Abstract
Objective
The study’s purpose was to test if subclinical atherosclerosis was associated with the risk of developing HI in a large cohort of middle-aged participants.
Methods
Study subjects were members of the Beaver Dam Offspring Study (BOSS), a longitudinal study of adult children of participants in the population-based Epidemiology of Hearing Loss Study (1993-present). BOSS examinations took place in 2005–2008 (baseline) and 2010–2013 (5-year follow-up). The 5-year incidence of hearing impairment was defined as a pure-tone average (PTA) of thresholds at 0.5, 1, 2 and 4 kHz > 25 decibels Hearing Level (dB HL) in either ear at follow-up among participants at risk (baseline PTA in both ears < = 25 dB HL; n=2,436, mean age = 47.7 years). Atherosclerosis was measured as the mean carotid intima-media thickness and the presence of carotid artery plaque.
Results
Among the 1,984 participants at-risk with a follow-up audiometric examination, the 5-year incidence of hearing impairment was 8.3% (95% Confidence Interval (C.I.) 7.1, 9.5). With multivariable adjustment, carotid intima-media thickness was positively associated with hearing impairment incidence (Odds Ratio (OR) = 1.18 per 0.1 mm, 95% C.I. 1.05, 1.32). The number of sites (0–6) with plaque was also positively associated with the incidence of impairment (OR = 1.19 per site, 95% C.I. 1.01, 1.41).
Conclusion
Atherosclerosis was associated with the 5-year incidence of hearing impairment in this predominantly middle-aged cohort. Interventions targeting atherosclerosis prevention may help to prevent or delay the onset of hearing impairment.
Keywords: hearing impairment, subclinical atherosclerosis, carotid intima-media thickness, carotid artery plaque, longitudinal cohort
Introduction
The risk of developing hearing impairment (HI) is high among older adults. In a population-based cohort study using audiometric threshold testing, the 10-yr cumulative incidence of HI was 22% among people ages 48–59 years of age at baseline and 73.7% among adults 70–79 years of age.1 Age-related HI is thought to be a slowly progressing degenerative process affecting signal transduction in the cochlea, neural transmission and central processing. People with HI report problems understanding speech, particularly in challenging listening conditions,2 and have poorer quality of life.3,4 Some studies have linked HI with depression, cognitive impairment, and mortality.5–8 For most adult onset or age-related hearing loss, there is no treatment which can restore hearing and hearing aid utilization rates are low,9–11 suggesting that efforts targeting primary prevention are needed to reduce the burden of hearing loss in aging populations. Therefore, it is important to identify modifiable risk factors that may be amenable to intervention.
Early histopathological studies suggested that sclerotic changes in the blood vessels of older ears contributed to degeneration in the inner ear and other cochlear changes.12 Rosen hypothesized low rates of cardiovascular disease (CVD), diabetes, and hypertension along with physically active lifestyles with low rates of smoking and noise exposure, and high fruit and fiber consumption may have contributed to the Mabaans’ retaining good hearing sensitivity at older ages.13,14 In a series of early ecologic studies, he found worse hearing in people living in areas with high rates of CVD compared to those living in areas with lower background rates of CVD.15,16 It has been observed that women with ischemic heart disease were more likely than controls without atherosclerosis to have HI.17 In the Framingham Heart Study, HI was associated with CVD events.18 Socioeconomic status, BMI and waist circumference, which are CVD risk factors, have been found to be associated with the incidence of hearing impairment in longitudinal studies.19–21
Other CVD risk factors (hypertension, type 2 diabetes, cigarette smoking, alcohol consumption, etc.) have been associated with HI in some, but not all, cross-sectional cohort studies.22–30 In the Health, Aging and Body Composition study, high resting heart rate, a marker of CVD risk and arterial stiffening, was associated with prevalent HI and, among women, higher pulse wave velocity, a marker of arterial stiffening, was associated with having poorer hearing.30 IMT has been associated with self-reported hearing loss.31
Recently, long-term high levels of serum C-reactive protein, a CVD risk marker also associated with IMT, were related to an increased 10-year risk of hearing impairment, particularly in adults less than 60 years of age at baseline.32 These studies add support to the hypothesis that underlying atherosclerotic changes may contribute to the risk of HI. The objective of the present longitudinal study of adults was to test if subclinical atherosclerosis was associated with the risk of developing HI.
Methods
Subjects
The Beaver Dam Offspring Study (BOSS) is a longitudinal cohort study of the adult children of participants in the population-based Epidemiology of Hearing Loss Study (EHLS, 1993-present).1,29 There were 3285 participants (ages 21–84 years) in the baseline BOSS in 2005–2008.29 A 5-yr follow-up study was conducted from 2010–2013. During the years between baseline and the follow-up examination, efforts were made to keep contact information current by sending materials such as newsletters to the participants. Participation in the 5-yr follow-up study was high (85%). Informed consent was obtained from all participants prior to each examination and approval for this research was obtained from the Health Sciences Institutional Review Board of the University of Wisconsin.
Hearing impairment was defined as a pure-tone average (PTA) of the hearing thresholds at 0.5, 1, 2, and 4 kHz greater than 25 decibels Hearing Level (dB HL) in at least one ear. For the purposes of this paper, only participants without HI at baseline (PTA ≤ 25 dB HL in right and left ears) were included in these analyses (at risk for incident hearing loss n=2,436). Of these, 1,984 (81.4%) had 5-yr follow-up audiometric testing data (mean length of follow-up = 4.9 years), 180 (7.4%) participated in BOSS2 but did not have complete audiometric data, 26 (1.1%) died prior to being seen, 4 (0.2%) were lost to follow-up, and 242 did not participate (9.9%). Incident hearing impairment was defined as a PTA > 25 dB HL in either ear at the 5-yr follow-up examination.
