Abstract
Three natural aromadendrane sesquiterpenes, (−)-epiglobulol, (−)-4β,7α-aromadendranediol, and (−)-4α,7α-aromadendranediol, have been synthesized in only seven steps in 12, 15, and 17 % overall yields, respectively, from (E,E)-farnesol by a stereodivergent gold(I)-catalyzed cascade reaction which forms the tricyclic aromadendrane core in a single step. These are the shortest total syntheses of these natural compounds.
Keywords: cyclization, gold, natural products, terpenoids, total synthesis
Aromadendranes are a family of hydroazulenes named after (+)-aromadendrene (1, Figure 1), the main component in the essential oil from Eucaliptus trees. The related sesquiterpenoids (−)-globulol (2), (−)-epiglobulol (3), (−)-4α,7α-aromadendranediol (4), and (−)-4β,7α-aromadendranediol (5) are widespread in plant species[1] and display antifungal,[2] antibacterial,[3] antiviral,[4] cytotoxic,[5] and other activities.[6] Interestingly, the antipodes of 1 and other aromadendrenes have been isolated from corals.[7] Aromadendranes with amino, isonitrile, isothiocyano, and urea functionalities at C4 have been found in sponges.[8] Diterpenoids with an aromadendrane structure are also natural products.[9]
Figure 1.
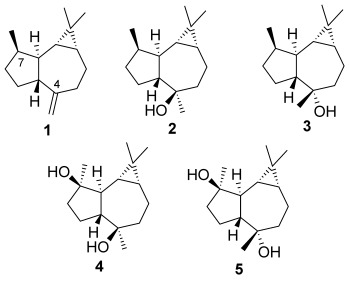
Naturally occurring aromadendranes.
The synthesis of members of this family of tricyclic sesquiterpenes has attracted significant interest.[10] (−)-Epiglobulol (3), isolated in hop[11] and many essential oils,[12] was prepared from 1 or the corresponding ketone (apoaromadendrone).[13] A first total synthesis of 3 from the chiral pool was accomplished in eight steps (4 % overall yield).[14] A recent synthesis of (±)-epiglobulol in 18 steps used a rhodium(I)-catalyzed hydroacylation/cycloisomerization as the key step.[15]
(−)-4α,7α-Aromadendranediol (4) was isolated from a marine coral Sinularia may[7] and the leaves of the Amazonian tree Xylopia brasiliensis.[2] A semisynthesis of 4 from (+)-spathulenol[7] and one total synthesis have been reported.[16] This total synthesis involved a three-reaction sequence in a three-component reaction to generate four stereogenic centers in one step and required ten steps to produce 4 in 23 % overall yield. (−)-4β,7α-Aromadendranediol (5) has been isolated from the leaves of Chloranthus glaber.[17] A semisynthesis of 5 from (+)-spathulenol has been reported.[7]
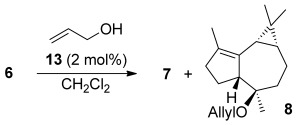
We developed a gold(I)-catalyzed cascade cyclization of the dienyne 6, a cascade consisting of a cyclization, 1,5-migration of the propargylic OR group, and intramolecular cyclopropanation, thus leading to tricyclic structures closely related to the aromadendrene sesquiterpenes (Scheme 1).[18] This reaction is stereospecific since (E)-6 gave the tricyclic product 7 having the relative configuration of 3 and 5, whereas the geometrical isomer of 6 led to 8, the C4 epimer of 7, having the configuration of 2 and 4. We recently applied a strategy based on a gold(I)-catalyzed cyclization/1,5-OR migration/intermolecular cyclopropanation for the first total synthesis of (+)-schisanwilsonene A. As part of our program on the synthesis of terpenoids by using new gold-catalyzed cyclization cascades,[19] we decided to target 3, 4, and 5, each of which present six stereogenic centers in a tricyclic skeleton. In principle, 3 and 5 could be synthesized from the dienyne (S,E)-6 (Scheme 1), whereas 4 would be prepared from geometric isomer (S,Z)-6. However, although enantioenriched (E)-6 could be readily prepared from (E,E)-farnesol (9), the starting material, (E,Z)-farnesol, required for the synthesis of (Z)-6 is not commercially available.[20]
Scheme 1.
