Abstract
Mycobacterium tuberculosis is the major causative agent of tuberculosis (TB). The gamma interferon (IFN-γ) release assay (IGRA) has been widely used to diagnose TB by testing cell-mediated immune responses but has no capacity for distinguishing between active TB and latent TB infection (LTBI). This study aims to identify a parameter that will help to discriminate active TB and LTBI. Whole-blood samples from 33 active TB patients, 20 individuals with LTBI, and 26 non-TB controls were applied to the commercial IFN-γ release assay, QuantiFERON-TB Gold In-Tube, and plasma samples were analyzed for interleukin-2 (IL-2), IL-6, IL-8, IL-10, IL-13, tumor necrosis factor-alpha (TNF-α), IFN-γ, monokine induced by IFN-γ (MIG), interferon gamma inducible protein 10 (IP-10), interferon-inducible T cell alpha chemoattractant (I-TAC), and monocyte chemoattractant protein 1 (MCP-1) by using a commercial cytometric bead array. The Mycobacterium tuberculosis antigen-specific production of most of the assayed cytokines and chemokines was higher in the active TB than in the LTBI group. The mitogen-induced responses were lower in the active TB than in the LTBI group. When the ratio of TB-specific to mitogen-induced responses was calculated, IL-2, IL-6, IL-10, IL-13, TNF-α, IFN-γ, MIG, and IP-10 were more useful in discriminating active TB from LTBI. In particular, most patients showed higher IP-10 production to Mycobacterium tuberculosis antigens than to mitogen at the individual level, and the ratio for IP-10 was the strongest indicator of active infection versus LTBI with 93.9% sensitivity and 90% specificity. In conclusion, the ratio of the TB-specific to the mitogen-induced IP-10 responses showed the most promising accuracy for discriminating active TB versus LTBI and should be further studied to determine whether it can serve as a biomarker that might help clinicians administer appropriate treatments.
INTRODUCTION
Mycobacterium tuberculosis, the major causative agent for tuberculosis (TB), is among the most successful human pathogens, infecting approximately 8.6 million people and leading to 1.3 million deaths each year (1). It is estimated that 2 billion people live with latent TB infection (LTBI) and are therefore a potential source of active TB (2, 3). Identifying LTBI is necessary in order to reduce the risk of development of the disease, while diagnosis of active TB can enable rapid treatment and disease control. To this end, diagnostic biomarkers that can accurately indicate disease status are needed (4, 5).
There is presently no diagnostic gold standard for LTBI. Until recently, the tuberculin skin test (TST) involving the intracutaneous injection of purified protein derivative (PPD) into the forearm was the only available method for diagnosing LTBI. However, PPD cross-reacts with nontuberculous mycobacteria as well as with Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine and has poor sensitivity in immunocompromised patients (6). The interferon gamma (IFN-γ) release assay (IGRA) has been widely used in clinical practice and public health policy for TB diagnosis (7). Commercial IGRAs such as the QuantiFERON-TB Gold In-Tube test (QFT-GIT) measure responses to M. tuberculosis-specific antigens, including early secretory antigenic target-6 (ESAT-6), culture filtrate protein 10 (CFP-10), and TB7.7 antigens, and discriminate between a M. tuberculosis infection and an immunity-induced response to BCG vaccination (8, 9). The IGRA therefore seems to be useful for the diagnosis of TB in individuals who have been recently vaccinated with BCG and those who are immunocompromised. However, IGRAs have only shown mediocre results in the latter group and are also unable to discriminate between active TB and LTBI (10).
In addition to IFN-γ, many cytokines and chemokines have been investigated as potential biomarkers for M. tuberculosis infection and disease status (11–18). The levels of several cytokines, including interleukin-6 (IL-6), IL-10, IL-15, chemokine (C-X-C) motif ligand (CXCL)/interferon gamma-inducible protein 10 (IP-10), and monocyte chemoattractant protein 2 (MCP-2), were significantly higher in TB patients than in healthy controls (7, 11, 18–21); although these finding suggest important roles for these factors in disease pathogenesis, they are not sufficient for diagnosing active as opposed to latent infections. Several studies have also suggested that biomarker combinations such as IFN-γ−tumor necrosis factor alpha (TNF-α), IFN-γ−IL-2, IFN-γ−IL-4, and IL-15−MCP-1 might be more sensitive than single markers (18, 22–24). However, a better biomarker to improve the sensitivity and specificity in discriminating between active TB and LTBI is still needed.
The current study was designed to develop a new biomarker for the diagnosis of different stages of M. tuberculosis infection. Participants were recruited following approval of the protocol by the ethics review committee. Interestingly, during our study, we found that the method for calculating the ratio of IP-10 levels in response to TB antigens and mitogen might be more sensitive in discriminating patients with active TB from individuals with LTBI or healthy controls without TB than that for measuring the concentrations of TB antigen-induced IP-10 or any other cytokines and chemokines that we have tested in this study.
