Abstract
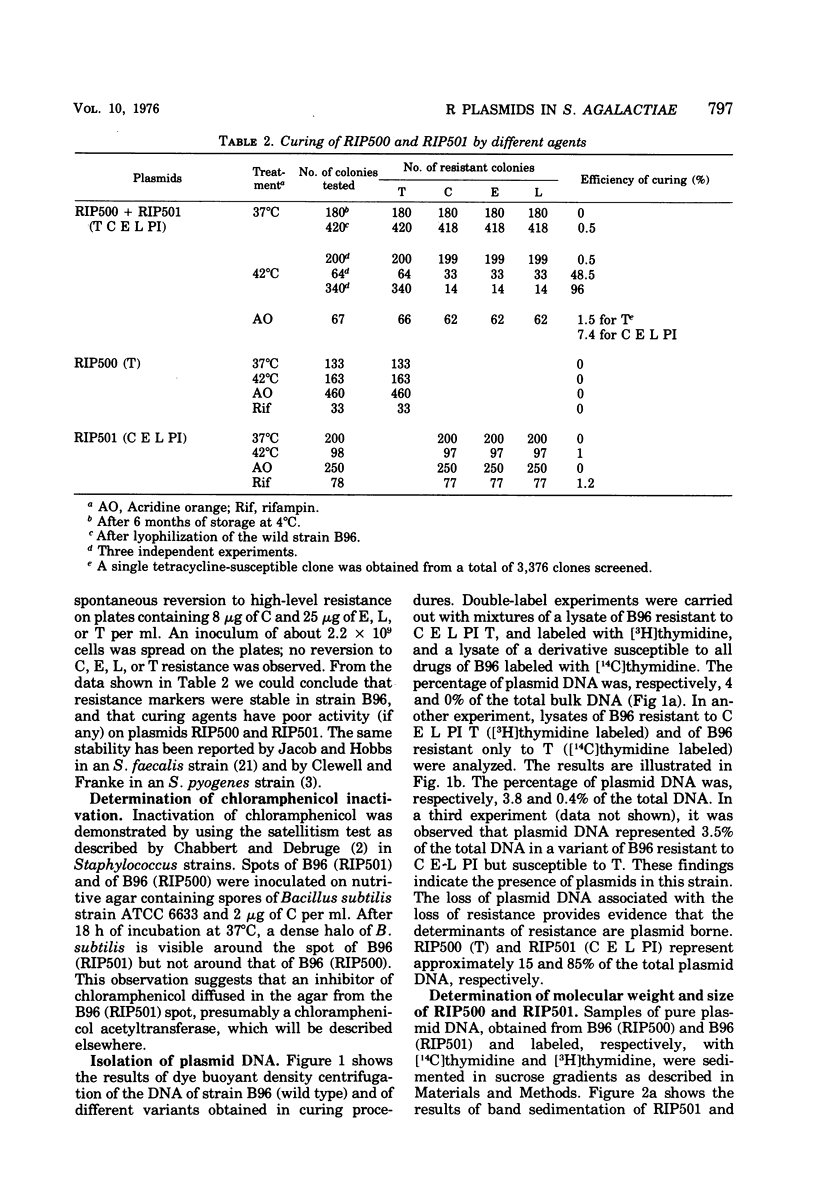
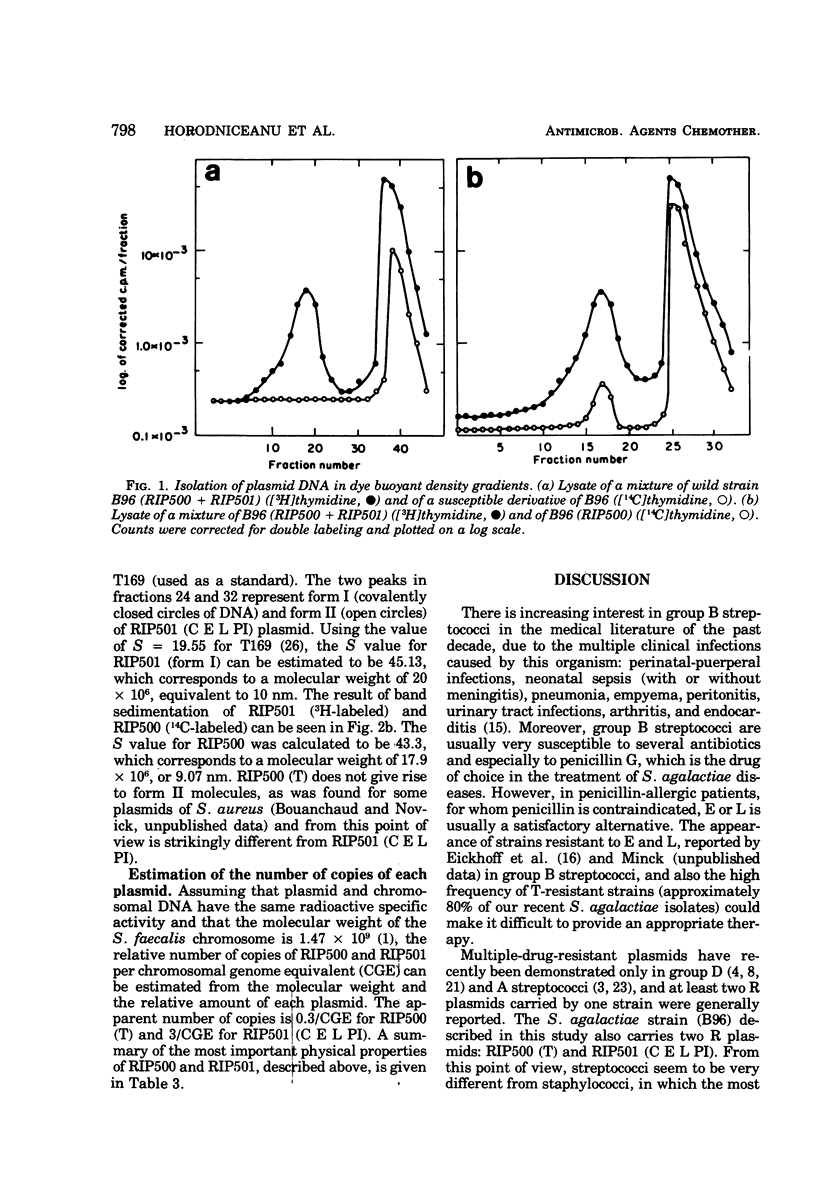
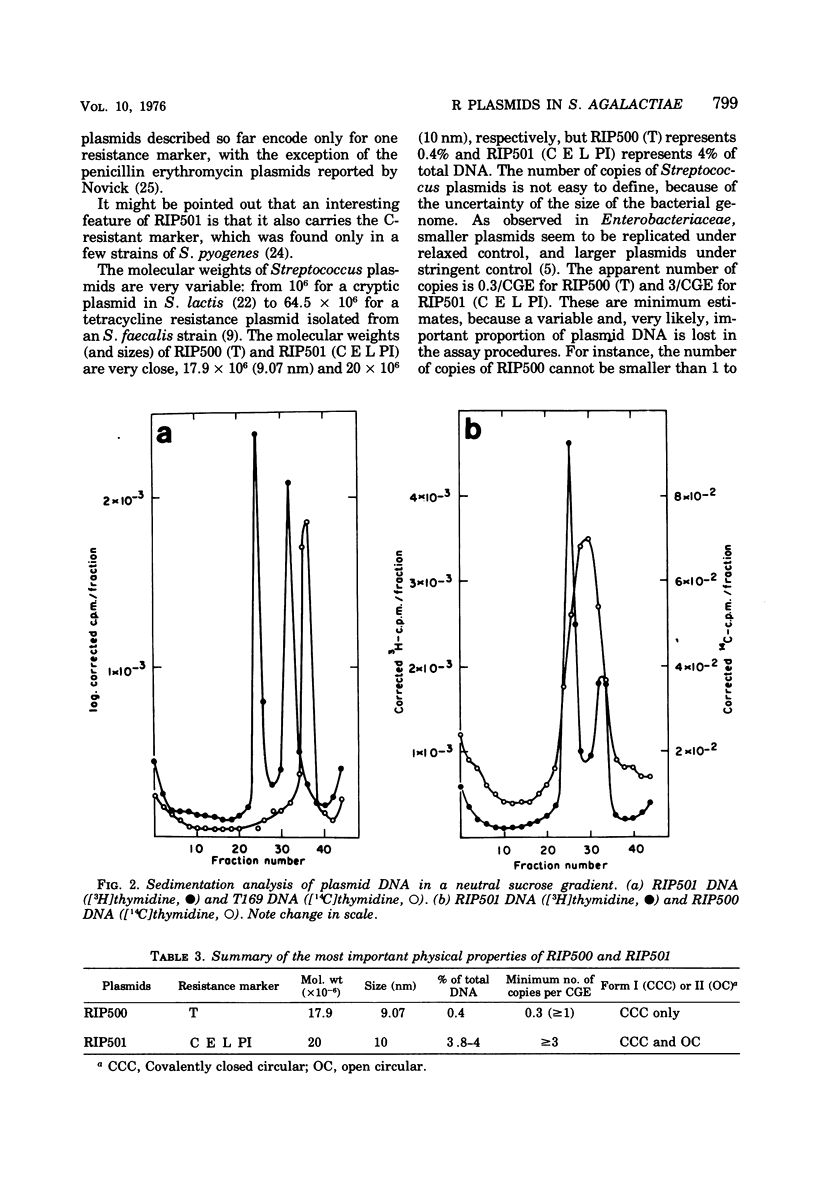
Two plasmids determining resistance to tetracycline (RIP500) and to chloramphenicol, erythromycin, lincomycin, and pristinamycin I (RIP501) were isolated from a strain of Streptococcus agalactiae. The frequency-of-resistance loss is very low for RIP500 (<3 × 104) but higher for RIP501 (the efficiency was dependent upon the curing agents and incubation temperature and varied between 0.5 and 96%). Derivatives susceptible to all drugs were also obtained. RIP500 and RIP501 have similar molecular weights (17.9 × 106 and 20 × 106, respectively) and represent different percentages of total deoxyribonucleic acid (0.4 and 4%, respectively). The number of copies of RIP500 and RIP501 per cell is different, and these plasmids are likely replicated under different kinds of control (stringent and/or relaxed). No plasmid deoxyribonucleic acid was found in a derivative of strain B96 susceptible to all drugs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bak A. L., Christiansen C., Stenderup A. Bacterial genome sizes determined by DNA renaturation studies. J Gen Microbiol. 1970 Dec;64(3):377–380. doi: 10.1099/00221287-64-3-377. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y., DEBRUGE J. La résistance naturelle des staphylocoques au chloramphénicol; fréquence des variants sensibles, production d'une substance antagoniste du chloramphénicol. Ann Inst Pasteur (Paris) 1956 Aug;91(2):225–230. [PubMed] [Google Scholar]