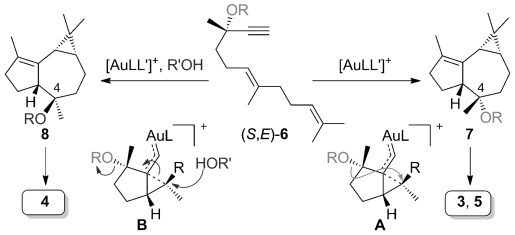
Gold-catalyzed formation of tricyclic cores of the aromadendranes by cyclization/1,5-OR migration/intramolecular cyclopropanation.
Herein we report a simple solution to this problem and it allows general access to this class of sesquiterpenes from (S,E)-6 as a common precursor by means of a stereodivergent gold(I)-catalyzed cascade process. The reaction can take place intramolecularly by 1,5-migration of OR in A and in the presence of an external nucleophile (via B), thus leading to 7 and 8, respectively, having opposite configurations at C4 (Scheme 1). Starting from (R,E)-6, enantiomeric aromadendranes can be similarly obtained. This proposal is based on our initial mechanistic study in the Z series, in which we found that the cyclopropyl gold(I) carbene intermediate could be trapped with methanol to form an epimeric compound as a minor product.[18] In these transformations, gold(I) acts as an artificial cyclase,[21] thus mimicking the action of terpene cyclases forming polycyclic skeletons by the selective activation of the alkyne terminus of a dienyne, to readily build a tricyclic skeleton with exquisite stereocontrol.[22, 23]
The dienyne (S,E)-6 (R=Bn) was prepared in four steps and 62 % overall yield by using a route similar to that used in the transformation of the lower homologue geraniol.[19a, 24] the transformation involved the known Sharpless asymmetric epoxidation of (E,E)-farnesol (9) to give the epoxide (S,S)-10 (88 % yield, 91:9 e.r.)[25, 26] (Scheme 2). Substitution of the primary alcohol by chloride with CCl4 and PPh3 gave 11, which was treated with nBuLi to yield the propargylic alcohol 12. Finally, benzylation under standard reaction conditions gave (S,E)-6.
Scheme 2.
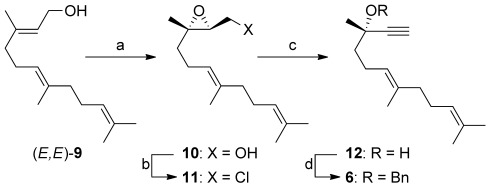
a) l-(+)-DIPT, Ti(OiPr)4, tBuOOH, 4 Å M.S., CH2Cl2, −48 °C, 88 %, 82 % ee;[25] b) PPh3, NaHCO3, CCl4, reflux, 6 h, 94 %; c) nBuLi, THF, −40 °C, 2 h, 82 %; d) BnBr, NaH, Bu4NI, THF, 23 °C 12 h, 91 %. DIPT=diisopropyl tartrate, M.S.=molecular sieves, THF=tetrahydrofuran.
Exposing (S,E)-6 to the cationic gold(I) complex [(JohnPhos)Au(MeCN)]SbF6 (13) for 5 minutes at room temperature gave 7 in 60 % yield (Scheme 3). Other gold(I) catalysts were also screened for this reaction, but the best results were obtained using complex 13.[27] The relative configuration of 7 (racemic series) was confirmed by X-ray diffraction.[28, 29] Debenzylation of 7 with H2 (1 atm) and Pd(OH)2/C gave the alcohol 14 (79 % yield), which was hydrogenated with [Ir(cod)(PCy3)py]BArF catalyst[30] under high pressure of H2 to give 3 in 40 % yield (95:5 e.r.). The synthesis 3 from 9 required seven steps and proceeded in 12 % overall yield.