Measurements
Audiometry
The same standardized methods were used at both examinations by trained and certified examiners. These included an extensive questionnaire, otoscopic evaluation, screening tympanogram (GSI-37 Autotyp, Grason-Stadler, Inc., Madison, WI), and pure-tone air and bone-conduction audiometry.29 GSI-61 clinical audiometers (Grason-Stadler, Inc., Madison, WI) equipped with TDH-50P headphones and ER-3A insert earphones were used. Audiometric testing was conducted according to the guidelines of the American Speech-Language-Hearing Association33 in sound-treated booths at the examination site in Beaver Dam, WI. Pure-tone air-conduction thresholds were obtained for each ear at 0.5, 1, 2, 3, 4, 6 and 8 kHz. Bone conduction thresholds were measured at 0.5, 2 and 4 kHz. Masking procedures were used as necessary to avoid cross-hearing effects of the better ear.
Clinical audiometers were calibrated every six months during the study period.34,35 Ambient noise levels were routinely monitored to ensure that testing conditions complied with ANSI standards.36 Participants (n=29) unable to travel to Beaver Dam for the follow-up examination were tested using insert earphones in quiet testing conditions at satellite clinics in Minneapolis, MN and Madison, WI.
Subclinical Atherosclerosis
Carotid intima media thickness (IMT) and plaque were measured from high resolution B-mode carotid artery ultrasound images (AU4, Esaote North America, Inc., Indianapolis, IN) obtained on the right and left sides using a modified Atherosclerosis Risk in Communities (ARIC) study protocol.37–39 Trained and certified graders measured the IMT of the near and far walls in 1.0 cm segments of the common carotid, bifurcation, and internal carotid artery. The mean IMT was the average of the 12 wall measurements. Plaque was considered present if, with acoustic shadowing there was a change in the wall shape or texture or an IMT> 1.5mm, or without acoustic shadowing at least two of the previously mentioned changes were present.37 The number of sites with plaque (0 to 6) was scored. IMT data were available for 1,953 of the 1,984 (98.4%) study participants and plaque count data were available for 1,861 (93.8%) participants.
Other measures
Blood pressure was measured with a Dinamap Procare 120 (GE Medical Systems, Milwaukee, WI). Three measures were taken with the participant sitting quietly. The average of the 2nd and 3rd sets of systolic and diastolic pressures were used in these analyses and in our definition of hypertension as systolic blood pressure ≥ 140 mmHg, or diastolic blood pressure ≥ 90 mmHg, or doctor diagnosis of hypertension with current blood pressure medication. Body mass index was calculated as weight in kilograms divided by height in meters squared. PWV (carotid to femoral) was measured using the Complior SP System (Alam Medical, Vincenees, France).40 Serum total cholesterol was measured using a double enzymatic process which produces hydrogen peroxide (Roche/Hitachi 911 System, Indianapolis, IN). After further treatment with peroxidase, 40-aminophenazone and phenol, a colored product was produced, which was then measured at 505 nm. Serum HDL cholesterol was measured the same way after precipitating off the other lipoprotein fractions. Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. Hemoglobin A1C was measured from whole blood using an A1C 2.2 Plus Glycohemoglobin Analyzer (Tosch, San Francisco, CA).
An interviewer administered questionnaire was used to obtain self-reported medical history, lifestyle exposures and sociodemographic factors. Participants were asked the highest degree or level of school completed which was coded as high school or less, some college or college graduate. Reported occupation was coded using census classifications and the group, “Managerial/professional”, was compared to the combination of all other classifications. Alcohol consumption was reported as the number of servings of beer, wine or hard liquor consumed during an average week in the past year which was then converted to grams of ethanol.
Baseline characteristics considered for inclusion as covariates in the multivariable analyses were age, sex, education, occupation, occupational noise exposure (job requiring speaking in a raised voice or louder to be heard at a distance of two feet), smoking status, exercise (regular activity long enough to work up a sweat at least once a week), ethanol consumption (grams/week), history of cardiovascular disease (doctor-diagnosed myocardial infarction, stroke, or angina), hypertension, diabetes (doctor diagnosis of diabetes or A1C ≥6.5%), body mass index (BMI< 25, 25–29, 30+ kg/m2), waist circumference (cm), non-high-density lipoprotein (non-HDL) cholesterol (mg/dl), statin use, and pulse wave velocity (PWV).
Statistical Analyses
All analyses were completed with the SAS System (SAS Institute, Inc., Gary, NC). Confidence intervals around incidence estimates were calculated with the normal approximation to the binomial distribution, except where small cell size required an exact binomial confidence interval. Potential confounders of the hearing impairment and subclinical atherosclerosis association were determined by first assessing the relationship of each covariate with the 5-year risk of hearing impairment in age- and sex-adjusted modified Poisson with robust error variances regression models.41 Full multivariable models and reduced models were tested using modified Poisson regression. Separate multivariable models were constructed for IMT and plaque because of the non-independence of these two measures of atherosclerosis. Because statin use may slow progression of atherosclerosis and reduce inflammation and prescription bias may influence use, we created stratified models to explore potential interactions between IMT (plaque) and statin use. Least squares regression models were used to evaluate the relationship of IMT and plaque at baseline with pure-tone average at the 5-yr follow-up examination, with similar model-building strategies, and were also used to assess frequency-specific trends in mean thresholds at follow-up by IMT tertile and plaque count. Finally, generalized estimating equation (GEE) models were used to test effects of right and left side atherosclerosis measures with ear-specific hearing.
