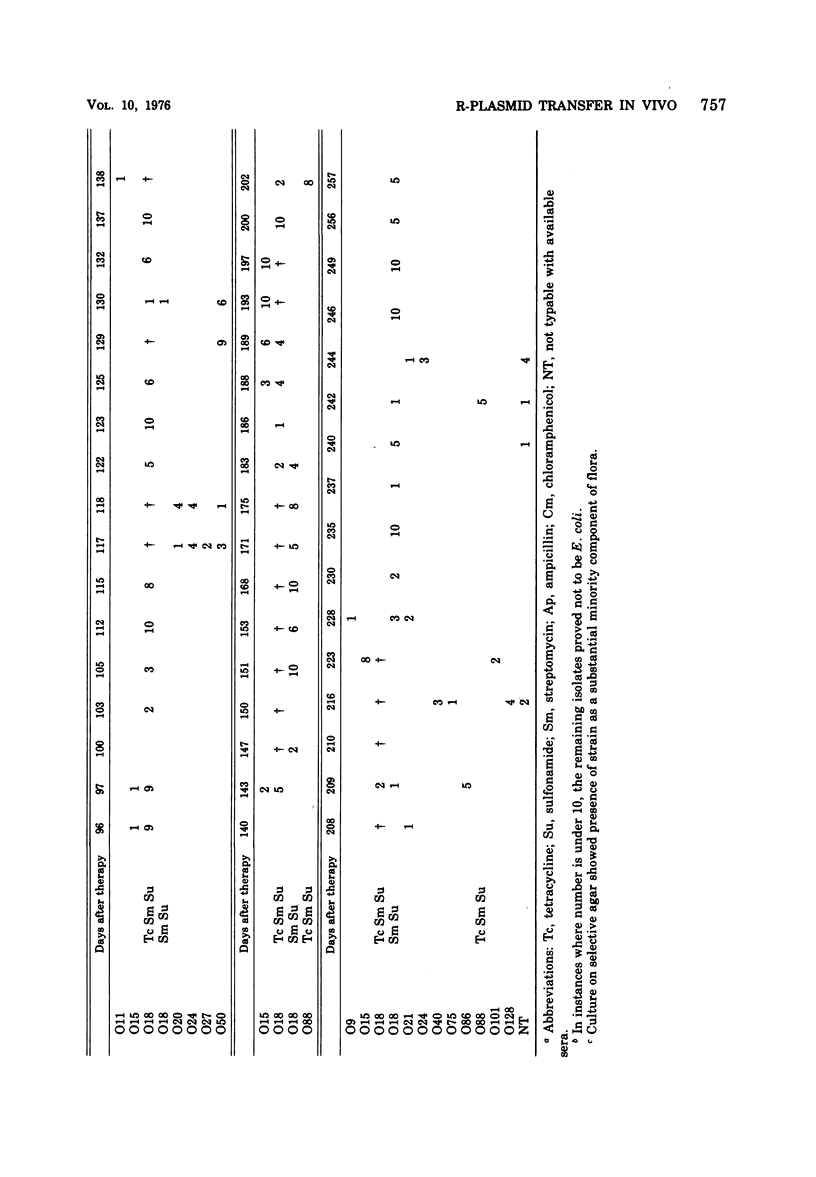



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Gillespie W. A., Richmond M. H. Chemotherapy and antibiotic-resistance transfer between Enterobacteria in the human gastro-intestinal tract. J Med Microbiol. 1973 Nov;6(4):461–473. doi: 10.1099/00222615-6-4-461. [DOI] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Trimethoprim resistance conferred by W plasmids in Enterobacteriaceae. J Gen Microbiol. 1972 Sep;72(2):349–355. doi: 10.1099/00221287-72-2-349. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Eidson M., Guerry P., Falkow S., Drusin L. M., Roberts R. B. Interbacterial transfer of R factor in the human intestine: in-vivo acquisition of R-factor-mediated kanamycin resistance by a multiresistant strain of Shigella sonnei. J Infect Dis. 1972 Jul;126(1):27–33. doi: 10.1093/infdis/126.1.27. [DOI] [PubMed] [Google Scholar]
- Grindley J. N., Anderson E. S. I-like resistance factors with the fi+ character. Genet Res. 1971 Jun;17(3):267–271. doi: 10.1017/s0016672300012295. [DOI] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Howe K., Linton A. H., Linton K. B., Richmond M. H. Distribution of R plasmids among the O-antigen types of Escherichia coli isolated from human and animal sources. Antimicrob Agents Chemother. 1975 Aug;8(2):122–131. doi: 10.1128/aac.8.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Richmond M. H. Antibiotic resistance and survival of E coli in the alimentary tract. Br Med J. 1975 Oct 11;4(5988):71–74. doi: 10.1136/bmj.4.5988.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Anderson E. S. A simple method for the preparation of large quantities of pure plasmid DNA. Biochim Biophys Acta. 1975 Apr 2;383(4):457–463. doi: 10.1016/0005-2787(75)90318-4. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Lowbury E. J., Babb J. R., Roe E. Clearance from a hospital of gram-negative bacilli that transfer carbenicillin-resistance to Pseudomonas aeruginosa. Lancet. 1972 Nov 4;2(7784):941–945. doi: 10.1016/s0140-6736(72)92469-5. [DOI] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W. Analytical isoelectric focusing of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1976 Feb;125(2):713–718. doi: 10.1128/jb.125.2.713-718.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pitton J. S., Anderson E. S. The inhibitory action of transfer factors on lysis of Escherichia coli K12 by phages mu 2 and phi 2. Genet Res. 1970 Oct 2;16(2):215–224. doi: 10.1017/s0016672300002433. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUSS J. H., Jr, SINSHEIMER R. L. Purification and properties of bacteriophage MS2 and of its ribonucleic acid. J Mol Biol. 1963 Jul;7:43–54. doi: 10.1016/s0022-2836(63)80017-0. [DOI] [PubMed] [Google Scholar]
- Smith H. W. Transfer of antibiotic resistance from animal and human strains of Escherichia coli to resident E. coli in the alimentary tract of man. Lancet. 1969 Jun 14;1(7607):1174–1176. doi: 10.1016/s0140-6736(69)92164-3. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thompson R., Hughes S. G., Broda P. Plasmid identification using specific endonucleases. Mol Gen Genet. 1974;133(2):141–149. doi: 10.1007/BF00264835. [DOI] [PubMed] [Google Scholar]
- Witchitz J. L., Chabbert Y. A. Résistance transférable à la gentamicine I. Expression du caractère de résistance. Ann Inst Pasteur (Paris) 1971 Dec;121(6):733–742. [PubMed] [Google Scholar]
- Zissler J. Integration-negative (int) mutants of phage lambda. Virology. 1967 Jan;31(1):189–189. doi: 10.1016/0042-6822(67)90030-x. [DOI] [PubMed] [Google Scholar]