Scheme 3.
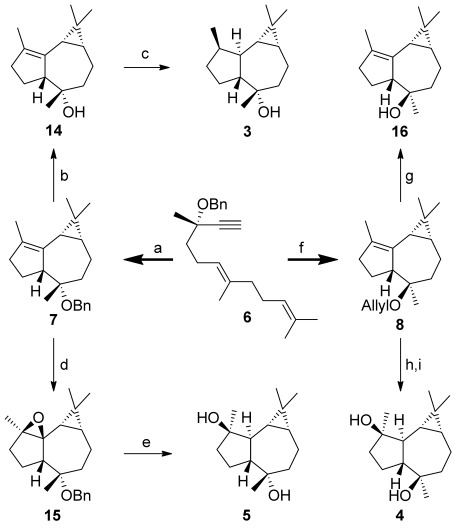
Reagents and conditions: a) [(JohnPhos)Au(MeCN)]SbF6 (13; 2 mol %), 23 °C, 5 min (60 %); b) H2, Pd(OH)2/C, 1:1 MeOH/THF, 23 °C, 4 h (79 %); c) [Ir(cod)(PCy3)py]BArF (15 mol %), H2 (80 atm), CH2Cl2, 40 °C, 4 days (40 %); d) oxone, NaHCO3, 18-crown-6, 1:1:2 acetone/CH2Cl2/H2O, 23 °C, 1 h (51 %); e) Li, EDA, 50 °C, 1 h (78 %); f) allyl alcohol (20 equiv), 13 (2 mol %), −30 °C, 15 min (56 % + 21 % 7); g) [Pd(PPh3)4] (5 mol %), K2CO3, MeOH, reflux 72 h (72 %); h) mCPBA, CH2Cl2, 0 to 23 °C (83 %); i) Li, EDA, 50 °C, 1.5 h (62 %). BArF=3,5-bis(trifluoromethyl)phenylborate, cod=1,5-cyclooctadiene, EDA=ethylenediamine, JohnPhos=(2-biphenyl)-di-tert-butylphosphine; mCPBA=m-chloroperbenzoic acid.
Epoxidation of 7 with dimethyldioxirane yielded 15 stereoselectivily. Epoxide opening and ether cleavage with Li in ethylenediamine[31] yielded 5 in 78 % (96:4 e.r.), which gave enantiopure material after crystallization. The synthesis of 5 from 9 was accomplished in seven steps with 15 % overall yield.
When the gold-catalyzed reaction of dienyne (S,E)-6 was performed in the presence of allyl alcohol as an external nucleophile, the allyl ether 8 was obtained with the opposite configuration at C4 compared to that of 7 (Table 1). While lowering the reaction temperature to −30 °C led to a 1:1 mixture of 7 and 8 (Table 1, entry 3), increasing the concentration of allyl alcohol to 20 equivalents favored the intermolecular pathway (Table 1, entry 5). Similar results were obtained with using only 1 mol % gold(I) catalyst (Table 1, entry 5). Under the optimized reaction conditions, 8 was obtained in 56 % yield, along with 7 (21 % yield; Scheme 3). Removal of the allylic ether with [Pd(PPh3)4] in MeOH gave the alcohol 16, whose structure was confirmed by X-ray crystal diffraction[29] in the racemic series (Figure 2).[26] Although 4 could be synthesized from 16, a more direct synthesis was completed from 8 by selective epoxidation with mCPBA from the convex face (83 % yield), followed by opening of the epoxide and allyl cleavage with Li in ethylenediamine to give 4 in 62 % yield (87:13 e.r.), yielding enantiopure 4 after crystallization. Spectral data and optical rotation of synthetic 4α,7α-aromadendranediol matched those reported for the natural compound. The relative and absolute configuration of 4 were confirmed by X-ray diffraction (Figure 2).[29] The synthesis of (+)-4α,7α-aromadendranediol was similarly carried out from (R,R)-10.[27]
Table 1.
