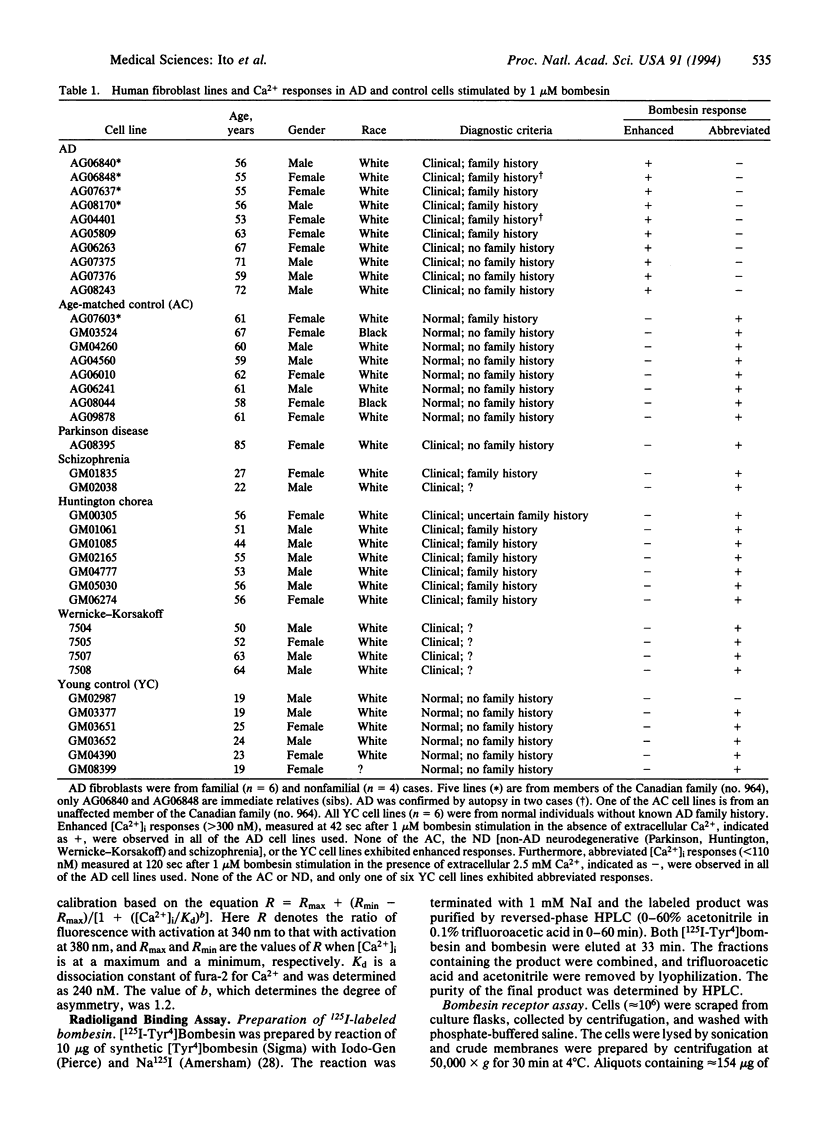
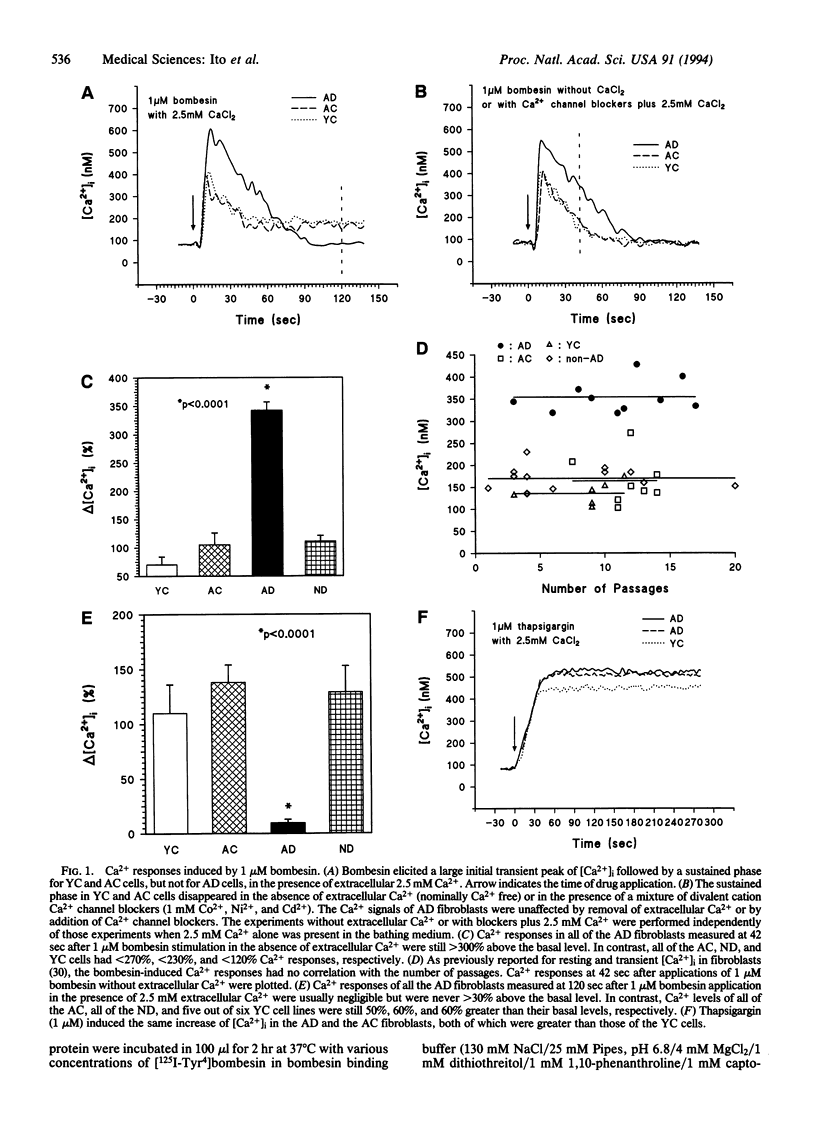
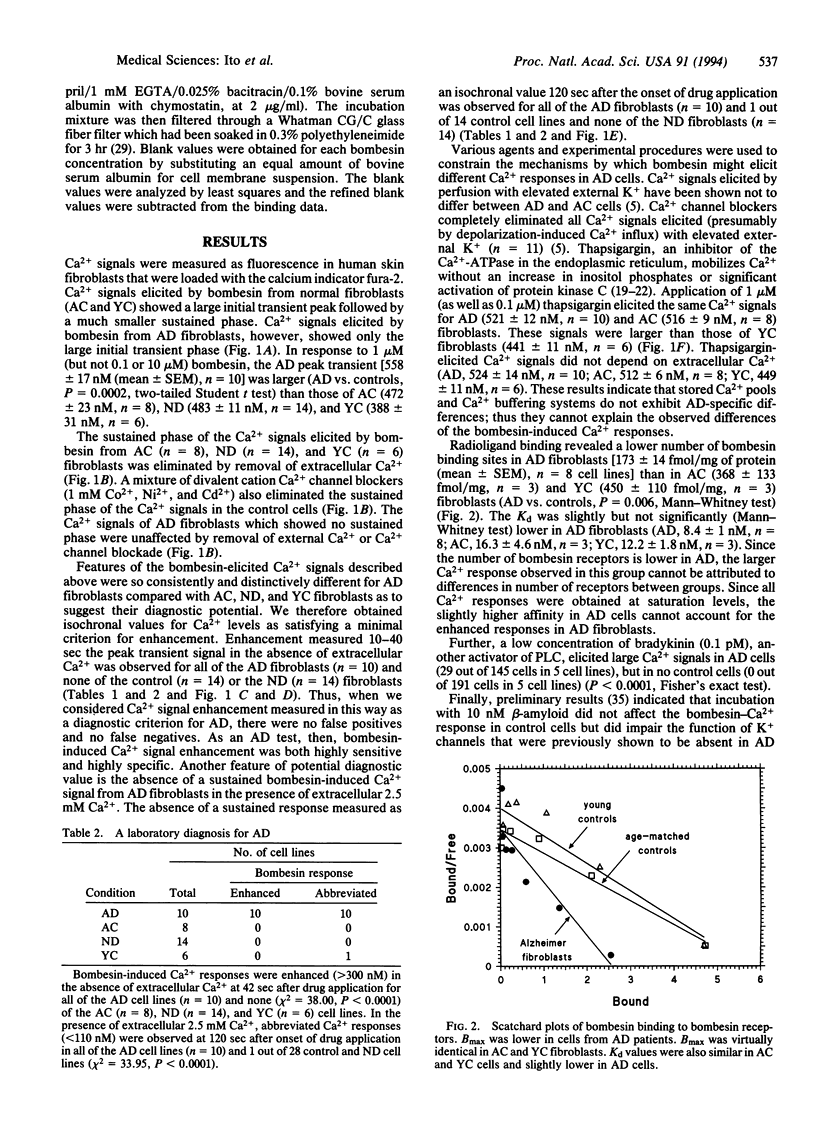
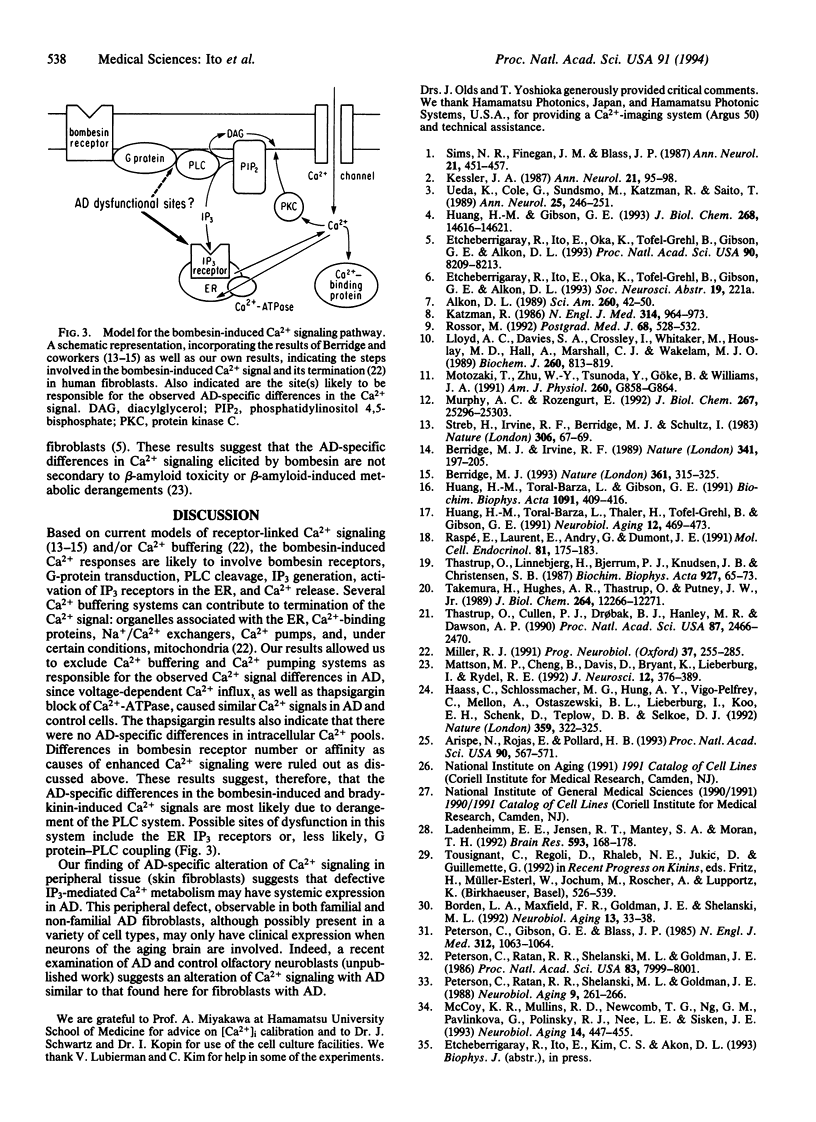
Abstract
The recent demonstration of K+ channel dysfunction in fibroblasts from Alzheimer disease (AD) patients and past observations of Ca(2+)-mediated K+ channel modulation during memory storage suggested that AD, which is characterized by memory loss and other cognitive deficits, might also involve dysfunction of intracellular Ca2+ mobilization. Bombesin-induced Ca2+ release, which is inositol trisphosphate-mediated, is shown here to be greatly enhanced in AD fibroblasts compared with fibroblasts from control groups. Bradykinin, another activator of phospholipase C, elicits similar enhancement of Ca2+ signaling in AD fibroblasts. By contrast, thapsigargin, an agent that releases Ca2+ by direct action on the endoplasmic reticulum, produced no differences in Ca2+ increase between AD and control fibroblasts. Depolarization-induced Ca2+ influx data previously demonstrated the absence of between-group differences of Ca2+ pumping and/or buffering. There was no correlation between the number of passages in tissue culture and the observed Ca2+ responses. Furthermore, cells of all groups were seeded and analyzed at the same densities. Radioligand binding experiments indicated that the number and affinity of bombesin receptors cannot explain the observed differences. These and previous observations suggest that the differences in bombesin and bradykinin responses in fibroblasts and perhaps other cell types are likely to be due to alteration of inositol trisphosphate-mediated release of intracellular Ca2+.