Results
The majority of participants were ages 35–64 years (Table 1). The 5-year incidence of hearing impairment was 8.3% (95% Confidence Interval (CI) 7.1, 9.5). Age-specific rates were higher in men than women and tended to be higher by age group. The risk of HI increased with age (OR for 5 yrs =1.42, 95% CI=1.29, 1.55) and men were more than twice as likely to develop HI as women (OR=2.28, 95% CI=1.64, 3.19).
Table 1.
5-yr Incidence of Hearing Impairment by Baseline Age and Sex: Beaver Dam Offspring Study
| Baseline Age (yrs) |
All | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N at risk |
n cases |
% (95% CI) |
N at risk |
n cases |
% (95% CI) |
N at risk |
n cases |
% (95% CI) |
|
| 21–34 | 129 | 2 | 1.6 (0.2, 5.5) | 52 | 2 | 3.9 (0.5, 13.2) | 77 | 0 | 0.0 (0.0, 4.7) |
| 35–44 | 609 | 30 | 4.9 (3.2, 6.6) | 266 | 19 | 7.1 (4.1, 10.2) | 343 | 11 | 3.2 (1.3, 5.1) |
| 45–54 | 776 | 62 | 8.0 (6.1, 9.9) | 342 | 39 | 11.4 (8.0, 14.8) | 434 | 23 | 5.3 (3.2, 7.4) |
| 55–64 | 384 | 50 | 13.0 (9.7, 16.4) | 154 | 33 | 21.4 (15.0, 27.9) | 230 | 17 | 7.4 (4.0, 10.8) |
| 65–79 | 86 | 20 | 23.3 (14.3, 32.2) | 31 | 5 | 16.1 (5.5, 33.7) | 55 | 15 | 27.3 (15.5, 39.0) |
| All | 1984 | 164 | 8.3 (7.1, 9.5) | 845 | 98 | 11.6 (9.4, 13.8) | 1139 | 66 | 5.8 (4.4, 7.2) |
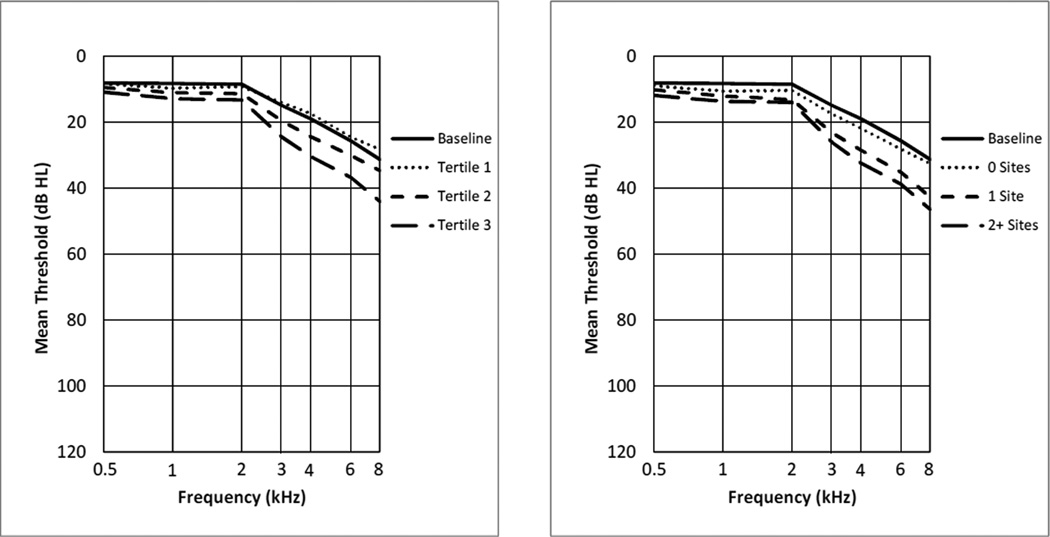
Figure 1 shows the audiograms at baseline and at follow-up by level of IMT or plaque. In unadjusted analyses, participants with thicker baseline IMT or greater number of sites with plaque at baseline had worse mean frequency-specific hearing thresholds at the 5-yr follow-up examination. These trends for IMT and plaque were statistically significant at all frequencies (p < .001). The audiogram patterns of the average thresholds were sloping, with worse hearing at higher frequencies, the typical sensorineural pattern of aging.
Figure 1.
Mean worse-ear hearing thresholds at baseline and at the 5-yr follow-up examination among those at-risk for developing hearing impairment. The thresholds at follow-up are shown by tertile of IMT at baseline (left) and by number of carotid artery sites with plaque at baseline (right).