Gold(I)-catalyzed addition of allyl alcohol to (S,E)-6.[a]
| Entry | AllylOH (equiv) | T [°C] | t [min] | 7/8[b] |
|---|---|---|---|---|
| 1 | 10 | 23 | 5 | 75:25 |
| 2 | 10 | 0 | 10 | 55:45 |
| 3 | 10 | −30 | 15 | 50:50 |
| 4 | 20 | −30 | 20 | 27:73 |
| 5[c] | 20 | −30 | 30 | 33:67 |
[a] 0.05 m. [b] Determined by GC-MS. [c] 1 mol % 13.
Figure 2.
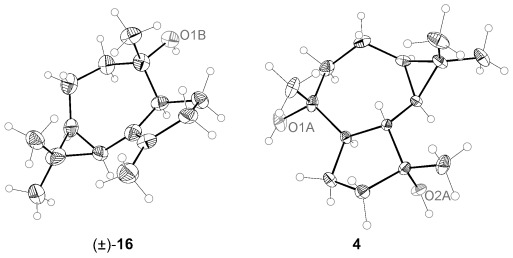
X-ray structures for (±)-16 and 4. Thermal ellipsoids are shown at 50 % probability.
The stereochemical divergent synthesis of 3 and 4 from (S,E)-6 confirms the proposal that this cascade cyclization process proceeds by intra- or intermolecular reactions of cyclopropyl gold(I) carbene-like intermediates such as A or B.[18, 32] The enantioselectivity is fully preserved in the formation of 3 and 5 via 7 by an intramolecular gold(I)-catalyzed 1,5-migration of a propargylic group. The intermolecular reaction of (S,E)-6 with allyl alcohol occurs with high enantioselectivity (ca. 96 %). In this case, the slight racemization is due to the competitive formation of a propargyl carbocation, presumably facilitated by the higher polarity of the reaction medium.
In summary, we have completed highly concise syntheses of three representative aromadendranes from a single precursor by a stereodivergent gold-catalyzed reaction which establishes four new stereogenic centers from a single one. The three natural sesquiterpenes (−)-epiglobulol (3), (−)-4α,7α-aromadendranediol (4), and (−)-4β,7α-aromadendranediol (5) have been synthesized in seven steps in 12, 17, and 15 % overall yields, respectively, from commercially available (E,E)-farnesol (9), and constitutes the shortest total syntheses of these natural compounds. This route could be extended for the enantioselective synthesis of any enantiomer of other aromadendranes and non-natural analogues.
Supporting Information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re-organized for online delivery, but are not copy-edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
miscellaneous_information
References
- 1.Gijsen HJM, Wijnberg JB, de Groot A. Prog. Chem. Org. Nat. Prod. 1995;64:149–193. doi: 10.1007/978-3-7091-9337-2_3. [DOI] [PubMed] [Google Scholar]
- 2.Moreira IC, Lago JHG, Young NCM, Roque NF. J. Braz. Chem. Soc. 2003;14:828–831. [Google Scholar]
- 3a.Gaspar-Marques C, Simões MF, Rodríguez B. J. Nat. Prod. 2004;67:614–621. doi: 10.1021/np030490j. [DOI] [PubMed] [Google Scholar]
- 3b.Yamakoshi Y, Murata M, Shimizu A, Homma S. Biosci. Biotechnol. Biochem. 1992;56:1570–1576. doi: 10.1271/bbb.56.1570. [DOI] [PubMed] [Google Scholar]
- 3c.Jacyno JM, Montemurro N, Bates AD, Cutler HG. J. Agric. Food Chem. 1991;39:1166–1168. [Google Scholar]
- 3d.Murata M, Yamakoshi Y, Homma S, Aida K, Hori K, Ohashi Y. Agric. Biol. Chem. 1990;54:3221–3226. [Google Scholar]