MATERIALS AND METHODS
Study population.
Participants were recruited from November 2010 to October 2012 following approval of the protocol by the Severance Hospital Ethics Review Committee (institutional review board [IRB] no. 4-2010-0213). All study subjects gave informed consent for the use of the samples obtained. The diagnosis of active pulmonary TB was based on all clinical, radiological, microbiological, and pathological results. Active TB was confirmed by culture of M. tuberculosis from respiratory specimens or by the presence of caseating granulomas in lung tissue. Patients with lymphocyte-predominant exudative effusions and adenosine deaminase levels of >40 IU/liter or those with a high likelihood of active TB based on clinical and radiological results were included if their conditions improved after antituberculosis treatment. In detail, among a total of 33 active TB patients, 25 patients were diagnosed as having active TB based on positive cultures of M. tuberculosis and 2 patients were diagnosed based on pathological results, which showed chronic granulomatous inflammation and positive PCRs. The remaining 6 patients were diagnosed based on the clinical and the radiological information. Since their chest computed tomography scans showed typical centrilobular nodules on their upper lungs, which resolved after a full-course of anti-TB treatment, they were finally classified as having active TB. The LTBI group consisted of household members with positive TST (>10 mm), who had been in close contact and lived with a patient with microbiologically confirmed active pulmonary TB for longer than 1 month (25). None of the household contacts showed the clinical symptoms or chest radiographic findings indicative of TB. The non-TB control group consisted of healthy adults with a negative TST, who were free of TB symptoms and did not have any contact with active pulmonary TB patients. All control subjects had normal chest X-ray results.
TST.
Skin tests were performed using RT-23 tuberculin (Statens Serum Institute, Copenhagen, Denmark). The induration was measured 48 to 72 h later, and the cutoff for a positive result in immunocompetent subjects was 10 mm, according to the Korean TB guideline (25).
IFN-γ determination by QFT-GIT assay.
The IFN-γ release assay was performed using the QFT-GIT assay kit. Briefly, 1 ml whole blood was drawn into the three QFT-GIT tubes precoated with saline (control), M. tuberculosis-specific antigens (ESAT-6, CFP-10, and TB7.7), or mitogen and incubated for 20 h at 37°C. After centrifugation, the supernatant was collected and stored frozen at −20°C until the IFN-γ concentration was determined by an enzyme-linked immunosorbent assay (ELISA) using a QFT Gold kit. The results were calculated using the manufacturer's QFT-GIT software. With the QFT Gold kits, we used 7 kinds of tubes with different lot numbers (80181, 80221, 80231, 80261, 80321, 80401, and 80451).
Multiplex analysis of cytokine production.
Levels of IL-2, IL-6, IL-8, IL-10, IL-13, TNF-α, IFN-γ, monokine induced by IFN-γ (MIG), IP-10, I-TAG, and MCP-1 were measured in the supernatants described above with commercial cytometric bead array human cytokine and chemokine flex sets (BD Biosciences, San Jose, CA, USA) according to the manufacturer's recommendations.
Data analysis.
Data were analyzed using GraphPad Prism version 5.02 (GraphPad Software, San Diego, CA, USA). Variables that were not normally distributed were compared across and within groups using the nonparametric Mann-Whitney test. Antigen-dependent and mitogen-induced biomarker production values were measured by subtracting the concentration measured in the control tube from those measured in the antigen and mitogen tubes, respectively. The diagnostic performances of the antigen-dependent and mitogen-induced biomarker values were compared with a receiver operating characteristic (ROC) curve analysis to determine the area under the curve (AUC) and the optimal cutoff levels.
RESULTS
Characteristics of the study population.
The study included 33 patients with active pulmonary TB, 20 subjects with LTBI, and 26 non-TB controls (Table 1). The LTBI group was slightly older than the other groups: median ages were 44 years (range, 22–60 years) for the LTBI group, 30 years (range, 20–63 years) for the active TB group, and 25 years (range, 22–54 years) for the non-TB control group. The male to female ratios were 4/16, 19/12, and 12/14, respectively, and BCG scars were seen in 18 (90%), 21 (63.6%), and 14 (53.8%) of the subjects, respectively. For the QFT-GIT assay, results for all of the non-TB controls were negative; positive results were observed in 11 (55%) subjects with LTBI and all 33 (100%) patients with active TB. We defined the LTBI group as TST-positive individuals who had been in close contact and lived with patients with microbiologically confirmed active pulmonary TB for longer than 1 month, according to the Korean TB guidelines (25).
TABLE 1.
