Summary
The CGG trinucleotide repeat within the FMR1 gene is associated with multiple clinical disorders, including fragile X-associated tremor/ataxia syndrome, fragile X-associated primary ovarian insufficiency, and fragile X syndrome. Differences in the distribution and prevalence of CGG repeat length and of AGG interruption patterns have been reported among different populations and ethnicities. In this study we characterized the AGG interruption patterns within 3,065 normal CGG repeat alleles from nine world populations including Australia, Chile, United Arab Emirates, Guatemala, Indonesia, Italy, Mexico, Spain, and United States. Additionally, we compared these populations with those previously reported, and summarized the similarities and differences. We observed significant differences in AGG interruption patterns. Frequencies of longer alleles, longer uninterrupted CGG repeat segments and alleles with greater than 2 AGG interruptions varied between cohorts. The prevalence of fragile X syndrome and FMR1 associated disorders in various populations is thought to be affected by the total length of the CGG repeat and may also be influenced by the AGG distribution pattern. Thus, the results of this study may be important in considering the risk of fragile X-related conditions in various populations.
Keywords: AGG interruptions, FMR1 allele, CGG repeat, expansion, ethnicity
1. Introduction
Fragile X syndrome (FXS) and FMR1 associated disorders are predominantly the result of an expansion of a trinucleotide repeat element located within the 5’ UTR of the Fragile X Mental Retardation 1 gene (FMR1). In normal individuals the triplet repeat number varies in length from 5 to 44 CGG repeats. Intermediate alleles are between 45 and 54 repeats, premutation alleles are between 55 and 200 CGG repeats and above 200 CGG repeats are full mutation alleles (1). FMR1 full mutations cause FXS, while premutation alleles lead to fragile X-associated tremor/ataxia syndrome (FXTAS) in an estimated 40% of males and 8–16% of females with the mutation, and fragile X-associated primary ovarian insufficiency (FXPOI) in approximately 20% of female premutation carriers (2).
The CGG repeat element, like other trinucleotide repeats, is prone to expansion during transmission from parent to child (3). While the mechanism that gives rise to CGG repeat expansion in FMR1 is not understood, evidence suggests repair of single-strand breaks in the meiotically arrested oocytes form loops, which may be incorporated into the DNA through mismatch repair resulting in an expansion (4).
Normal alleles most frequently have 2 AGG interruptions, less frequently they have 1 AGG interruption or 0 AGG interruptions, and rarely greater than 2 AGG interruptions. Within normal alleles the patterns most commonly seen are 9 or 10 CGG repeat segments between interruptions (5,6). The 9-A-9-A-9 and 10-A-9-A-9 AGG interruption patterns predominate in all populations that have been studied, evidence that these two patterns were present 200,000 years ago during early divergence of human races or that a strong selection pressure exists at this locus (7).
In intermediate and premutation alleles the AGG interruptions tend to occur at the 5′ end of the locus and the pure CGG stretch, defined as the longest stretch of uninterrupted CGG repeats, is located at the 3′ end (8,9). The loss of AGG interruptions appear to have occurred multiple times during human evolution (10) but can be a late event in the mutation pathway that leads to expansion (11). It is rare for AGG interruptions to be lost during transmission, but observation of its occurrence has been reported (12–14).
A normal allele without an AGG interruption has been shown to have an increase mutational rate compared to an allele of similar size containing an AGG interruption (15–17). Differences in the distribution of AGG interruption patterns between ethnicities, has been reported, including differences in the frequency of alleles that exceed 35 CGG repeats in length and lack AGG interruptions. These higher frequencies are associated with increased prevalence of FXS (18). Conversely, highly interspersed CGG repeat alleles have been observed in the Basque, Native American, and Asian populations, which also have lower estimated FXS prevalence rates (19,20).
The presence of AGG interruptions does not seem to affect the transcriptional or translational expression of the FMR1 gene (21–24). However, the presence of AGG interruptions in both intermediate and premutation alleles has been shown to decrease the rate of instability (any change in CGG repeat size) and magnitude of size change in both paternal and maternal transmissions (12,25,26).
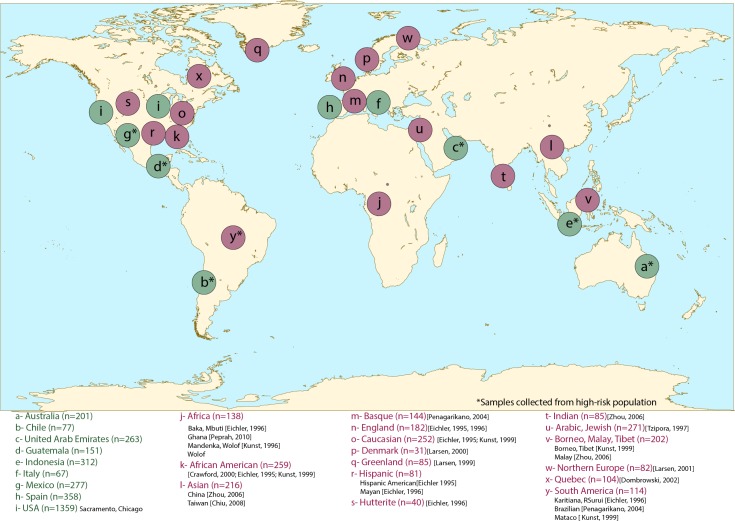
While the distribution of CGG repeat total length has been reported in a number of populations (27), fewer studies have reported the distribution of AGG interruptions within populations. This study reports on the AGG interruption patterns in a total of 3,065 normal alleles (9–40 CGG repeats) from 1,989 participants (males: n = 794; females: n = 1,195) from 9 countries: Australia, Chile, Emirates, Guatemala, Indonesia, Italy, Mexico, Spain, and USA. We compare these results with previous studies that reported AGG interruption patterns in global populations (Figure 1).