- 4a.Nishizawa M, Emura M, Kan Y, Yamada H, Ogawa K, Hamanaka N. Tetrahedron Lett. 1992;21:2983–2986. [Google Scholar]
- 4b.De Tommasi N, Pizza C, Conti C, Orsi N, Stein ML. J. Nat. Prod. 1990;53:830–835. doi: 10.1021/np50070a009. [DOI] [PubMed] [Google Scholar]
- 5a.Su Z-S, Yin S, Zhou Z-W, Wu Y, Diang J, Yue J-M. J. Nat. Prod. 2008;71:1410–1413. doi: 10.1021/np800240v. [DOI] [PubMed] [Google Scholar]
- 5b.Tada H, Yasuda F. Chem. Pharm. Bull. 1985;33:1941–1945. [Google Scholar]
- 6a.Matsuo A, Atsumi K, Nakayama M, Hayashi S. J. Chem. Soc. Perkin Trans. 1. 1981:2816–2824. [Google Scholar]
- 6b.Hubert TD, Wiemer DF. Phytochemistry. 1985;24:1197–1198. [Google Scholar]
- 6c.Asakawa Y, Toyota M, Takemoto T, Kubo I, Nakanishi K. Phytochemistry. 1980;19:2147–2154. [Google Scholar]
- 6d.Pérez-Hernández N, Ponce-Monter H, Ortiz MI, Cariño-Cortés R, Joseph-Nathan P. Z. Naturforsch. C. 2009;64:840–846. doi: 10.1515/znc-2009-11-1214. [DOI] [PubMed] [Google Scholar]
- 6e.Wu TS, Chan Y, Leu Y. Chem. Pharm. Bull. 2000;3:357–361. doi: 10.1248/cpb.48.357. [DOI] [PubMed] [Google Scholar]
- 7a.Beechan CM, Djerassi C, Eggert H. Tetrahedron. 1978;34:2503–2508. [Google Scholar]
- 8a.Fattorusso E, Magno S, Mayol L, Santacroce C, Sica D. Tetrahedron Lett. 1975;31:269–270. [Google Scholar]
- 8b.da Silva CC, Almagro V, Zukerman-Schpector J, Castellano EE, Marsaioli AJ. J. Org. Chem. 1994;59:2880–2881. [Google Scholar]
- 8c.Kozawa S, Ishiyama H, Fromont J, Kobayashi J. J. Nat. Prod. 2008;71:445–447. doi: 10.1021/np0703139. [DOI] [PubMed] [Google Scholar]
- 8d.Ishiyama H, Kozawa S, Aoyama K, Mikami Y, Fromont J, Kobayashi J. J. Nat. Prod. 2008;71:1301–1303. doi: 10.1021/np800164s. [DOI] [PubMed] [Google Scholar]
- 9.Anjaneyulu ASR, Krishnamurthy MVR, Rao GV. Tetrahedron. 1997;53:9301–9312. [Google Scholar]
- 10a.Büchi G, Hofheinz W, Paukstelis JV. J. Am. Chem. Soc. 1966;88:4113–4114. doi: 10.1021/ja00969a053. [DOI] [PubMed] [Google Scholar]
- 10b.Buechi G, Hofheinz W, Paukstelis JV. J. Am. Chem. Soc. 1969;91:6473–6478. doi: 10.1021/ja00969a053. [DOI] [PubMed] [Google Scholar]
- 10c.Marshall JA, Ruth JA. J. Org. Chem. 1974;39:1971–1973. [Google Scholar]
- 10d.Gijsen HJM, Wijnberg JBPA, Stork GA, de Groot A. Tetrahedron. 1991;47:4409–4416. [Google Scholar]
- 10e.Gijsen HJM, Wijnberg JBPA, Stork GA, de Groot A, de Waard MA, van Nistelrooy JGM. Tetrahedron. 1992;48:2465–2476. [Google Scholar]
- 10f.Gijsen HJM, Wijnberg JBPA, van Ravenswaay C, de Groot A. Tetrahedron. 1994;50:4733–4744. [Google Scholar]
- 10g.Gijsen HJM, Wijnberg JBPA, de Groot A. Tetrahedron. 1994;50:4745–4754. [Google Scholar]
- 10h.Gwaltney SL, II, Shea KJ. Tetrahedron Lett. 1996;37:949–952. [Google Scholar]
- 10i.Guillermo R, Hanson JR, Truneh A. J. Chem. Res. Synop. 1997:28–29. [Google Scholar]