Characteristics of the study subjects for the multicytokine assay
| Characteristica | Result forb: |
||
|---|---|---|---|
| Controls (n = 26) | LTBI subjects (n = 20) | Active TB patients (n = 33) | |
| Age (median [range]) (yrs) | 25 (22–54) | 44 (22–60) | 30 (20–63) |
| Sex (male/female) | 12/14 | 4/16 | 19/12 |
| Presence of a BCG scar (n [%]) | 14 (53.8) | 18 (90) | 21 (63.6) |
| Positive TST (n [%]) | 20 (100) | 3 (75) (29 NDc) | |
| Pulmonary TB diagnosis | |||
| Positive AFB culture (n [%]) | 25 (75.8) | ||
| Positive QFT-GIT result (n [%]) | 0 (0) | 11 (55) | 33 (100) |
AFB, acid-fast bacilli; BCG, bacille Calmette-Guérin; QFT-GIT, QuantiFERON-TB Gold In-Tube test; TST, tuberculin skin test.
LTBI, latent tuberculosis infection; TB, tuberculosis.
ND, not determined.
Comparison of cytokine and chemokine levels in response to TB antigens or mitogen.
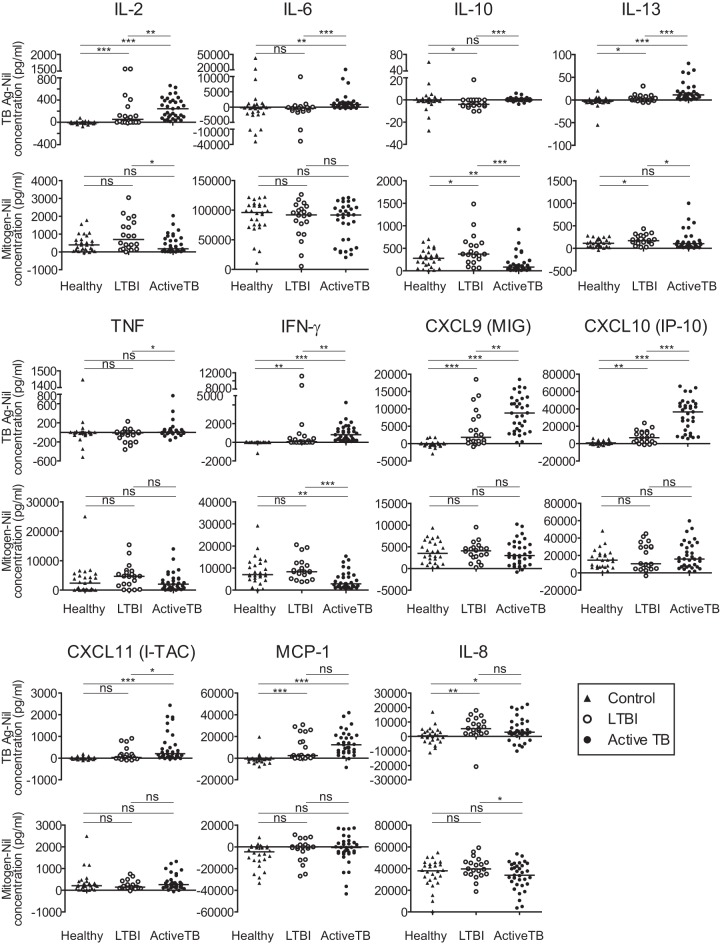
To identify new biomarkers for detecting active TB and discriminating LTBI, the levels of 11 cytokines and chemokines were evaluated (Fig. 1). The baseline levels of all cytokines, as observed in unstimulated whole-blood samples from each group, were subtracted from the corresponding levels in TB antigen- and mitogen-stimulated samples.
FIG 1.
Cytokine levels in active TB and LTBI. Whole blood was stimulated with M. tuberculosis-specific antigens (ESAT-6, CFP-10, and TB7.7) or with mitogen for 24 h, and the levels of TB-specific and mitogen-induced cytokines and chemokines were measured in active TB patients, LTBI subjects, and non-TB healthy control subjects. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
The median levels of IL-2, IL-6, IL-13, MIG, IP-10, I-TAG, MCP-1, and IL-8 were higher in active TB patients than in non-TB controls, in accordance with IFN-γ responses measured by the QFT-GIT assay. In addition, IL-2, IL-6, IL-10, IL-13, TNF-α, MIG, IP-10, and I-TAG levels were higher in active TB patients than in LTBI subjects, which was also consistent with the IFN-γ response. The levels of these factors differed significantly between samples from non-TB controls and active TB patients and between samples from active TB patients and LTBI subjects, implying that they are potentially useful biomarkers.
Ratio of TB-specific to mitogen-induced responses.