- Clewell D. B., Franke A. E. Characterization of a plasmid determining resistance to erythromycin, lincomycin, and vernamycin Balpha in a strain Streptococcus pyogenes. Antimicrob Agents Chemother. 1974 May;5(5):534–537. doi: 10.1128/aac.5.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. R. The elimination of urease activity in Streptococcus faecium as evidence for plasmid-coded urease. J Gen Microbiol. 1976 Jan;92(1):49–58. doi: 10.1099/00221287-92-1-49. [DOI] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Croissant O., Blangy D. Identification of two plasmids determining resistance to tetracycline and to erythromycin in group D streptococcus. Mol Gen Genet. 1974;132(3):181–192. doi: 10.1007/BF00269391. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J. M., Lipinski A. E. Resistance of group A beta-hemolytic streptococci to lincomycin and erythromycin. Antimicrob Agents Chemother. 1972 Apr;1(4):333–339. doi: 10.1128/aac.1.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICKHOFF T. C., KLEIN J. O., DALY A. K., INGALL D., FINLAND M. NEONATAL SEPSIS AND OTHER INFECTIONS DUE TO GROUP B BETA-HEMOLYTIC STREPTOCOCCI. N Engl J Med. 1964 Dec 10;271:1221–1228. doi: 10.1056/NEJM196412102712401. [DOI] [PubMed] [Google Scholar]
- Freifelder D. Studies with Escherichia coli sex factors. Cold Spring Harb Symp Quant Biol. 1968;33:425–434. doi: 10.1101/sqb.1968.033.01.049. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bouanchaud D. H. Genetic and physical studies of recombinant plasmids formed between an R plasmid of compatibility group FI and sex factor F of HfrH. J Bacteriol. 