Figure 1.
The distribution of 25 global populations with AGG interruption patterns described. AGG interruption patterns were compared between the 9 newly characterized cohorts (a-i, in green) and with previously published studies (j-y, in red). Populations from previous studies were combined if geographical proximity was present to increase sample sizes. Cohorts with samples collected from high-risk populations are denoted with an asterisk, total sample size for each cohort and the studies reporting their AGG patterns are provided next to the cohort's name.
Our findings indicate that variations in CGG repeat allele sizes and AGG interruption pattern distributions exist between populations. Two populations (Australia and Indonesia), from the nine newly described, had a higher frequency of long pure CGG repeat stretches (greater than 20 pure CGG repeats), and the USA population had a lower frequency of these long pure stretches. These differences may be important when considering the burden of FMR1 associated disorders in different populations.
2. Materials and Methods
2.1. Participants
Genomic DNA from unrelated individuals with at least one normal FMR1 allele was included in this study (n = 3,065 alleles). These samples were previously screened to determine the prevalence rates of expanded alleles. Cohorts from Australia (n = 201) (28), Chile (n = 77), the United Arab Emirates (n = 263), Guatemala (n = 151) (29), Indonesia (n = 312) (30), Italy (n = 67), Mexico (n = 277), Spain (n = 358) (31), and the United States (n = 1,359) (32) were included. Individuals were recruited from the general population for the Italy, Spain, and United States samples. From the USA cohort, participants were from two different geographical areas: Sacramento (California) and Chicago (Illinois). The remaining samples were recruited from high-risk populations including intellectual disabilities, individuals with a family history of FXS and individuals with Parkinsonism. DNA isolation and AGG interruption genotyping were performed at the UC Davis MIND Institute Molecular Laboratory as previously described (25,32), except 67 alleles extracted and genotyped in Italy, following IRB approved protocols at the correspondent institutions. Only AGG interruption patterns of unrelated normal alleles less than or equal to 40 CGG repeats in length, therefore within the normal size range (33–35), were included in the study.
2.2. Statistical analysis
Distributions of categorical variables were compared among countries using chi-square tests. Chi-square test p-values were obtained by Monte Carlo simulation when the sample size assumptions for use of the chi-square distribution were not met.
In order to identify specific AGG interspersion patterns, total CGG lengths, pure CGG stretches, or AGG interruptions whose frequency in a given population was significantly higher or lower than would be expected under homogeneity, the adjusted residuals from the chi-square table (36) were compared to a standard normal distribution and the resulting p-values were adjusted for multiple testing using the Bonferroni correction. All analyses were conducted using R, version 2.13.0 (37).
3. Results
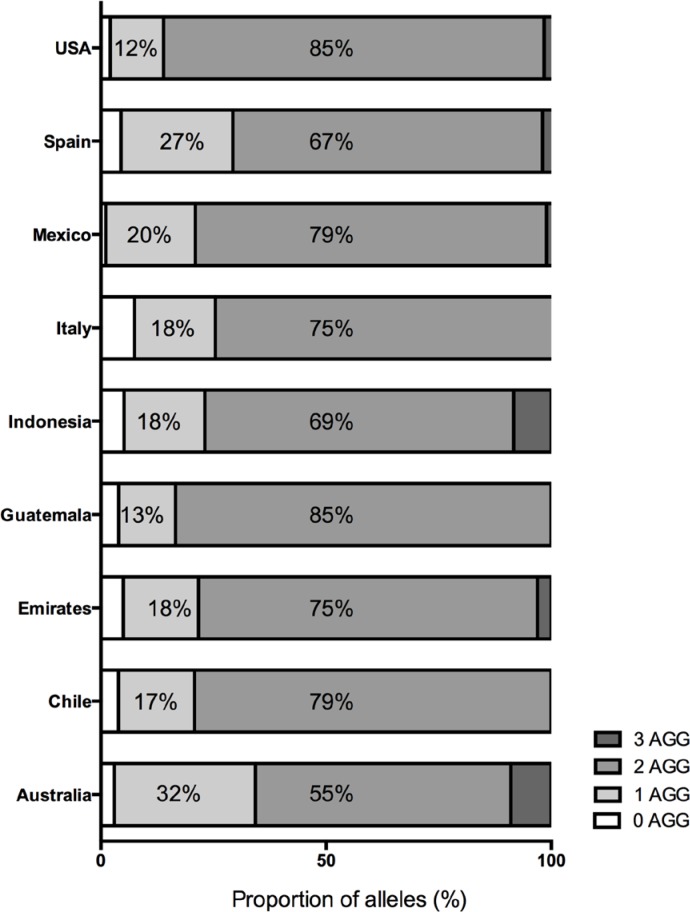
In the nine populations we determined the number and position of the AGG interruption within each CGG alleles and thus determined the AGG interruption pattern in 3,065 alleles. We observed 30 different CGG repeat lengths ranging from 9 to 40 CGG repeats and 231 different AGG interruption patterns, each allele contained no AGG interruptions up to 3 AGG interruptions. Consistent with previous population based studies of the CGG repeat locus, 29 and 30 CGG repeats were the most common allele sizes in all 9 populations. Indonesia was the only population with a greater proportion of alleles with 29 (39%) than 30 (28%) CGG repeats. Two AGG interruptions were present in at least 56% of the alleles genotyped for each population; 1 AGG interruption occurred in at least 11% of the alleles genotyped (Figure 2, Table 1).