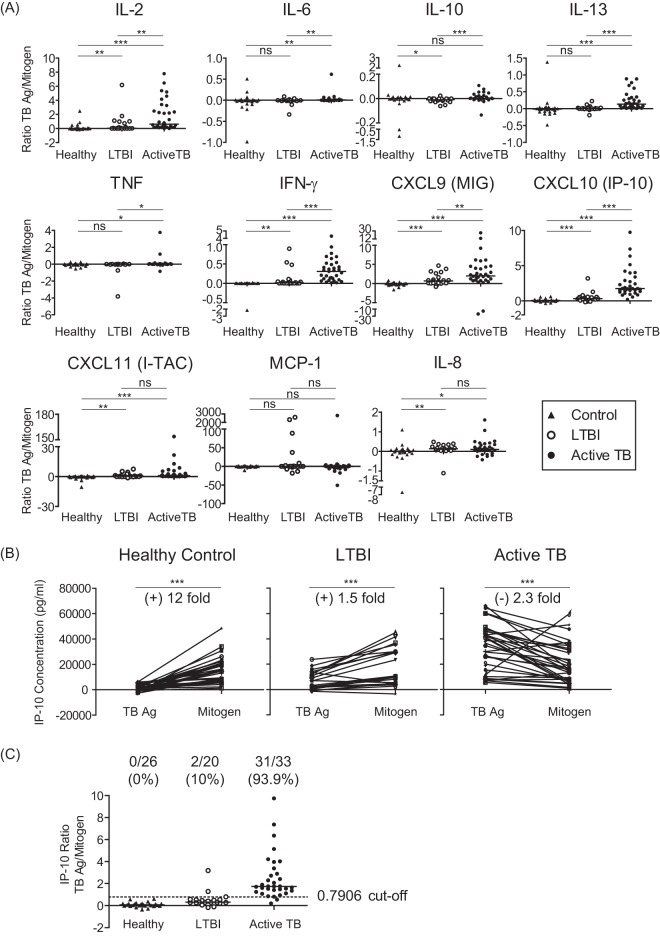
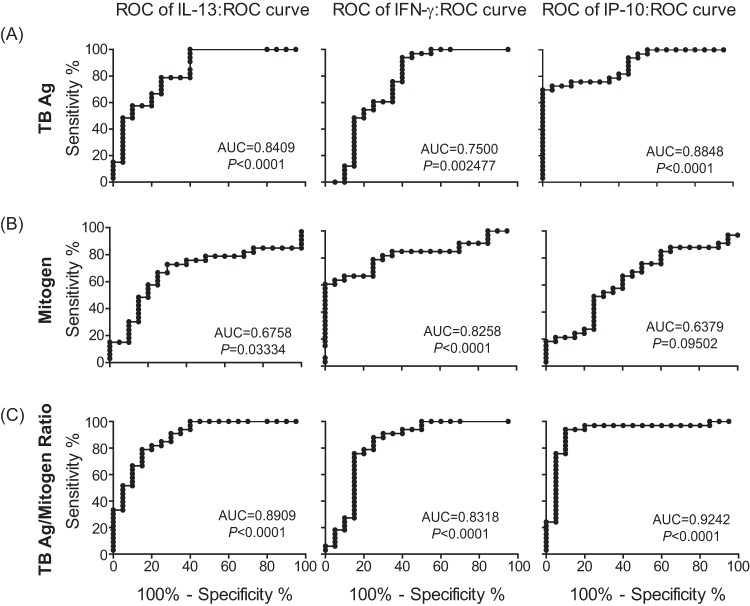
While analyzing the responses after stimulation of whole-blood samples with TB antigens or mitogen, we found that the levels of IL-2, IL-10, IL-13, IL-8, and IFN-γ in response to mitogen were relatively lower in active TB patients than in LTBI individuals (Fig. 1). Even though this phenomenon had been previously observed in some studies (13, 26), it has not been seriously considered. Based on our finding, the ratio of TB antigen-specific to mitogen-induced nonspecific responses was therefore calculated in individual samples (Fig. 2A). Interestingly, the ratios for IL-2, IL-6, IL-13, TNF-α, MIG, IP-10, I-TAG, IL-8, and IFN-γ were markedly higher in active TB patients than in non-TB controls. In addition, the ratios for IL-2, IL-6, IL-10, IL-13, TNF-α, MIG, IP-10, and IFN-γ were higher in active TB patients than in LTBI individuals. The diagnostic performances of the biomarkers were evaluated using ROC curves. The AUC is widely recognized as a measure of the discriminatory ability of a diagnostic test; in general, ROC curves with an AUC of 0.8 are considered to have good diagnostic ability. The biomarkers with potential (AUC values of >0.8) are summarized in Table 2 and Fig. 3. ROC curves corresponding to the TB-specific responses of IL-13, IFN-γ, and IP-10 revealed AUCs of 0.8409, 0.75, and 0.8848, respectively (Table 2), which were statistically significant. However, the ratios of TB-specific to mitogen-induced responses were associated with higher AUC values (0.8909, 0.8318, and 0.9242, respectively) that were also statistically significant compared to those of the TB-specific responses (Table 2). Importantly, the AUC for the IP-10 ratio displayed the highest value among the AUCs for the tested parameters, including the TB-specific IP-10 response, indicating that the IP-10 ratio is the most valuable diagnostic marker to discriminate active TB versus LTBI. This tendency was still observed even though we used tubes with different lot numbers, indicating that our finding is not due to lot-to-lot differences (data not shown).