Adjusting for age and sex, the following baseline characteristics were significantly associated with a higher risk of developing HI: Education (some college vs. college graduate), non-managerial/professional jobs, BMI, IMT, and plaque (Table 2); the association between waist circumference and HI was of borderline statistical significance. In the full multivariable model with IMT adjusting for all covariates, age, sex, and IMT were associated with HI risk (Table 3a). In the final, reduced multivariable model adjusting for age and sex, the relative risk of HI associated with a difference of 0.1 mm in the IMT was 1.14 (95% CI = 1.04, 1.24) and BMI was marginally associated with increased risk (RR per kg/m2=1.02, 95% CI = 1.00,1.05). Similarly, in the final, reduced multivariable model for plaque count, adjusting for age, sex, and education, the number of sites with plaque was positively associated with the incidence of impairment (RR = 1.16 per site, 95% CI = 1.01, 1.32; Table 3b). Similar effects for IMT and plaque were found using GEE models to include side-specific measures of atherosclerosis and hearing (data not shown).
Table 2.
Baseline Characteristics by 5-year Incidence of Hearing Impairment
| Baseline Characteristic | Incident Case | Age-sex-adjusted Relative Riska (95%CI) |
|
|---|---|---|---|
| No (N=1820) | Yes (N=164) | ||
| n (%) | n (%) | ||
| Men | 747 (41.0) | 98 (59.8) | 2.08 (1.55, 2.79) |
| Education | |||
| High school or less | 551 (30.4) | 58 (34.6) | 1.31 (0.90, 1.92) |
| Some college | 619 (34.2) | 63 (38.9) | 1.45 (1.01, 2.10) |
| College graduate | 641 (35.4) | 43 (26.5) | 1.00 (ref) |
| Smoking | |||
| Current | 1015 (55.8) | 86 (52.4) | 1.14 (0.76, 1.71) |
| Past | 502 (27.6) | 50 (30.5) | 0.92 (0.66, 1.28) |
| Never | 303 (16.7) | 28 (17.1) | 1.00 (ref) |
| Exercise (at least 1×/week) | 1140 (62.7) | 90 (55.2) | 0.80 (0.60, 1.06) |
| Cardiovascular disease | 43 (2.4) | 10 (6.1) | 1.42 (0.79, 2.57) |
| Hypertension | 586 (32.2) | 82 (50.0) | 1.26 (0.93, 1.70) |
| Diabetes | 83 (4.6) | 15 (9.2) | 1.34 (0.84, 2.15) |
| Statin Use | 244 (13.4) | 35 (21.3) | 0.99 (0.70, 1.42) |
| Current noisy job | 260 (14.3) | 27 (16.5) | 1.32 (0.90, 1.94) |
| Non managerial/professional job | 1168 (66.4) | 120 (74.1) | 1.42 (1.02, 1.99) |
| Mean (SD) | Mean (SD) | ||
| Age, per 5 yr | 47.5 (9.1) | 53.2 (9.2) | 1.36 (1.26, 1.46) |
| Alcohol, per 25 g/wk | 54.6 (99.7) | 64.7 (109.4) | 1.00 (0.97, 1.04) |
| Systolic blood pressure per 10 mm | 124.5 (17.4) | 129.5 (18.2) | 1.03 (0.95, 1.12) |
| Diastolic blood pressure per 10 mm | 73.3 (9.7) | 75.0 (9.8) | 1.01 (0.87, 1.18) |
| A1C per 1 % | 5.36 (0.64) | 5.55 (0.66) | 1.14 (0.99, 1.30) |
| BMI per 1 kg/m2 | 30.0 (6.5) | 31.7 (7.5) | 1.02 (1.00, 1.05) |
| Waist, per 10 cm | 98.8 (16.3) | 104.9 (18.7) | 1.10 (1.00, 1.22) |
| Non-HDL cholesterol, per 20mg/dL | 153.4 (38.1) | 151.0 (37.7) | 0.95 (0.87, 1.02) |
| IMT, per 0.1 mm | 0.64 (0.12) | 0.72 (0.18) | 1.15 (1.05, 1.25) |
| Plaque count, per site | 0.3 (0.8) | 0.7 (1.2) | 1.17 (1.03, 1.33) |
| PWV Femoral, per m/s | 8.0 (5.4) | 8.5 (4.6) | 1.00 (0.97, 1.03) |
Bold face indicates statistically significant results (p < 0.05).
Table 3.