Figure 2.
Distribution of number of AGG interruptions. For the nine newly characterized populations the proportion of alleles with 0 to 3 AGG interruptions is graphically represented. Alleles with 2 AGG interruptions were the most common in each cohort, followed by 1 AGG interruption. Four AGG interruptions were observed in Australia, United Arab Emirates, Indonesia, and Spain only. Within the nine populations no alleles were identified with more than 3 AGG interruptions.
Table 1. Summary of allele structure in nine populations.
| Items | Australia | Chile | Emirates | Guatemala | Indonesia | Italy | Mexico | Spain | USA |
|---|---|---|---|---|---|---|---|---|---|
| Total length | |||||||||
| 29 | 23 (11%) | 19 (25%) | 58 (22%) | 47 (31%) | 122 (39%) | 11 (16%) | 96 (35%) | 56 (16%) | 413 (30%) |
| 30 | 72 (36%) | 41 (53%) | 104 (40%) | 71 (47%) | 86 (28%) | 30 (45%) | 106 (38%) | 143 (40%) | 677 (50%) |
| Other | 106 (53%) | 17 (22%) | 101 (38%) | 33 (22%) | 104 (33%) | 26 (39%) | 75 (27%) | 159 (44%) | 269 (20%) |
| Pure Stretch | |||||||||
| 9 | 34 (17%) | 17 (22%) | 57 (22%) | 43 (28%) | 139 (45%) | 11 (16%) | 96 (35%) | 56 (16%) | 422 (31%) |
| 10 | 100 (50%) | 48 (62%) | 136 (52%) | 75 (50%) | 78 (25%) | 35 (52%) | 127 (46%) | 190 (53%) | 756 (56%) |
| Other | 67 (33%) | 12 (16%) | 70 (27%) | 33 (22%) | 95 (30%) | 21 (31%) | 54 (19%) | 112 (31%) | 181 (13%) |
| Number of AGG | |||||||||
| 0 | 6 (3%) | 3 (4%) | 13 (5%) | 6 (4%) | 16 (5%) | 5 (7%) | 3 (1%) | 16 (4%) | 28 (2%) |
| 1 | 63 (31%) | 13 (17%) | 44 (17%) | 19 (13%) | 56 (18%) | 12 (18%) | 55 (20%) | 89 (25%) | 161 (12%) |
| 2 | 114 (57%) | 61 (79%) | 198 (75%) | 126 (83%) | 214 (69%) | 50 (75%) | 216 (78%) | 246 (69%) | 1148 (84%) |
| 3 | 18 (9%) | 0 (0%) | 8 (3%) | 0 (0%) | 26 (8%) | 0 (0%) | 3 (1%) | 7 (2%) | 22 (2%) |
3.1. The distribution of total CGG length, pure CGG stretch, and number of AGG interruptions differs between populations
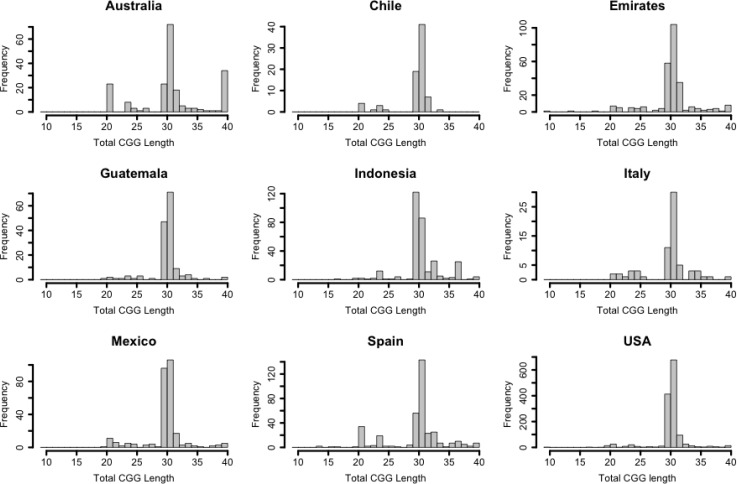
The mode of total CGG length was 30 in subjects from all countries examined except in Indonesia where it was 29 (Figure 3). The relative proportions of subjects with a total of 29 CGG repeats, 30 CGG repeats, or a value other than 29 or 30 differed significantly by country (p < 0.001) (Table 1). Likewise, the relative proportions of subjects with a pure stretch of 9 CGG repeats, 10 CGG repeats, or a value other than 9 or 10 differed significantly by country (p < 0.001). Examination of adjusted residuals suggests that significantly more alleles from Australia (p < 0.001), Emirates (p = 0.007) and Spain (p < 0.001) had total CGG lengths other than 29 or 30 and Australia (p < 0.001) and Spain (p < 0.001) had pure stretch lengths other than 9 or 10 repeats. Further, significantly more alleles from Indonesia (p < 0.001) had a total length of 29 CGG repeats, and significantly fewer USA (p < 0.001) alleles had total CGG lengths other than 29 and 30. Indonesia had significantly fewer alleles with a pure stretch of 10 CGG repeats (p < 0.001), Spain had fewer alleles with a pure stretch of 9 CGG repeats (p < 0.001) and USA had more alleles with 10 pure CGG repeats (p < 0.001) than was expected under homogeneity, where homogeneity would assume the same allele frequencies are present between populations.