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Memory storage and neural systems. Sci Am. 1989 Jul;261(1):42–50. doi: 10.1038/scientificamerican0789-42. [DOI] [PubMed] [Google Scholar]
- Arispe N., Rojas E., Pollard H. B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Borden L. A., Maxfield F. R., Goldman J. E., Shelanski M. L. Resting [Ca2+]i and [Ca2+]i transients are similar in fibroblasts from normal and Alzheimer's donors. Neurobiol Aging. 1992 Jan-Feb;13(1):33–38. doi: 10.1016/0197-4580(92)90005-i. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Ito E., Oka K., Tofel-Grehl B., Gibson G. E., Alkon D. L. Potassium channel dysfunction in fibroblasts identifies patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8209–8213. doi: 10.1073/pnas.90.17.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. Amyloid beta-peptide is produced by cultured cells during normal metabolism. Nature. 1992 Sep 24;359(6393):322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- Huang H. M., Gibson G. E. Altered beta-adrenergic receptor-stimulated cAMP formation in cultured skin fibroblasts from Alzheimer donors. J Biol Chem. 1993 Jul 15;268(20):14616–14621. [PubMed] [Google Scholar]
- Huang H. M., Toral-Barza L., Gibson G. E. Interactions between inositol phosphates and cytosolic free calcium following bradykinin stimulation in cultured human skin fibroblasts. Biochim Biophys Acta. 1991 Feb 19;1091(3):409–416. doi: 10.1016/0167-4889(91)90208-f. [DOI] [PubMed] [Google Scholar]