| a: Multivariable Models for 5-yr Incidence of Hearing Impairment with IMT (Relative Risk & 95% Confidence Interval) | ||
|---|---|---|
| Risk Factors | Full Model | Reduced Model |
| Age (per 5 yr) | 1.27 (1.16, 1.39) | 1.27 (1.17, 1.38) |
| Men (vs. Women) | 1.91 (1.37, 2.65) | 1.87 (1.38, 2.54) |
| < College graduate | 1.24 (0.87, 1.77) | -- |
| Smoking (vs Never) | ||
| Current | 0.92 (0.59, 1.44) | -- |
| Past | 0.85 (0.60, 1.21) | -- |
| Exercise (at least 1×/week) | 0.86 (0.63, 1.17) | -- |
| Cardiovascular disease | 1.17 (0.62, 2.23) | -- |
| Hypertension | 1.03 (0.73, 1.44) | -- |
| Diabetes | 0.79 (0.43, 1.44) | -- |
| Current noisy job | 1.17 (0.78, 1.76) | -- |
| Alcohol, per 25 g/wk | 1.01 (0.98, 1.04) | -- |
| BMI per 1 kg/m2 | 1.02 (1.00, 1.05) | 1.02 (1.00, 1.05) |
| Non-HDL cholesterol, per 20mg/dL | 0.93 (0.86, 1.02) | -- |
| IMT (per 0.1 mm) | 1.12 (1.03, 1.23) | 1.14 (1.04, 1.24) |
| b: Multivariable Models for 5-yr Incidence of Hearing Impairment with Plaque Count (Relative Risk & 95% Confidence Interval) | ||
|---|---|---|
| Risk Factors | Full Model | Reduced Model |
| Age (per 5 yr) | 1.28 (1.17, 1.40) | 1.30 (1.20, 1.41) |
| Men (vs. Women) | 1.92 (1.38, 2.69) | 1.99 (1.46, 2.72) |
| < College graduate | 1.32 (0.91, 1.92) | 1.41 (0.99, 2.01) |
| Smoking (vs Never) | ||
| Current | 0.86 (0.53, 1.37) | -- |
| Past | 0.87 (0.61, 1.23) | -- |
| Exercise (at least 1×/week) | 0.81 (0.59, 1.11) | -- |
| Cardiovascular disease | 1.17 (0.57, 2.39) | -- |
| Hypertension | 1.05 (0.74, 1.49) | -- |
| Diabetes | 0.86 (0.45, 1.64) | -- |
| Current noisy job | 1.16 (0.76, 1.77) | -- |
| Alcohol, per 25 g/wk | 1.02 (0.99, 1.05) | -- |
| BMI per 1 kg/m2 | 1.03 (1.00, 1.06) | -- |
| Non-HDL cholesterol, per 20mg/dL | 0.96 (0.88, 1.05) | -- |
| Plaque (per site) | 1.14 (0.98, 1.32) | 1.16 (1.01, 1.32) |
There was no indication of effect modification by statin use status (data not shown). The associations between baseline characteristics and 5-yr follow-up PTA on a continuous scale were also evaluated. Several baseline CVD risk factors were significantly associated with follow-up PTA (Table 4). In final models including age, sex, education, smoking, exercise, waist circumference, diabetes, and non-HDL cholesterol at baseline, each additional 0.1 mm in baseline IMT was associated with a 0.32 dB higher PTA at the 5-yr follow-up examination (p = .04). Similarly, adjusting for age, sex, education, smoking, exercise, and waist circumference, each additional site with baseline plaque was associated with a 0.49 dB higher PTA at follow-up (p = .03); diabetes and non-HDL cholesterol were not retained in this final model.
Table 4.
Risk Factors Associated with Pure Tone Averagea at 5-year Follow-up Examination
| Model 1 (IMT) | Model 2 (Plaque) | |||||
|---|---|---|---|---|---|---|
| Risk Factors | Beta | se | P | Beta | se | P |
| Age (per 5 yr) | 1.41 | 0.11 | < .001 | 1.41 | 0.10 | < .001 |
| Men (vs. Women) | 3.39 | 0.35 | < .001 | 3.35 | 0.35 | < .001 |
| < College graduate | 1.10 | 0.35 | .002 | 1.31 | 0.36 | .001 |
| Smoking | ||||||
| Current | 1.36 | 0.47 | .004 | 1.22 | 0.48 | .01 |
| Past | 0.65 | 0.39 | .09 | 0.49 | 0.39 | .22 |
| Never | (ref) | ---- | ---- | (ref) | ---- | ---- |
| Exercise (at least 1×/week) | −1.04 | 0.34 | .003 | −0.96 | 0.35 | .006 |
| Waist (per 10 cm) | 0.51 | 0.11 | < .001 | 0.43 | 0.11 | < .001 |
| Diabetes | −1.65 | 0.80 | .04 | ---- | ---- | ---- |
| Non-HDL (per 20 mg/dL) | −0.22 | 0.09 | .02 | ---- | ---- | ---- |
| IMT (per 0.1 mm) | 0.32 | 0.16 | .04 | ---- | ---- | ---- |
| Plaque (per site) | ---- | ---- | ---- | 0.49 | 0.22 | .03 |
Average of hearing thresholds at .5, 1, 2, and 4 kHz in the worse ear.
Discussion
Subclinical atherosclerosis, measured by carotid artery mean IMT and plaque, was found to be related to about a 15% increased risk of hearing impairment during a 5-year follow-up period and with lower hearing sensitivity at follow-up in this large, primarily middle-aged cohort. These relationships were found to be independent of other hearing impairment risk factors and cardiovascular risk factors. This is the first study to report an association between measures of subclinical atherosclerosis and the incidence of HI. A similar relationship was suggested in a cross-sectional study with a slightly older study population than the present and with self-reported hearing loss data.31 These findings are consistent with early histopathological studies showing arteriosclerotic changes throughout the auditory system in older people12,42, 43 and a cross-sectional study suggesting associations between other markers of arterial stiffness and prevalent hearing impairments.30 The impact of a 0.2 mm difference in IMT on risk of hearing loss in this study was similar to the effects of five years of aging.