FIG 2.
Ratio of TB-specific to mitogen-induced responses. Whole blood was stimulated with M. tuberculosis-specific antigens (ESAT-6, CFP-10, and TB7.7) or with mitogen for 24 h, and the levels of TB-specific and mitogen-induced cytokines and chemokines were measured in active TB patients, LTBI subjects, and non-TB healthy control subjects. (A) Ratios of TB-specific to mitogen-induced responses are shown. (B) Comparison of TB-specific and mitogen-induced IP-10 levels. (C) The optimal cutoff value and positive cases are indicated on the graph. *P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
TABLE 2.
AUC values obtained from the receiver operating characteristic curve analysis
| Analytical criteriona | Targetb | AUCc | 95% CId | P |
|---|---|---|---|---|
| TB Ag | IL-13 | 0.8409 | 0.714–0.927 | <0.0001 |
| IFN-γ | 0.7500 | 0.612–0.859 | 0.002477 | |
| IP-10 | 0.8848 | 0.767–0.956 | <0.0001 | |
| TB Ag/mitogen ratio | IL-13 | 0.8909 | 0.775–0.960 | <0.0001 |
| IFN-γ | 0.8318 | 0.704–0.920 | <0.0001 | |
| IP-10 | 0.9242 | 0.818–0.979 | <0.0001 |
TB, tuberculosis; Ag, antigen,
IL-13, interleukin-13; IFN-γ, interferon-gamma; IP-10, interferon gamma-induced protein 10/chemokine (C-X-C) motif ligand-10.
AUC, area under the curve.
CI, confidence interval.
FIG 3.
ROC curves for TB-specific and mitogen-induced cytokine responses. The AUCs of TB-specific (A) and mitogen-induced (B) IL-13, IFN-γ, and IP-10 levels and the ratios of TB-specific to mitogen-induced responses (C) were compared between active TB patients and LTBI. The AUCs and the P values are indicated on the graphs.
The TB-specific IP-10 levels were higher in active TB patients than in LTBI subjects, whereas no differences were observed for mitogen-induced levels between the two groups. The TB-specific and mitogen-induced IP-10 levels were compared for each individual to investigate the reason that the IP-10 ratio showed a greater difference than the IP-10 level for TB antigen in discriminating active TB versus LTBI (Fig. 2B). Interestingly, most of the active TB patients showed a higher response to TB antigen stimulation but few to mitogen induction. However, healthy controls and LTBI subjects had lower TB-specific than mitogen-induced IP-10 levels, accounting for the significant difference in the IL-10 ratios between the groups.
The optimal cutoff values for the discrimination between active TB versus LTBI were determined by ROC curve analysis and are summarized in Table 3. The cutoffs for the median values of TB-specific IL-13, IFN-γ, and IP-10 were 0, 172.84, and 23780.88, respectively; these were able to discriminate 100%, 93.4%, and 69.7% of the true active TB patients (sensitivity) and 60%, 60%, and 100% of the true LTBI subjects (specificity), respectively. Cutoffs for the ratios of TB-specific to nonspecific responses were 0.0363, 0.0454, and 0.7906, respectively, and were able to discriminate 78.79%, 87.88%, and 93.34% of the true active TB cases and 85%, 75%, and 90% of the true LTBI cases, respectively. Unexpectedly, the IP-10 cutoff value of 0.7906 was able to distinguish between active TB and LTBI with 93.4% sensitivity and 90% specificity (Fig. 2C). Based on this optimal cutoff value, 31 of 33 active TB patients were positively identified, but none of the non-TB healthy controls and only 2 of 20 LTBI subjects were positive. The 2 active TB patients who were identified as negative had lower TB-specific than mitogen-induced IP-10 levels, but no other unusual characteristics.
TABLE 3.
Optimal cutoff values with associated sensitivities and specificities to discriminate between LTBI and active TB
| Analytical criteriona | Targetb | Cutoff | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| TB Ag | IL-13 | 0 | 100 | 60 |
| IFN-γ | 172.84 | 93.94 | 60 | |
| IP-10 | 23,780.88 | 69.7 | 100 | |
| TB Ag/mitogen ratio | IL-13 | 0.0363 | 78.79 | 85 |
| IFN-γ | 0.0454 | 87.88 | 75 | |
| IP-10 | 0.7906 | 93.94 | 90 |
TB, tuberculosis; Ag, antigen,
IL-13, interleukin-13; IFN-γ, interferon-gamma; IP-10, interferon gamma-induced protein 10/chemokine (C-X-C) motif ligand-10.