- Huang H. M., Toral-Barza L., Thaler H., Tofel-Grehl B., Gibson G. E. Inositol phosphates and intracellular calcium after bradykinin stimulation in fibroblasts from young, normal aged and Alzheimer donors. Neurobiol Aging. 1991 Sep-Oct;12(5):469–473. doi: 10.1016/0197-4580(91)90075-u. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N Engl J Med. 1986 Apr 10;314(15):964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Kessler J. A. Deficiency of a cholinergic differentiating factor in fibroblasts of patients with Alzheimer's disease. Ann Neurol. 1987 Jan;21(1):95–98. doi: 10.1002/ana.410210117. [DOI] [PubMed] [Google Scholar]
- Ladenheim E. E., Jensen R. T., Mantey S. A., Moran T. H. Distinct distributions of two bombesin receptor subtypes in the rat central nervous system. Brain Res. 1992 Oct 16;593(2):168–178. doi: 10.1016/0006-8993(92)91305-x. [DOI] [PubMed] [Google Scholar]
- Lloyd A. C., Davies S. A., Crossley I., Whitaker M., Houslay M. D., Hall A., Marshall C. J., Wakelam M. J. Bombesin stimulation of inositol 1,4,5-trisphosphate generation and intracellular calcium release is amplified in a cell line overexpressing the N-ras proto-oncogene. Biochem J. 1989 Jun 15;260(3):813–819. doi: 10.1042/bj2600813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matozaki T., Zhu W. Y., Tsunoda Y., Göke B., Williams J. A. Intracellular mediators of bombesin action on rat pancreatic acinar cells. Am J Physiol. 