- 10j.Moreno-Dorado FJ, Lamers YMAW, Mironov G, Wijnberg JBPA, de Groot A. Tetrahedron. 2003;59:7743–7750. [Google Scholar]
- 10k.Lamers YMAW, Rusu G, Wijnberg JBPA, de Groot A. Tetrahedron. 2003;59:9361–9369. [Google Scholar]
- 11.Tressl R, Engel K-H, Kossa M, Koppler H. J. Agric. Food Chem. 1983;31:892–897. [Google Scholar]
- 12a.Elaissia A, Medinia H, Khoujab ML, Simmonds M, Lynend F, Farhat F, Chemli R, Harzallah-Skhiri F. Chem. Biodiversity. 2011;8:352–361. doi: 10.1002/cbdv.201000102. [DOI] [PubMed] [Google Scholar]
- 12b.Özel MZ, Gögüs F, Lewis AC. J. Chromatogr. Sci. 2008;46:157–161. doi: 10.1093/chromsci/46.2.157. [DOI] [PubMed] [Google Scholar]
- 12c.Nazemiyeh H, Razavi SM, Hajiboland R, Delazar A, Esna-asharii S, Bamdad R, Nahar L, Sarker SD. Chem. Nat. Compd. 2007;43:736–737. [Google Scholar]
- 12d.Stefanello MEA, Pascoal ACRF, Salvador MJ. Chem. Biodiversity. 2011;8:73–94. doi: 10.1002/cbdv.201000098. [DOI] [PubMed] [Google Scholar]
- 13.Gijsen HJM, Kanai K, Stork GA, Wijnberg JBPA, Orru RVA, Seelen CGJM, van der Kerk SM, de Groot A. Tetrahedron. 1990;46:7237–7246. [Google Scholar]
- 14.Caine D, Gupton JT., III J. Org. Chem. 1975;40:809–810. [Google Scholar]
- 15.Oonishi Y, Taniuchi A, Mori M, Sato Y. Tetrahedron Lett. 2006;47:5617–5621. [Google Scholar]
- 16.Simmons B, Walji AM, MacMillan DWC. Angew. Chem. 2009;121:4413–4417. [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:4349–4353. doi: 10.1002/anie.200900220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takeda Y, Yamashita H, Matsumoto T, Terao H. Phytochemistry. 1993;33:713–715. [Google Scholar]
- 18.Jiménez-Nuñez E, Raducan M, Lauterbach T, Molawi K, Solorio CR, Echavarren AM. Angew. Chem. 2009;121:6268–6271. doi: 10.1002/anie.200902248. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2009;48:6152–6155. doi: 10.1002/anie.200902248. [DOI] [PubMed] [Google Scholar]
- 19a.Jiménez-Núñez E, Molawi K, Echavarren AM. Chem. Commun. 2009:7327–7329. doi: 10.1039/b920119j. [DOI] [PubMed] [Google Scholar]
- 19b.Molawi K, Delpont N, Echavarren AM. Angew. Chem. 2010;122:3595–3597. doi: 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2010;49:3517–3519. doi: 10.1002/anie.201000890. [DOI] [PubMed] [Google Scholar]
- 19c.Gaydou M, Miller RE, Delpont N, Ceccon J, Echavarren AM. Angew. Chem. 2013;125:6524–6527. doi: 10.1002/anie.201302411. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52:6396–6399. doi: 10.1002/anie.201302411. [DOI] [PubMed] [Google Scholar]