Intima-media thickness and plaque count in the carotid artery may be markers of generalized vascular disease including microvascular disease involving the stria vascularis in the lateral wall of the cochlea. The stria vascularis is the location of the electrochemical pump involved in the endolymphatic potential which is instrumental in the amplification function of the cochlea.44 Animal models have demonstrated age-related degeneration of the stria vascularis45 perhaps related to microvasculature changes such as reductions in capillary densities and vessel diameters.46,47 A relationship between strial atrophy and age has also been found in an investigation using human temporal bones.48 A reduction in blood flow to the stria vascularis may therefore contribute to a loss in the endolymphatic potential resulting in diminishment of cochlear amplification. Blood flow reduction may also be associated with the histopathological changes observed with age in the organ of Corti, such as the loss of hair cells.42 Another possible mechanism for the involvement of atherosclerosis with HI is through its association with oxidative stress which has been found to be related to HI.20 In addition to localized changes in the cochlea, it is possible that these generalized vascular changes affect neural signal transmission and processing contributing to changes in auditory function with aging. Alternatively, it is possible that IMT is serving as a marker of biologic or arterial age, rather than as a marker of hypoperfusion. If this is the case, it is possible that other imaging modalities such as coronary artery calcium, which are better markers of aging, may also show an association with HI risk.49–51 In models including both age and IMT or plaque, the age effect was slightly attenuated but remained significant and PWV, a marker of arterial stiffening was not associated with HI, suggesting that the associations of IMT and plaque with HI risk may reflect hypoxia/ischemia. HI may be part of the spectrum of atherosclerosis-related disorders; therefore, future studies focused on building risk prediction models for HI should include measures of vascular burden and multiple imaging modalities to measure arterial aging.51,52
Some but not all CVD and atherosclerotic risk factors also were associated with HI. BMI was significantly associated with HI risk and larger baseline waist circumference was associated with worse PTAs at follow-up. Adiposity, which is strongly related to risk of cardiovascular events, may indicate increased risk for HI as suggested by reports from some longitudinal studies.20,21 Socioeconomic status (SES) measured by education or occupation, also was associated with incidence of HI (lower risk with higher SES level), as has been reported in older longitudinal cohort studies.1,53 Other CVD risk factors such as smoking, alcohol consumption, exercise, and non-HDL cholesterol were not significantly related to HI incidence, similar to other reports.54 Because HI in adults develops slowly over decades, it may be difficult to detect the small contributions of these factors to incidence with only five years of follow-up.
Major strengths of the present study include the prospective design in which hearing sensitivity was measured at two time points with the same, standard audiometric methods, thus providing the opportunity to evaluate the relationship between atherosclerosis and the 5-year risk of hearing impairment. Carotid artery imaging was performed and graded using standard protocols followed by trained and certified exam personnel. The study population was large with a very high retention rate at the 5-year follow-up (85%) and with minimal missing data. Because the majority of the study population was middle-aged or younger, it was possible to investigate the relationship between early atherosclerotic changes and the development of hearing impairment, perhaps at ages before other hearing loss-related factors begin to exert a strong effect on risk. We have shown that incident HI is common in people in their forties, which suggests that studies aimed at preventing hearing loss need to target early to middle adult years, before significant and potentially irreversible auditory damage occurs. However, the relatively young age of the study population and the short follow-up time may be limitations because the numbers of participants with diabetes or a history of CVD and its related conditions were low resulting in possible inadequate power for detecting associations. Another limitation of the study is that the BOSS cohort is primarily non-Hispanic white which impacts the generalizability of the observed incidence rates. Finally, residual confounding may have been present so that the observed association between atherosclerosis and hearing impairment was related to the influence of an undetermined, shared risk factor.
Conclusion
The risk of developing hearing impairment over a 5 year follow-up period in a predominantly middle-aged group of participants was increased about 15% in the presence of atherosclerosis. This relationship was independent of other hearing impairment risk factors. These findings, coupled with previous histopathological studies of the cochlear microvasculature,45–47 suggest an important role for vascular health in auditory functioning. Interventions targeting the prevention of atherosclerosis may have additional impact on the prevention or delay in onset of hearing impairment.
Highlights.
Strategies targeting modifiable risk factors for hearing impairment are needed.
We investigated subclinical atherosclerosis and 5-year risk of hearing impairment.
Study population was primarily middle-aged.
Risk of hearing impairment increased about 15% in presence of atherosclerosis.
Acknowledgments
The authors thank the participants for their continued commitment to the study.
Funding/Support: The project described was supported by R01AG021917 (KJ Cruickshanks) from the National Institute on Aging, National Eye Institute, and National Institute on Deafness and Other Communication Disorders and an unrestricted grant from Research to Prevent Blindness.
Role of the Sponsor: The funding agencies had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: All authors declare that they have no conflict of interest.
Contributor Information
Mary E Fischer, Email: fischer@episense.wisc.edu.
Carla R Schubert, Email: schubert@episense.wisc.edu.
David M Nondahl, Email: nondahl@episense.wisc.edu.
Dayna S Dalton, Email: dalton@episense.wisc.edu.
Guan-Hua Huang, Email: ghuang@stat.nctu.edu.tw.
Brendan J Keating, Email: bkeating@mail.med.upenn.edu.
Barbara EK Klein, Email: kleinb@epi.ophth.wisc.edu.
Ronald Klein, Email: kleinr@epi.ophth.wisc.edu.
Ted S Tweed, Email: Tedt@tds.net.
Karen J. Cruickshanks, Email: cruickshanks@episense.wisc.edu.