1991 Jun;260(6 Pt 1):G858–G864. doi: 10.1152/ajpgi.1991.260.6.G858. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy K. R., Mullins R. D., Newcomb T. G., Ng G. M., Pavlínková G., Polinsky R. J., Nee L. E., Sisken J. E. Serum- and bradykinin-induced calcium transients in familial Alzheimer's fibroblasts. Neurobiol Aging. 1993 Sep-Oct;14(5):447–455. doi: 10.1016/0197-4580(93)90103-i. [DOI] [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Murphy A. C., Rozengurt E. Pasteurella multocida toxin selectively facilitates phosphatidylinositol 4,5-bisphosphate hydrolysis by bombesin, vasopressin, and endothelin. Requirement for a functional G protein. J Biol Chem. 1992 Dec 15;267(35):25296–25303. [PubMed] [Google Scholar]
- Peterson C., Gibson G. E., Blass J. P. Altered calcium uptake in cultured skin fibroblasts from patients with Alzheimer's disease. N Engl J Med. 1985 Apr 18;312(16):1063–1065. doi: 10.1056/NEJM198504183121618. [DOI] [PubMed] [Google Scholar]
- Peterson C., Ratan R. R., Shelanski M. L., Goldman J. E. Altered response of fibroblasts from aged and Alzheimer donors to drugs that elevate cytosolic free calcium. Neurobiol Aging. 1988 May-Jun;9(3):261–266. doi: 10.1016/s0197-4580(88)80063-0. [DOI] [PubMed] [Google Scholar]
- Peterson C., Ratan R. R., Shelanski M. L., Goldman J. E. Cytosolic free calcium and cell spreading decrease in fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7999–8001. doi: 10.1073/pnas.83.20.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raspé E., Laurent E., Andry G., Dumont J. E. ATP, bradykinin, TRH and TSH activate the Ca(2+)-phosphatidylinositol cascade of human thyrocytes in primary culture. Mol Cell Endocrinol. 1991 Oct;81(1-3):175–183. doi: 10.1016/0303-7207(91)90216-f. [DOI] [PubMed] [Google Scholar]