- 19d.Pitaval A, Leboeuf D, Ceccon J, Echavarren AM. Org. Lett. 2013;15:4580–4583. doi: 10.1021/ol402188b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu JS, Kleckley TS, Wiemer DF. Org. Lett. 2005;7:4803–4806. doi: 10.1021/ol0513239. A mixture of isomers is also available. (ZZ)- or (EZ)-farnesol are just available as analytical standards. Although (EZ)-farnesol can be prepared in two steps from nerylacetone (10:1 EZEE mixture, 55 % yield): pure nerylacetone is commercially available only in milligram amounts. [DOI] [PubMed] [Google Scholar]
- 21.Willot M, Christmann M. Nat. Chem. 2010;2:519–520. doi: 10.1038/nchem.715. [DOI] [PubMed] [Google Scholar]
- 22a.Fürstner A, Morency L. Angew. Chem. 2008;120:5108–5111. [Google Scholar]
- Angew. Chem. Int. Ed. 2008;47:5030–5033. doi: 10.1002/anie.200800934. Selected examples of gold(I)-catalyzed polycyclizations. [DOI] [PubMed] [Google Scholar]
- 22b.Toullec PY, Blarre T, Michelet V. Org. Lett. 2009;11:2888–2891. doi: 10.1021/ol900864n. [DOI] [PubMed] [Google Scholar]
- 22c.Sethofer SG, Mayer T, Toste FD. J. Am. Chem. Soc. 2010;132:8276–8277. doi: 10.1021/ja103544p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.Schafroth MA, Sarlah D, Krautwald S, Carreira EM. J. Am. Chem. Soc. 2012;134:20276–20278. doi: 10.1021/ja310386m. For lead references on late transition metal catalyzed polycyclizations of polyenes. [DOI] [PubMed] [Google Scholar]
- 23b.Jeker OF, Kravina AG, Carreira EM. Angew. Chem. 2013;125:12388–12391. doi: 10.1002/anie.201307187. [DOI] [PubMed] [Google Scholar]
- Angew. Chem. Int. Ed. 2013;52:12166–12169. doi: 10.1002/anie.201307187. [DOI] [PubMed] [Google Scholar]
- 23c.Geier MJ, Gagné MR. J. Am. Chem. Soc. 2014;136:3032–3035. doi: 10.1021/ja500656k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohapatra DK, Pramanik C, Chorghade MS, Gurjar MK. Eur. J. Org. Chem. 2007:5059–5063. doi: 10.1021/jo070560h. [DOI] [PubMed] [Google Scholar]
- 25.Tanuwidjaja J, Ng S-S, Jamison TF. J. Am. Chem. Soc. 2009;131:12084–12085. doi: 10.1021/ja9052366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The syntheses of (±)-epiglobulol and (±)-4α,7α-aromadendranediol was also carried out from a 1.2:1 mixture of geranyl- and nerylacetone. See the Supporting Information for details.
- 27. See the Supporting Information for additional details.
- 28. Racemic 7 was prepared in four steps from a 1.2:1 mixture of geranyl- and nerylacetone.[27]
- 29. CCDC 983695 (4), 983696 [(±)-7)], 983697 [(±)-16)] contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
- 30.Wüstenberg B, Pfaltz A. Adv. Synth. Catal. 2008;350:174–178. [Google Scholar]
- 31.Brown HC, Ikegami S, Kawakami JH. J. Org. Chem. 1970;35:3243–3245. doi: 10.1021/jo00830a023. [DOI] [PubMed] [Google Scholar]
- 32a.Jiménez-Núñez E, Echavarren AM. Chem. Rev. 2008;108:3326–3350. doi: 10.1021/cr0684319. [DOI] [PubMed] [Google Scholar]
- 32b.Obradors C, Echavarren AM. Acc. Chem. Res. 2014;47:902–912. doi: 10.1021/ar400174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
miscellaneous_information