DISCUSSION
Commercial IGRAs such as the QFT-GIT are widely used to diagnose M. tuberculosis infection, but they cannot distinguish active TB disease from LTBI (10, 27). In the current study, several cytokines and chemokines were evaluated in active TB patients, LTBI subjects, and non-TB healthy controls for their discriminatory potential for the enhanced diagnosis of active TB infection compared to that of the conventional QFT-GIT assay. In accordance with previous findings, IL-2, IL-13, MIG, IP-10, and IFN-γ were present at higher levels in active TB patients than in LTBI subjects and non-TB healthy controls. In addition, IL-6 and IL-10, TNF-α, and I-TAG levels were higher in the active TB than in the LTBI group. However, these markers were unable to distinguish between the groups accurately. Moreover, single cytokines may only reflect inflammation; thus, mitogen-induced responses were assumed to reflect the subjects' immune status. When the ratio of the TB-specific to mitogen-induced responses was calculated, ratios for IL-13, IP-10, and IFN-γ were higher than the corresponding values for TB antigen-stimulated responses. In particular, the IP-10 ratio, when the optimal cutoff value was applied, identified 93.9% of the active TB and 90% of the LTBI subjects out of the TB-related subjects.
Several studies tried to identify potential biomarkers for diagnosing active TB and discriminating between active TB and LTBI. Wang et al. reported that the IL-2/IFN-γ ratio discriminated between active TB and LTBI with a sensitivity of 77.2% and a specificity of 87.2% (24). In addition, Frahm et al. reported that the combination of IL-15 and MCP-1 identified active TB and LTBI with a sensitivity of 83% and a specificity of 88% (18). In our study, we found that the IP-10 ratio of TB-specific to mitogen-induced responses displayed better sensitivity and specificity (93.9% and 90%, respectively) in identifying active TB from LTBI than other reported parameters. Therefore, the use of the IP-10 ratio might support an improved diagnosis of active TB disease when combined with the standard diagnostic methods, including microbiology, molecular tests, and clinical and radiological assessments. IP-10 (also known as CXCL10 or IFN-γ-induced protein 10) is a chemokine that is expressed at a high level in inflammatory diseases such as systemic lupus erythematosus, thyroid disease, acute coronary syndrome, and allergies (28–30). IP-10 is expressed in the bronchial epithelium (31) and in inflamed tissues (32) of active TB patients, who demonstrate upregulation of the helper T (Th) 1-type cytokine IFN-γ. IP-10 secretion may play an important role in recruiting activated T cells, which is necessary for protective immunity. In addition, chemokines and their receptors, including IP-10, contribute to the formation and maintenance of granulomas in TB (33). IP-10 plays a role in M. tuberculosis infection, and several studies have shown that antigen-stimulated IP-10 responses have a sensitivity similar to that of QFT-GIT for detecting active TB (12, 13, 34, 35). Our results also showed a higher level of IP-10 in active TB patients, consistent with previously published studies; IP-10 can therefore serve as a potential diagnostic biomarker.
Since there is no gold standard for diagnosing LTBI, the biomarker studies to discriminate LTBI from active TB, including our study, are often confronted with a difficulty in defining an LTBI group. In the current study, we defined the LTBI group as persons with no sign of active TB, who had close contact with infectious TB patients and positive TST according to the Korean TB guideline (25). Since 45% of the LTBI individuals defined in our study were QFT negative, it would be worthwhile to compare the responses after dividing the LTBI group into those who were QFT positive and negative. Indeed, we found that the ratio of IP-10 in response to TB antigens and mitogen is higher in QFT-positive than in QFT-negative individuals, suggesting that the TB antigen-specific IFN-γ response and IP-10 production have a certain relation (see Fig. S1 in the supplemental material). However, although we restricted the LTBI group to the persons positive for both the TST and QFT, it was still obvious that the ratio of TB antigen-induced and mitogen-induced IP-10 productions is the most reliable parameter to discriminate between the LTBI individuals and active TB patients with high values for both sensitivity and specificity (see Table S1 in the supplemental material). These data indicate that the method using the IP-10 ratio might be applicable to help in the diagnosis for distinguishing active TB from LTBI, irrespective of the QFT response in defining the LTBI group.