Figure 3.
Histogram of total CGG length for 9 populations. The most common total length of CGG repeat sizes for the 9 populations are 30 and 29 CGG repeats, 30 is the most common for every population except Indonesia. Populations show difference in less prominent modes including some previously identified (20 CGG repeats, 23 CGG repeats, and 36 CGG repeats).
The proportion of alleles with 3 AGG interruptions differs significantly by country (p < 0.001) (Table 1); examination of adjusted residuals suggests that significantly more alleles from Indonesia (p < 0.001) and Australia (p < 0.001), and significantly fewer from USA (p = 0.005) had AGG interruptions than was expected under homogeneity.
3.2. AGG interspersion patterns by country
The most common AGG interspersion pattern was 10-A-9-A-9 in all countries except Indonesia. In Indonesia the most common AGG interspersion pattern was 9-A-9-A-9, 10-A-9-A-9 was the second most common pattern. The distribution of AGG interspersion patterns differed significantly by country (p < 0.001) and the most common patterns are shown in Supplementary Table 1 (http://www.irdrjournal.com/docindex.php?year=2014&kanno=4). Examination of adjusted residuals suggested that significantly more alleles from Australia had the patterns 9-A-9-A-9-A-9 (p < 0.001, 8%), 9-A-9-A-19 (p = 0.012, 2%), and 10-A-9 (p < 0.001, 11%); significantly more alleles from the Emirates had the pattern 10-A-10-A-9 (p < 0.001, 6%), 11-A-9-A-9 (p = 0.022, 2%), and 9-A-10-A-9 (p < 0.001, 7%). Indonesia had significantly more alleles with 30 CGG repeats and no AGG interruptions (p = 0.010, 2%), 9-A-13 (p < 0.001, 3%), 9-A-22 (p < 0.001, 3%), 9-A-9-A-9 (p = 0.004, 35%), and 9-A-9-A-6-A-9 (p < 0.001, 6%) patterns; significantly more Spanish alleles had the patterns 10-A-9 (p < 0.001, 9%), 13-A-9 (p = 0.013, 4%) and 9-A-12-A-9 (p < 0.001, 4%), and significantly more USA alleles had the pattern 10-A-9-A-9 (p < 0.001, 44%) than was expected under homogeneity. Likewise, fewer alleles from Australia and Spain had the pattern 9-A-9-A-9 (both p < 0.001, 9% and 14%, respectively), fewer alleles from Indonesia have the pattern 10-A-9-A-9 (p < 0.001, 21%), fewer alleles from USA had the pattern 10-A-9 (p < 0.001, 2%) than was expected under homogeneity.
In 201 normal alleles genotyped from Australia, we observed 74 AGG interruption patterns (Supplementary Table 1; http://www.irdrjournal.com/docindex.php?year=2014&kanno=4). A larger proportion of alleles were in the high normal range than observed in the other populations, between 32 and 40 CGG repeats in length, and approximately 9% of the genotyped alleles contained three AGG interruptions. In 77 normal alleles genotyped from Chile, 17 AGG interruption patterns were present. In 151 normal alleles genotyped from Guatemala 39 AGG interruption patterns were observed however no remarkable patterns were observed. In 263 normal alleles genotyped from the United Arab Emirates, 77 different AGG interruption patterns were observed out of which twenty were only observed in the Emirates population. Approximately 1% of the alleles had 3 AGG interruptions. In the 312 normal alleles genotyped from Indonesia, 60 AGG interruption patterns were observed. A large portion of normal alleles with 3 AGG interruptions (8%), with the majority of these alleles having the 9-A-9-A-6-A-9 pattern (6.4% of patterns) was observed in Indonesia. The 9-A-9-A-6-A-9 pattern and CGG length of 36 repeats has been observed in previous studies to occur within Indonesian and Asian cohorts (30,38,39). Fifty AGG interruption patterns were observed in 277 FMR1 alleles genotyped from Mexico. Six distinct AGG interruption patterns were observed only in the Mexico cohort, although these were each observed only once. Eighty-four AGG interruption patterns were observed in 358 normal alleles from Spain. Eleven AGG interruption patterns were only observed in the Spain cohort. Twenty-four AGG interruption patterns were observed in 67 normal CGG repeat alleles from Italy. Three patterns were observed in the Italy cohort only.
3.3. Regional differences in frequencies of AGG interruption patterns were observed within the USA samples (Chicago and Sacramento area)
The largest cohort of this study was from the United States, and consisted of samples from a larger collection of newborn blood spots that were collected in both the Sacramento and Chicago area (32). The Chicago cohort was comprised of individuals identified as Caucasian (n = 153 alleles), African American (n = 225 alleles), Hispanic (n = 223 alleles), Asian (n = 42 alleles), Southeast Asian (n = 14 alleles), Native American (n = 14 alleles), and other (n = 5 alleles). The Sacramento cohort was comprised of individuals identified as Caucasian (n = 156 alleles), African American (n = 24 alleles), Hispanic (n = 123 alleles), Asian (n = 42 alleles), Pacific Islander (n = 6 alleles), Native American (n = 4 alleles), and other (n = 328 alleles).