1976 Jan;125(1):58–67. doi: 10.1128/jb.125.1.58-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H. Genetics of resistance to macrolide antibiotics and lincomycin in natural isolates of Streptococcus pyogenes. Mol Gen Genet. 1974;135(4):349–367. doi: 10.1007/BF00271149. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Plasmid distribution and evidence for a proteinase plasmid in Streptococcus lactis C2-1. Appl Microbiol. 1975 Apr;29(4):546–548. doi: 10.1128/am.29.4.546-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae M., Inoue M., Mitsuhashi S. Artificial elimination of drug resistance from group A beta-hemolytic streptococci. Antimicrob Agents Chemother. 1975 May;7(5):719–720. doi: 10.1128/aac.7.5.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders E., Foster M. T., Scott D. Group A beta-hemolytic streptococci resistant to erythromycin and lincomycin. N Engl J Med. 1968 Mar 7;278(10):538–540. doi: 10.1056/NEJM196803072781005. [DOI] [PubMed] [Google Scholar]
- Toala P., McDonald A., Wilcox C., Finland M. Susceptibility of group D streptococcus (enterococcus) to 21 antibiotics in vitro, with special reference to species differences. Am J Med Sci. 1969 Dec;258(6):416–430. doi: 10.1097/00000441-196912000-00006. [DOI] [PubMed] [Google Scholar]
- Yag Y., Franke A. E., Clewell D. B. Plasmid-determined resistance to erythromycin: comparison of strains of streptococcus faecalis and streptococcus pyogenes with regard to plasmid hmology and resistance inducibility. Antimicrob Agents Chemother. 1975 Jun;7(6):871–873. doi: 10.1128/aac.7.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]