References
- 1.Cruickshanks KJ, Nondahl DM, Tweed TS, et al. Education, occupation, noise exposure history and the 10-yr cumulative incidence of hearing impairment in older adults. Hear Res. 2010;264(1–2):3–9. doi: 10.1016/j.heares.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiley TL, Cruickshanks KJ, Nondahl DM, Tweed TS. Self-reported hearing handicap and audiometric measures in older adults. J Am Acad Audiol. 2000;11(2):67–75. [PubMed] [Google Scholar]
- 3.Dalton DS, Cruickshanks KJ, Klein BEK, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. 2003;43(5):661–668. doi: 10.1093/geront/43.5.661. [DOI] [PubMed] [Google Scholar]
- 4.Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. 2000;40(3):320–326. doi: 10.1093/geront/40.3.320. [DOI] [PubMed] [Google Scholar]
- 5.Gopinath B, Wang JJ, Schneider J, et al. Depressive symptoms in older adults with hearing impairments: the Blue Mountains Study. J Am Geriatr Soc. 2009;57(7):1306–1308. doi: 10.1111/j.1532-5415.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 6.Ulhmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. 1989;261(13):1916–1919. [PubMed] [Google Scholar]
- 7.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med. 2013;173(4):293–299. doi: 10.1001/jamainternmed.2013.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karpa MJ, Gopinath B, Beath K, et al. Associations between hearing impairment and mortality risk in older persons: the Blue Mountains Hearing Study. Ann Epidemiol. 2010;20(6):452–459. doi: 10.1016/j.annepidem.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BE, Klein R. Low prevalence of hearing aid use among older adults with hearing loss: the Epidemiology of Hearing Loss Study. J Am Geriatr Soc. 1998;46(9):1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- 10.Fischer ME, Cruickshanks KJ, Wiley TL, Klein BE, Klein R, Tweed TS. Determinants of hearing aid acquisition in older adults. Am J Public Health. 2011;101(8):1449–1455. doi: 10.2105/AJPH.2010.300078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nash SD, Cruickshanks KJ, Huang GH, et al. Unmet hearing health care needs: the Beaver Dam offspring study. Am J Public Health. 2013;103(6):1134–1139. doi: 10.2105/AJPH.2012.301031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fieandt H von, Saxen A. Pathologie und klinik der altersschwerhörigkeit. Acta Oto-Laryngologica. 1937;(Supplementum XXIII):1–85. [Google Scholar]
- 13.Rosen S, Bergman M, Plester D, El-Mofty A, Satti MH. Presbycusis study of a relatively noise-free population in the Sudan. Ann Otol Rhinol Laryngol. 1962;71:727–743. doi: 10.1177/000348946207100313. [DOI] [PubMed] [Google Scholar]
- 14.Rosen S, Olin P. Hearing loss and coronary heart disease. Arch Otolaryngol. 1965;82:236–243. doi: 10.1001/archotol.1965.00760010238004. [DOI] [PubMed] [Google Scholar]
- 15.Rosen S. Hearing studies in selected urban and rural populations. Trans NY Acad Sci. 1966;29(1):9–21. doi: 10.1111/j.2164-0947.1966.tb02246.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosen S, Preobrajensky N, Khechinashvili S, Glazunov I, Kipshidze N, Rosen HV. Epidemiologic hearing studies in the USSR. Arch Otolaryngol. 1970;91(5):424–428. doi: 10.1001/archotol.1970.00770040618005. [DOI] [PubMed] [Google Scholar]
- 17.Susmano A, Rosenbush SW. Hearing loss and ischemic heart disease. Am J Otol. 1988;9(5):403–408. [PubMed] [Google Scholar]
- 18.Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119(2):156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- 19.Cruickshanks KJ, Tweed TS, Wiley TL, et al. The 5-year incidence and progression of hearing loss: the Epidemiology of Hearing Loss Study. Arch Otolaryngol Head Neck Surg. 2003;129(10):1041–1046. doi: 10.1001/archotol.129.10.1041. [DOI] [PubMed] [Google Scholar]
- 20.Curhan SG, Eavey R, Wang M, Stampfer MJ, Curhan GC. Body mass index, waist circumference, physical activity, and risk of hearing loss in women. Am J Med. 2013;126(12):1142.e1–1142.e8. doi: 10.1016/j.amjmed.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linssen AM, van Boxtel MP, Joore MA, Anteunis LJ. Predictors of hearing acuity: cross-sectional and longitudinal analysis. J Gerontol A Biol Sci Med Sci. 2014;69(6):759–765. doi: 10.1093/gerona/glt172. [DOI] [PubMed] [Google Scholar]
- 22.Brant LJ, Gordon-Salant S, Pearson JD, et al. Risk factors related to age-associated hearing loss in the speech frequencies. J Am Acad Audiol. 1996;7(3):152–160. [PubMed] [Google Scholar]
- 23.Cruickshanks KJ, Klein R, Klein BEK, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: the Epidemiology of Hearing Loss Study. JAMA. 1998;279(21):1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- 24.Dalton DS, Cruickshanks KJ, Klein R, Klein BEK, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998;21(9):1540–1544. doi: 10.2337/diacare.21.9.1540. [DOI] [PubMed] [Google Scholar]