A potential diagnostic biomarker that is able to discriminate active TB from LTBI, depending on the IP-10 cutoff value, was identified in this study. This parameter reflects individuals' immune status, thereby increasing the detection rate and improving diagnostic accuracy. Based on the bias in sensitivity and specificity evidenced by the cutoff value, the ratio of TB-specific to mitogen-induced responses might be a reliable diagnostic biomarker for active TB. Although additional studies with a larger sample size are needed to confirm the clinical utility of this ratio, these findings present a promising method, which can discriminate between active TB and LTBI, to support the current diagnostic methods for TB. This potential biomarker might help the detection of patients with real active TB within 2 h with improved sensitivity and specificity, thereby improving the accuracy of diagnosis and advancing the time of therapy.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Korean Health Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (grants A101750 and HI13C0833), and by the Bio & Medical Technology Development Program (grant 2012M3A9B4028264) of the National Research Foundation of Korea (NRF), funded by the Korean government (MEST).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JCM.02758-14.
REFERENCES
- 1.Eurosurveillance editorial team. 2013. WHO publishes global tuberculosis report 2013. Euro Surveill 18(43):pii=20615 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20615. [PubMed] [Google Scholar]
- 2.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 3.Lin PL, Flynn JL. 2010. Understanding latent tuberculosis: a moving target. J Immunol 185:15–22. doi: 10.4049/jimmunol.0903856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufmann SH. 2010. Novel tuberculosis vaccination strategies based on understanding the immune response. J Intern Med 267:337–353. doi: 10.1111/j.1365-2796.2010.02216.x. [DOI] [PubMed] [Google Scholar]
- 5.Parida SK, Kaufmann SH. 2010. The quest for biomarkers in tuberculosis. Drug Discov Today 15:148–157. doi: 10.1016/j.drudis.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Nahid P, Pai M, Hopewell PC. 2006. Advances in the diagnosis and treatment of tuberculosis. Proc Am Thorac Soc 3:103–110. doi: 10.1513/pats.200511-119JH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.John SH, Kenneth J, Gandhe AS. 2012. Host biomarkers of clinical relevance in tuberculosis: review of gene and protein expression studies. Biomarkers 17:1–8. doi: 10.3109/1354750X.2011.628048. [DOI] [PubMed] [Google Scholar]
- 8.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. 2005. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis 24:529–536. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 9.Jasmer RM, Nahid P, Hopewell PC. 2002. Clinical practice. Latent tuberculosis infection. N Engl J Med 347:1860–1866. [DOI] [PubMed] [Google Scholar]
- 10.Lange C, Pai M, Drobniewski F, Migliori GB. 2009. Release assays for the diagnosis of active tuberculosis: sensible or silly? Eur Respir J 33:1250–1253. doi: 10.1183/09031936.00019709. [DOI] [PubMed] [Google Scholar]
- 11.Mattos AM, Almeida Cde S, Franken KL, Alves CC, Abramo C, de Souza MA, L'Hotellier M, Alves MJ, Ferreira AP, Oliveira SC, Ottenhoff TH, Teixeira HC. 2010. Increased IgG1, IFN-γ, TNF-α and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol 22:775–782. doi: 10.1093/intimm/dxq429. [DOI] [PubMed] [Google Scholar]
- 12.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Kofoed K, Eugen-Olsen J, Ravn P. 2007. CXCL10/IP-10 release is induced by incubation of whole blood from tuberculosis patients with ESAT-6, CFP10 and TB77. Microbes Infect 9:806–812. doi: 10.1016/j.micinf.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Ruhwald M, Bodmer T, Maier C, Jepsen M, Haaland MB, Eugen-Olsen J, Ravn P, TBNET. 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J 32:1607–1615. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 14.Sargentini V, Mariotti S, Carrara S, Gagliardi MC, Teloni R, Goletti D, Nisini R. 2009. Cytometric detection of antigen-specific IFN-gamma/IL-2 secreting cells in the diagnosis of tuberculosis. BMC Infect Dis 9:99. doi: 10.1186/1471-2334-9-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruhwald M, Bjerregaard-Andersen M, Rabna P, Eugen-Olsen J, Ravn P. 2009. IP-10, MCP-1, MCP-2, MCP-3, and IL-1RA hold promise as biomarkers for infection with M. tuberculosis in a whole blood based T-cell assay. BMC Res Notes 2:19. doi: 10.1186/1756-0500-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong JY, Jung GS, Kim H, Kim YM, Lee HJ, Cho SN, Kim SK, Chang J, Kang YA. 2012. Efficacy of inducible protein 10 as a biomarker for the diagnosis of tuberculosis. Int J Infect Dis 16:e855–859. doi: 10.1016/j.ijid.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Anbarasu D, Raja CP, Raja A. 2013. Multiplex analysis of cytokines/chemokines as biomarkers that differentiate healthy contacts from tuberculosis patients in high endemic settings. Cytokine 61:747–754. doi: 10.1016/j.cyto.