There were 105 AGG interruption patterns observed in 1,359 normal alleles. Twenty-five AGG interruption patterns were observed in the USA cohort and were not observed in the other 9 populations.
The most common total CGG length in both Sacramento and Chicago was 30, pure CGG stretch was 10, and number of AGG interruptions was 2 (Supplementary Figure 1; http://www.irdrjournal.com/docindex.php?year=2014&kanno=4). Compared to Chicago, Sacramento had a higher frequency of alleles with total CGG lengths of 30 and pure CGG stretches of 10 than would be expected under homogeneity (both, p < 0.001). The two cities were similar in the proportion of alleles with a pure stretch that was greater than 20 CGG repeats (p = 0.2322), and a total length that was greater than 35 CGG repeats (p = 0.7471).
Supplementary PDF file supplied by authors.
The proportion of alleles with 3 interruptions did not differ significantly between Sacramento and Chicago (p = 0.8271). The most common pattern in both cities was 10-A-9-A-9. However, the overall distribution of AGG interspersion patterns differed significantly between Sacramento and Chicago (p < 0.001). Examination of adjusted residuals reveals that more alleles in Sacramento had the pattern 10-A-9-A-9 (p < 0.001) and more alleles in Chicago had the pattern 9-A-9-A-9 (p = 0.043) than would be expected under homogeneity.
3.4. Previously studied populations
The data from the 9 populations were compared with data from previously published studies including samples collected and sequenced from Quebec (11), Taiwan (40), Norway, Saami, Nenets (41), Greenland (42), African American (43), Denmark (16), Basque (44), Caucasian, Mataco, Tibet, Navajo, Borneo, Mandenka, Wolof, African American (19), Brazil (45), China, Malay, India (17), and sub-saharah West Africa (46). AGG interruption patterns were determined by mnl I digestion for samples collected from England, Hispanic American, African American, and Asian American (5), Tunisian Jews, Sephardic Jews, Ashkenazic Jews, and Arabs (18), Suriu, Mayan, Karitiana, Baka, Mbuti, and Hutterite (7).
Collections were combined as indicated in Figure 1 in order to increase sample sizes; alleles from the Navajo population were excluded because they did not reach a sufficient sample size. The distribution of total CGG repeats length, pure CGG stretch, and number of AGG interruptions was significantly different in the 25 global populations. Seven populations had higher proportions of alleles with more than 35 CGG repeats (Asian, p < 0.001; Australia, p < 0.001; Caucasian, p = 0.013; Denmark, p = 0.001; Greenland, p < 0.001; India, p < 0.001; and Indonesia, p = 0.007). Four populations had a larger proportion of alleles with less than 35 CGG repeats (Chile, p = 0.016; Guatemala, p = 0.016; Hispanic American, p = 0.044; and USA, p < 0.001). When pure CGG repeat stretch was compared in the 25 populations, 6 populations (Australia, p < 0.001; Africa, p = 0.001; African American, p = 0.043; Basque, p = 0.043; Indonesia, p = 0.001; and Jewish & Arabic, p < 0.001) had higher frequencies of alleles with greater than 20 pure CGG repeats. Seven populations (Asia, p < 0.036; Greenland, p = 0.014; Hispanic American, p = 0.006; Malay, p = 0.004; and USA, p < 0.001) had higher frequencies of alleles with less than 20 pure CGG repeats. The populations with highly interspersed alleles included Asia (p < 0.001), Australia (p = 0.002), Greenland (p < 0.001), India (p < 0.001), Indonesia (p < 0.001), and Malay (p = 0.034); the USA had significantly less AGG interruptions then expected under homogeneity (p < 0.001).
4. Discussion
Differences in the frequency of AGG interruption patterns within the CGG repeat locus of FMR1 have been previously reported to vary between ethnicities, and suggested that such differences can affect the mutation rate of this locus. We have genotyped 3,065 alleles from 9 global cohorts to investigate how AGG interruption patterns vary between geographic and ethnic populations. The distribution of CGG repeat total length, and AGG interruption patterns were found to be significantly different between populations. Consistent with previous studies two AGG interruption patterns, 10-A-9-A-9 and 9-A-9-A-9, were the most common in all nine populations reported in this study, and in the 14 previously published population studies (Supplementary Table 1; http://www.irdrjournal.com/docindex.php?year=2014&kanno=4). 10-A-9-A-9 was the most common allele for all populations except in the African American, Asia, Indonesia, and Malay, Borneo, and Tibet cohort where 9-A-9-A-9 was the most common pattern. The frequency of the 9-A-9-A-9 pattern in Asian ethnic groups was consistent with what has previously been shown (17,40), and in the African American group the 9-A-9-A-9 pattern was only 1% higher in frequency than the 10-A-9-A-9 pattern. It is unknown whether these two patterns have a biological advantage, however, CGG repeat length in the normal allele has been shown to alter translational efficiency (47) with the highest translational efficiency occurring at 30 CGG repeats. Thus, the common lengths may provide alleles within the optimum size range with the lowest mutation rate.