- 25.Popelka MM, Cruickshanks KJ, Wiley TL, et al. Moderate alcohol consumption and hearing loss: a protective effect. J Am Geriatr Soc. 2000;48(10):1273–1278. doi: 10.1111/j.1532-5415.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 26.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Surveys, 1999–2004. Ann Intern Med. 2008;149(1):1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fransen E, Topsakal V, Hendrickx JJ, et al. Occupational noise, smoking and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol. 2008;9(3):264–276. doi: 10.1007/s10162-008-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999 to 2002. Otol Neurotol. 2009;30(2):139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- 29.Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors. The Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011;137(5):432–439. doi: 10.1001/archoto.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helzner EP, Patel AS, Pratt S, et al. Hearing sensitivity in older adults: associations with cardiovascular risk factors in the health, aging and body composition study. J Am Geriatr Soc. 2011;59(6):972–979. doi: 10.1111/j.1532-5415.2011.03444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John U, Baumeister SE, Kessler C, Völzke H. Associations of carotid intima-media thickness, tobacco smoking and overweight with hearing disorder in a general population sample. Atherosclerosis. 2007;195(1):e144–e199. doi: 10.1016/j.atherosclerosis.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Nash SD, Cruickshanks KJ, Klein R, et al. Long-term variability of inflammatory markers and associated factors in a population-based cohort. J Am Geriatr Soc. 2013;61(8):1269–1276. doi: 10.1111/jgs.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Speech-Language-Hearing Association (ASHA) Guidelines for Manual Pure-Tone Threshold Audiometry. 2005 Available from www.asha.org/policy. [Google Scholar]
- 34.American National Standards Institute. New York, NY: ANSI; 2004. Specification for Audiometers. (ANSI S3.6-2004) [Google Scholar]
- 35.American National Standards Institute. New York, NY: ANSI; 2010. Specification for Audiometers. (ANSI S3.6-2010) [Google Scholar]
- 36.American National Standards Institute. New York, NY: ANSI; 1999. Maximum Permissible Ambient Noise Levels for Audiometric Test Rooms. (ANSI S3.1-1999) [Google Scholar]
- 37.Zhong W, Cruickshanks KJ, Huang GH, et al. Carotid atherosclerosis and cognitive function in midlife: the Beaver Dam Offspring Study. Atherosclerosis. 2011;219(1):330–333. doi: 10.1016/j.atherosclerosis.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bond MG, Barnes RW, Riley WA, et al. High-resolution B-mode ultrasound scanning methods in the Atherosclerosis Risk in Communities Study (ARIC) J Neuroimaging. 1991;1(2):68–73. [PubMed] [Google Scholar]
- 39.Riley WA, Barnes RW, Bond MG, et al. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. J Neuroimaging. 1991;1(4):168–172. [PubMed] [Google Scholar]
- 40.Zhong W, Cruickshanks KJ, Schubert CR, et al. Pulse wave velocity and cognitive function in older adults. Alzheimer Dis Assoc Disord. 2014;28(1):44–49. doi: 10.1097/WAD.0b013e3182949f06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 42.Johnsson LG, Hawkins JE., Jr Vascular changes in the human inner ear associated with aging. Ann Otol Rhinol Laryngol. 1972;81(3):364–376. doi: 10.1177/000348947208100307. [DOI] [PubMed] [Google Scholar]
- 43.Makishima K. Arteriolar sclerosis as a cause of presbycusis. Otolaryngology. 1978;86(2):ORL322–ORL326. doi: 10.1177/019459987808600225. [DOI] [PubMed] [Google Scholar]
- 44.Gates GA, Mills JH. Presbycusis. Lancet. 2005;366(9491):1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- 45.Spiess AC, Lang H, Schulte BA, Spicer SS, Schmiedt RA. Effects of gap junction uncoupling in the gerbil cochlea. Laryngoscope. 2002;112(9):1635–1641. doi: 10.1097/00005537-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 46.Gratton MA, Schulte BA. Alterations in microvasculature are associated with atrophy of the stria vascularis in quiet-aged gerbils. Hear Res. 1995;82:44–52. doi: 10.1016/0378-5955(94)00161-i. [DOI] [PubMed] [Google Scholar]
- 47.Di Girolamo S, Quaranta N, Picciotti P, Torsello A, Wolf F. Age-related histopathological changes of the stria vascularis: an experimental model. Audiology. 2001;40(6):322–326. [PubMed] [Google Scholar]
- 48.Suzuki T, Nomoto Y, Nakagawa T, et al. Age-dependent degeneration of the stria vascularis in human cochleae. Laryngoscope. 2006;116:1846–1850. doi: 10.1097/01.mlg.0000234940.33569.39. [DOI] [PubMed] [Google Scholar]
- 49.Tota-Maharaj R, Blaha MJ, McEvoy JW, et al. Coronary artery calcium for the prediction of mortality in young adults < 45 years old and elderly adults > 75 years old. Eur Heart J. 2012;33(23):2955–2962. doi: 10.1093/eurheartj/ehs230. [DOI] [PubMed] [Google Scholar]
- 50.McClelland RL, Nasir K, Budoff M, Blumenthal RS, Kronmal RA. Arterial age as a function of coronary artery calcium (from the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Cardiol. 2009;103(1):59–63. doi: 10.1016/j.amjcard.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaha MJ. The future of CV risk prediction: multisite imaging to predict multiple outcomes. JACC Cardiovasc Imaging. 2014;7(1):1054–1056. doi: 10.1016/j.jcmg.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Heinzel S, Liepelt-Scarfone I, Roeben B, et al. A neurodegenerative vascular burden index and the impact on cognition. Front Aging Neurosci. 2014;6:161. doi: 10.3389/fnagi.2014.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell P, Gopinath B, Wang JJ, et al. Five-year incidence and progression of hearing impairment in an older population. Ear Hear. 2011;32(2):251–257. doi: 10.1097/AUD.0b013e3181fc98bd. [DOI] [PubMed] [Google Scholar]
- 54.Gopinath B, Flood VM, McMahon CM, Burlutsky G, Smith W, Mitchell P. The effects of smoking and alcohol consumption on age-related hearing loss: the Blue Mountains Hearing Study. Ear Hear. 2010;31(2):277–282. doi: 10.1097/AUD.0b013e3181c8e902. [DOI] [PubMed] [Google Scholar]