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Frahm M, Goswami ND, Owzar K, Hecker E, Mosher A, Cadogan E, Nahid P, Ferrari G, Stout JE. 2011. Discriminating between latent and active tuberculosis with multiple biomarker responses. Tuberculosis 91:250–256. doi: 10.1016/j.tube.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Broser M, Rom WN. 1994. Activation of the interleukin 6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by nuclear factors NF-IL6 and NF-κB. Proc Natl Acad Sci U S A 91:2225–2229. doi: 10.1073/pnas.91.6.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. 1999. Interaction of Mycobacterium tuberculosis-induced transforming growth factor β1 and interleukin-10. Infect Immun 67:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su WL, Perng WC, Huang CH, Yang CY, Wu CP, Chang FY, Chen JH. 2010. Identification of cytokines in whole blood for differential diagnosis of tuberculosis versus pneumonia. Clin Vaccine Immunol 17:771–777. doi: 10.1128/CVI.00526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemeth J, Winkler HM, Karlhofer F, Selenko-Gebauer N, Graninger W, Winkler S. 2010. T cells co-producing Mycobacterium tuberculosis-specific type 1 cytokines for the diagnosis of latent tuberculosis. Eur Cytokine Netw 21:34–39. doi: 10.1684/ecn.2009.0182. [DOI] [PubMed] [Google Scholar]
- 23.Wassie L, Demissie A, Aseffa A, Abebe M, Yamuah L, Tilahun H, Petros B, Rook G, Zumla A, Andersen P, Doherty TM, VACSEL/VACSIS Study Group . 2008. Ex vivo cytokine mRNA levels correlate with changing clinical status of Ethiopian TB patients and their contacts over time. PLoS One 3:e1522. doi: 10.1371/journal.pone.0001522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Diao N, Lu C, Wu J, Gao Y, Chen J, Zhou Z, Huang H, Shao L, Jin J, Weng X, Zhang Y, Zhang W. 2012. Evaluation of the diagnostic potential of IP-10 and IL-2 as biomarkers for the diagnosis of active and latent tuberculosis in a BCG-vaccinated population. PLoS One 7:e51338. doi: 10.1371/journal.pone.0051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korean Centers for Disease Control and Prevention. 2014. Guidelines for tuberculosis, vol 2. Korea Centers for Disease Control and Prevention, Seoul, Republic of Korea. [Google Scholar]
- 26.Mueller H, Detjen AK, Schuck SD, Gutschmidt A, Wahn U, Magdorf K, Kaufmann SH, Jacobsen M. 2008. Mycobacterium tuberculosis-specific CD4+, IFNγ+, and TNFα+ multifunctional memory T cells coexpress GM-CSF. Cytokine 43:143–148. doi: 10.1016/j.cyto.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Menzies D, Pai M, Comstock G. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 146:340–354. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 28.Lit LC, Wong CK, Tam LS, Li EK, Lam CW. 2006. Raised plasma concentration and ex vivo production of inflammatory chemokines in patients with systemic lupus erythematosus. Ann Rheum Dis 65:209–215. doi: 10.1136/ard.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romagnani P, Rotondi M, Lazzeri E, Lasagni L, Francalanci M, Buonamano A, Milani S, Vitti P, Chiovato L, Tonacchera M, Bellastella A, Serio M. 2002. Expression of IP-10/CXCL10 and MIG/CXCL9 in the thyroid and increased levels of IP-10/CXCL10 in the serum of patients with recent-onset Graves' disease. Am J Pathol 161:195–206. doi: 10.1016/S0002-9440(10)64171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawamura A, Miura S, Fujino M, Nishikawa H, Matsuo Y, Tanigawa H, Tomita S, Tsuchiya Y, Matsuo K, Saku K. 2003. CXCR3 chemokine receptor-plasma IP10 interaction in patients with coronary artery disease. Circ J 67:851–854. doi: 10.1253/circj.67.851. [DOI] [PubMed] [Google Scholar]
- 31.Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. 1997. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. Am J Respir Crit Care Med 155:1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 32.Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. 1999. The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells. J Immunol 162:3549–3558. [PubMed] [Google Scholar]
- 33.Rhoades ER, Cooper AM, Orme IM. 1995. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun 63:3871–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruhwald M, Aabye MG, Ravn P. 2012. IP-10 release assays in the diagnosis of tuberculosis infection: current status and future directions. Expert Rev Mol Diagn 12:175–187. doi: 10.1586/erm.11.97. [DOI] [PubMed] [Google Scholar]
- 35.Petrucci R, Abu Amer N, Gurgel RQ, Sherchand JB, Doria L, Lama C, Ravn P, Ruhwald M, Yassin M, Harper G, Cuevas LE. 2008. Interferon γ, interferon γ-induced-protein 10, and tuberculin responses of children at high risk of tuberculosis infection. Pediatr Infect Dis J 27:1073–1077. doi: 10.1097/INF.0b013e31817d05a3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.