We combined the AGG interruption pattern results of the 9 population cohorts genotyped for this study to the 16 cohorts from previous published studies. The results showed that six populations had a higher frequency of alleles with a total length greater than 35 repeats, and five populations had a higher frequency of alleles with an uninterrupted stretch greater than 20 repeats. Australia, Denmark, and Quebec had both, suggesting that an increased frequency of expanded alleles, intermediate, premutation, and full mutation alleles may be present in these populations. It should be noted that as the Australian cohort was part of a high risk screening study, a sample bias affecting these results could be present given that intermediate prevalence rates were found to be increased compared to the general population (28). However, only alleles not greater than 40 CGG repeats were included in this study and importantly the distribution of CGG repeat length was not statistically different from the one observed in a group of 3,091 alleles (1,091 male and 2,000 female alleles) derived from Australian newborns from the state of Victoria (p = 0.3052). In these two population-based samples the frequencies of GZ alleles were 1.3% (> 40 CGG repeats) and 0.4% (> 44 CGG repeats), in male newborns; and 5.5% (> 40 CGG repeats) and 1.4% (> 44 CGG repeats), in female newborns (unpublished data). The frequency of premutation alleles was 0.3% in both male and female samples. In Canada prevalence estimates for intermediate alleles is 1:86 in females, and for premutation alleles is 1:813 in males and 1:241 in females (Dombrowski et al., 2002). No prevalence estimates are available for the Denmark population. These prevalence rates are not higher than those estimated in other populations and also we do not have any information regarding whether these prevalence rates are increasing or decreasing with generation.
Guatemala, Hispanic American, Mexico, and USA cohorts had a smaller proportion of alleles in the high normal range, and a smaller proportion of alleles with greater than 20 uninterrupted CGG repeats, suggesting increased stability of the normal allele in these populations. However, both Guatemala and Mexico cohorts were collected for high risk screening studies, and sample collection bias may also be present in these two populations. In the USA population prevalence rates for intermediate alleles were estimated to be 1:112 for males and 1:66 for females, and for premutation alleles were estimated to be 1:430 for males and 1:209 for females (32). The Hispanic American, Guatamala, and Mexico populations do not have estimated prevalence rates. The prevalence estimates for the USA population are neither in agreement or disagreement with the population having an increased stability compared to the other studied populations.
A comparison of Sacramento and Chicago showed similarities in the distribution of AGG interruption patterns, and the proportion of alleles in the high normal and intermediate range, and with more than 20 uninterrupted CGG repeats (Supplementary Figure 1; http://www.irdrjournal.com/docindex.php?year=2014&kanno=4). Interestingly, Sacramento has an increased prevalence of premutation alleles (males, 1:305; females, 1:172) when compared to Chicago (males, 1:308; females, 1:894) (32). Both cohorts were collected and genotyped in the same study and were collected as part of a pilot study newborn screening for FXS.
One limitation of this study is the possible sampling bias within the six newly described population cohorts that were collected from high-risk screening studies. A sample bias may also likely be present in the Sacramento and Chicago cohorts that make up the USA population because AGG interruption data was available mainly for samples that were genotyped by the CGG linker PCR assay (32) when initial genotyping of females resulted in only one allele. Another limitation of this study, and of the other published studies, is represented by the small sample sizes. The expected variability introduced by sampling error inhibits strong comparisons between prevalence rates of intermediate, premutation, and full mutation alleles and AGG interruption pattern distribution; limitations that could be reduced with increasing cohort sizes.
The AGG interruption patterns within the CGG repeat locus of FMR1 further characterize the alleles beyond repeat length. The results of the study agree with what is known about the CGG repeat distribution in the nine countries, including increased frequency of the 9-A-9-A-6-A-9 pattern in Asian ethnicities where the 36 CGG repeat length is more frequent. Population structure is important to consider when studying the CGG repeat locus, sub-populations have consistently shown significant differences in the literature, including differences between ethnic and geographic groups. Our results suggest that AGG interruption pattern distributions in populations could be associated with differing prevalence of categorically non-normal alleles, however larger cohort sizes and more prevalence rates will be needed for many ethnicities to confirm these observations.
Acknowledgements
The project described was supported by the NICHD grant HD02274, and by the National Center for Advancing Translational Science, National Institutes of Health, through grant # UL1 TR000002. This work is dedicated to the memory of Matteo.