- Rossor M. Alzheimer's disease. Postgrad Med J. 1992 Jul;68(801):528–532. doi: 10.1136/pgmj.68.801.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims N. R., Finegan J. M., Blass J. P. Altered metabolic properties of cultured skin fibroblasts in Alzheimer's disease. Ann Neurol. 1987 May;21(5):451–457. doi: 10.1002/ana.410210507. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Linnebjerg H., Bjerrum P. J., Knudsen J. B., Christensen S. B. The inflammatory and tumor-promoting sesquiterpene lactone, thapsigargin, activates platelets by selective mobilization of calcium as shown by protein phosphorylations. Biochim Biophys Acta. 1987 Jan 19;927(1):65–73. doi: 10.1016/0167-4889(87)90066-8. [DOI] [PubMed] [Google Scholar]
- Tousignant C., Regoli D., Rhaleb N. E., Jukic D., Guillemette G. 125I-Tyr,D-Arg[Hyp3,D-Phe7,Leu8]BK, a radiolabelled B2 antagonist specifically interacts with two distinct binding sites on epithelial membranes of guinea pig ileum. Agents Actions Suppl. 1992;38(Pt 1):526–539. doi: 10.1007/978-3-0348-7321-5_64. [DOI] [PubMed] [Google Scholar]
- Uéda K., Cole G., Sundsmo M., Katzman R., Saitoh T. Decreased adhesiveness of Alzheimer's disease fibroblasts: is amyloid beta-protein precursor involved? Ann Neurol. 1989 Mar;25(3):246–251. doi: 10.1002/ana.410250307. [DOI] [PubMed] [Google Scholar]