References
- 1. Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, Hirsch B, Jacky P, McDowell GA, Popovich B, Watson M, Wolff DJ. Technical standards and guidelines for fragile X: The first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Quality Assurance Subcommittee of the Laboratory Practice Committee. Genet Med. 2001; 3:200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013; 12:786-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: Resolution of the Sherman paradox. Cell. 1991; 67:1047-1058. [DOI] [PubMed] [Google Scholar]
- 4. McMurray CT. Mechanisms of trinucleotide repeat instability during human development. Nat Rev Genet. 2010; 11:786-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eichler EE, Hammond HA, Macpherson JN, Ward PA, Nelson DL. Population survey of the human FMR1 CGG repeat substructure suggests biased polarity for the loss of AGG interruptions. Hum Mol Genet. 1995; 4:2199-2208. [DOI] [PubMed] [Google Scholar]
- 6. Kunst CB, Warren ST. Cryptic and polar variation of the fragile X repeat could result in predisposing normal alleles. Cell. 1994; 77:853-861. [DOI] [PubMed] [Google Scholar]
- 7. Eichler EE, Nelson DL. Genetic variation and evolutionary stability of the FMR1 CGG repeat in six closed human populations. Am J Med Genet. 1996; 64:220-225. [DOI] [PubMed] [Google Scholar]
- 8. Eichler EE, Holden JJ, Popovich BW, Reiss AL, Snow K, Thibodeau SN, Richards CS, Ward PA, Nelson DL. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994; 8:88-94. [DOI] [PubMed] [Google Scholar]
- 9. Snow K, Tester DJ, Kruckeberg KE, Schaid DJ, Thibodeau SN. Sequence analysis of the fragile X trinucleotide repeat: Implications for the origin of the fragile X mutation. Hum Mole Genet. 1994; 3:1543-1551. [DOI] [PubMed] [Google Scholar]
- 10. Eichler EE, Macpherson JN, Murray A, Jacobs PA, Chakravarti A, Nelson DL. Haplotype and interspersion analysis of the FMR1 CGG repeat identifies two different mutational pathways for the origin of the fragile X syndrome. Hum Mol Genet. 1996; 5:319-330. [DOI] [PubMed] [Google Scholar]
- 11. Dombrowski C, Levesque S, Morel ML, Rouillard P, Morgan K, Rousseau F. Premutation and intermediate-size FMR1 alleles in 10572 males from the general population: Loss of an AGG interruption is a late event in the generation of fragile X syndrome alleles. Hum Mol Genet. 2002; 11:371-378. [DOI] [PubMed] [Google Scholar]
- 12. Nolin SL, Sah S, Glicksman A, et al. Fragile X AGG analysis provides new risk predictions for 45–69 repeat alleles. Am J Med Genet A. 2013; 161A:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez-Carvajal I, Lopez Posadas B, Pan R, Raske C, Hagerman PJ, Tassone F. Expansion of an FMR1 grey-zone allele to a full mutation in two generations. J Mol Diapn. 2009; 11:306-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Terracciano A, Pomponi MG, Marino GM, Chiurazzi P, Rinaldi MM, Dobosz M, Neri G. Expansion to full mutation of a FMR1 intermediate allele over two generations. Eur Hum Genet. 2004; 12:333-336. [DOI] [PubMed] [Google Scholar]
- 15. Kunst CB, Leeflang EP, Iber JC, Arnheim N, Warren ST. The effect of FMR1 CGG repeat interruptions on mutation frequency as measured by sperm typing. J Med Genet. 1997; 34:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsen LA, Armstrong JS, Grønskov K, Hjalgrim H, Macpherson JN, Brøndum-Nielsen K, Hasholt L, Nørgaard-Pedersen B, Vuust J. Haplotype and AGG-interspersion analysis of FMR1 (CGG)n alleles in the Danish population: Implications for multiple mutational pathways towards fragile X alleles. Am J Med Genet. 2000; 93:99-106. [DOI] [PubMed] [Google Scholar]
- 17. Zhou Y, Tang K, Law HY, Ng IS, Lee CG, Chong SS. FMR1 CGG repeat patterns and flanking haplotypes in three Asian populations and their relationship with repeat instability. AnnHum Genet. 2006; 70:784-796. [DOI] [PubMed] [Google Scholar]
- 18. Falik-Zaccai TC, Shachak E, Yalon M, Lis Z, Borochowitz Z, Macpherson JN, Nelson DL, Eichler EE. Predisposition to the fragile X syndrome in Jews of Tunisian descent is due to the absence of AGG interruptions on a rare Mediterranean haplotype. Am J Hum Genet. 1997; 60:103-112. [PMC free article] [PubMed] [Google Scholar]
- 19. Kunst CB, Zerylnick C, Karickhoff L, Eichler E, Bullard J, Chalifoux M, Holden JJ, Torroni A, Nelson DL, Warren ST. FMR1 in global populations. Am J Hum Genet. 1996; 58:513-522. [PMC free article] [PubMed] [Google Scholar]
- 20. Arrieta MI, Ramírez JM, Télez M, Flores P, Criado B, Barasoain M, Huerta I, González AJ. Analysis of the Fragile X trinucleotide repeat in Basques: Association of premutation and intermediate sizes, anchoring AGGs and linked microsatellites with Unstable Alleles. Curr Genomics. 2008; 9:191-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ludwig AL, Raske C, Tassone F, Garcia-Arocena D, Hershey JW, Hagerman PJ. Translation of the FMR1 mRNA is not influenced by AGG interruptions. Nucleic Acids Res. 2009; 37:6896-6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peprah E, He W, Allen E, Oliver T, Boyne A, Sherman SL. Examination of FMR1 transcript and protein levels among 74 premutation carriers. J Human Genet. 2010; 55:66-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yrigollen CM, Tassone F, Durbin-Johnson B, Tassone F. The role of AGG interruptions in the transcription of FMR1 premutation alleles. PloS One. 2011; 6:e21728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tassone F, Beilina A, Carosi C, Albertosi S, Bagni C, Li L, Glover K, Bentley D, Hagerman PJ. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007; 13:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yrigollen CM, Durbin-Johnson B, Gane L, Nelson DL, Hagerman R, Hagerman PJ, Tassone F. AGG interruptions within the maternal FMR1 gene reduce the risk of offspring with fragile X syndrome. Genet Med. 2012; 14:729-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yrigollen CM, Martorell L, Durbin-Johnson B, Naudo M, Genoves J, Murgia A, Polli R, Zhou L, Barbouth D, Rupchock A, Finucane B, Latham GJ, Hadd A, Berry-Kravis E, Tassone F. AGG interruptions and maternal age affect FMR1 CGG repeat allele stability during transmission. J Neurodev Disord. 2014;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peprah E. Fragile X syndrome: The FMR1 CGG repeat distribution among world populations. Ann Hum Genet. 2012; 76:178-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loesch DZ, Tassone F, Lo J, Slater HR, Hills LV, Bui MQ, Silburn PA, Mellick GD. New evidence for, and challenges in, linking small CGG repeat expansion FMR1 alleles with Parkinson's disease. Cli Genet. 2013; 84:382-385. [DOI] [PubMed] [Google Scholar]
- 29. Yuhas J, Walichiewicz P, Pan R, Zhang W, Casillas EM, Hagerman RJ, Tassone F. High-risk fragile x screening in Guatemala: Use of a new blood spot polymerase chain reaction technique. Genet Test Mol Biomarkers. 2009; 13:855-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winarni TI, Utari A, Mundhofir FE, Tong T, Durbin-Johnson B, Faradz SM, Tassone F. Identification of expanded alleles of the FMR1 gene among high-risk population in Indonesia by using blood spot screening. Genet Test Mol Biomarkers. 2012; 16:162-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fernandez-Carvajal I, Walichiewicz P, Xiaosen X, Pan R, Hagerman PJ, Tassone F. Screening for expanded alleles of the FMR1 gene in blood spots from newborn males in a Spanish population. J Mol Diagn. 2009; 11:324-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tassone F, Iong KP, Tong TH, Lo J, Gane LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012; 4:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, Tassone F. Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. J Med Genet. 2007; 44:200-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hall D, Tassone F, Klepitskaya O, Leehey M. Fragile X-associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. 2012; 27:296-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg J, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet B Neuropsychiatr Genet. 2012; 159B:589-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Agresti A. An introduction to categorical data analysis Hoboken, NJ: Wiley-Interscience; 2007; 2nd:[xvii, 372 p. ill. 25 cm.]. [Google Scholar]
- 37. Team RC. R: A language and environment for statistical computing. 3.0.3 ed. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 38. Faradz SM, Pattiiha MZ, Leigh DA, Jenkins M, Leggo J, Buckley MF, Holden JJ. Genetic diversity at the FMR1 locus in the Indonesian population. Ann Hum Genet. 2000; 64:329-339. [DOI] [PubMed] [Google Scholar]
- 39. Faradz SM, Leggo J, Murray A, Lam-Po-Tang PR, Buckley MF, Holden JJ. Distribution of FMR1 and FMR2 alleles in Javanese individuals with developmental disability and confirmation of a specific AGG-interruption pattern in Asian populations. Ann Hum Genet. 2001; 65:127-135. [DOI] [PubMed] [Google Scholar]
- 40. Chiu HH, Tseng YT, Hsiao HP, Hsiao HH. The AGG interruption pattern within the CGG repeat of the FMR1 gene among Taiwanese population. J Genet. 2008; 87:275-277. [DOI] [PubMed] [Google Scholar]
- 41. Larsen LA, Vuust J, Nystad M, Evseeva I, Van Ghelue M, Tranebjaerg L. Analysis of FMR1 (CGG)n alleles and DXS548-FRAXAC1 haplotypes in three European circumpolar populations: Traces of genetic relationship with Asia. Eur J Hum Genet. 2001; 9:724-727. [DOI] [PubMed] [Google Scholar]
- 42. Larsen LA, Armstrong JS, Grønskov K, Hjalgrim H, Brøndum-Nielsen K, Hasholt L, Nørgaard-Pedersen B, Vuust J. Analysis of FMR1 (CGG)n alleles and FRAXA microsatellite haplotypes in the population of Greenland: Implications for the population of the New World from Asia. Eur J Hum Genet. 1999; 7:771-777. [DOI] [PubMed] [Google Scholar]
- 43. Crawford DC, Meadows KL, Newman JL, Taft LF, Scott E, Leslie M, Shubek L, Holmgreen P, Yeargin-Allsopp M, Boyle C, Sherman SL. Prevalence of the fragile X syndrome in African-Americans. Am J Med Genet. 2002; 110:226-233. [DOI] [PubMed] [Google Scholar]
- 44. Penagarikano O, Gil A, Télez M, Ortega B, Flores P, Veiga I, Peixoto A, Criado B, Arrieta I. A new insight into fragile X syndrome among Basque population. Am J Med Genet A. 2004; 128A:250-255. [DOI] [PubMed] [Google Scholar]
- 45. Angeli CB, Capelli LP, Auricchio MT, Leal-Mesquita ER, Ribeiro-dos-Santos AK, Ferrari I, Oliveira SF, Klautau-Guimarães Mde N, Vianna-Morgante AM, Mingroni-Netto RC. AGG interspersion patterns in the CGG repeat of the FMR1 gene and linked DXS548/FRAXAC1 haplotypes in Brazilian populations. Am J Med Genet A. 2005; 132A:210-214. [DOI] [PubMed] [Google Scholar]
- 46. Peprah EK, Allen EG, Williams SM, Woodard LM, Sherman SL. Genetic diversity of the fragile X syndrome gene (FMR1) in a large Sub-Saharan West African population. Ann Hum Genet. 2010; 74:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Hum Mol Genet. 2003; 12:3067-3074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary PDF file supplied by authors.