Abstract
Tamoxifen is an endocrine therapy which is administered to up to 70% of all breast cancer patients with oestrogen receptor alpha (ERα) expression. Despite the initial response, most patients eventually acquire resistance to the drug. MicroRNAs (miRNAs) are a class of small non-coding RNAs which have the ability to post-transcriptionally regulate genes. Although the role of a few miRNAs has been described in tamoxifen resistance at the single gene/target level, little is known about how concerted actions of miRNAs targeting biological networks contribute to resistance. Here we identified the miRNA cluster, C19MC, which harbours around 50 mature miRNAs, to be up-regulated in resistant cells, with miRNA-519a being the most highly up-regulated. We could demonstrate that miRNA-519a regulates tamoxifen resistance using gain- and loss-of-function testing. By combining functional enrichment analysis and prediction algorithms, we identified three central tumour-suppressor genes (TSGs) in PI3K signalling and the cell cycle network as direct target genes of miR-519a. Combined expression of these target genes correlated with disease-specific survival in a cohort of tamoxifen-treated patients. We identified miRNA-519a as a novel oncomir in ER+ breast cancer cells as it increased cell viability and cell cycle progression as well as resistance to tamoxifen-induced apoptosis. Finally, we could show that elevated miRNA-519a levels were inversely correlated with the target genes' expression and that higher expression of this miRNA correlated with poorer survival in ER+ breast cancer patients. Hence we have identified miRNA-519a as a novel oncomir, co-regulating a network of TSGs in breast cancer and conferring resistance to tamoxifen. Using inhibitors of such miRNAs may serve as a novel therapeutic approach to combat resistance to therapy as well as proliferation and evasion of apoptosis in breast cancer. Published by John Wiley & Sons, Ltd. © 2014 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: microRNAs, tamoxifen resistance, C19MC cluster, breast cancer, cell cycle network
Introduction
Up to 70% of all breast cancer patients overexpress the nuclear receptor oestrogen receptor-α (ER-α) 1, making it an excellent candidate for endocrine therapy. Tamoxifen is an ER antagonist that competitively inhibits the interaction of oestrogen with the ER, thus repressing ER activity 2,3, and is commonly administered in adjuvant first-line treatment of ERα + patients. Unfortunately, long-term efficacy has been limited by disease relapse and the occurrence of resistance 4. Improving response to tamoxifen and combating resistance are critical and urgent priorities in current breast cancer treatment regimes.
Tamoxifen treatment induces cell cycle arrest via the regulation of genes such as cyclin D1, retinoblastoma protein (RB1), and the cyclin-dependent kinase inhibitors CDKN1A/CIP1/WAF1/p21 and p27/KIP1 5, and induces apoptosis by decreasing BCL2 and increasing BAX expression 6. Therefore, genes that control the cell cycle and apoptosis have the potential to impact drug sensitivity and resistance. For example, overexpression of cyclin D1, a direct transcriptional target of ER signalling, conferred resistance to tamoxifen in vitro 7 and was up-regulated in de novo tamoxifen-resistant cells 8. Similarly, a recent screen by Gonzalez-Malerva et al determined negative regulators of the cell cycle to be down-regulated in tamoxifen resistance 9. Thus, combinatorial targeting of cell cycle genes may be a potential route to overcome resistance.
MicroRNAs (miRNAs) are 20- to 22-nucleotide-long non-coding RNAs which mostly anneal in the 3′UTR of protein coding mRNAs at sequences that have imperfect or perfect complementarity, leading to post-transcriptional silencing or mRNA degradation, respectively, of the target genes. Each miRNA can have thousands of target genes, determined by their seed sequence at 2–8 nucleotides. Up to 50% of mammalian miRNAs are found in clusters, which are often co-transcribed from one promoter as a polycistronic miRNA precursor 10. There has been a recent surge of evidence linking miRNAs and resistance to cancer therapy 11. Recently, our group uncovered the involvement of miRNA-375 in resistance to tamoxifen. Using our in vitro model of tamoxifen resistance, we demonstrated that miRNA-375 regulated tamoxifen resistance and associated EMT-like properties, partially through targeting the oncogene metadherin (MTDH) 12. While the role of a few miRNAs has been described in tamoxifen resistance at the single gene/target level 12,13, little is known about how concerted actions of miRNAs contribute to resistance. miRNAs have great therapeutic potential, due to their ability to simultaneously co-repress many target genes. Work from our group and others has demonstrated the global effect of miRNAs and their ability to co-regulate proteins within a functional network 14,15. Since the emergence of drug resistance is multifactorial, miRNAs in combination with targeted therapies may be a potential strategy to overcome resistance.
In the present study, we identified a large cluster of miRNAs, C19MC, which were up-regulated in tamoxifen-resistant cells. We showed that inhibition of miRNA-519a sensitized tamoxifen-resistant (TamR) cells to tamoxifen, while overexpressing the miRNA in wild-type (WT) cells conferred resistance to tamoxifen. We identified three tumour-suppressor genes (TSGs) acting in the cell cycle and PI3K pathway as direct targets of miRNA-519a. Finally, elevated miRNA-519a levels correlated inversely with the expression of the target genes and correlated positively with poorer disease-free survival, exclusively in ER + patients.
Materials and methods
Cell culture
Cells were cultured as previously described 12. Briefly, MCF-7 WT and TamR cells were maintained in MEM without phenol red, supplemented with 10% FBS, 1% Pen/Strep, 1% l-glutamine, and 1% sodium pyruvate (Invitrogen, Carlsbad, CA, USA). MCF-7 TamR cells were cultured in 5 µm 4-hydroxytamoxifen (Sigma Aldrich, St Louis, MO, USA). HEK-293FT cells (Invitrogen) were grown in DMEM high-glucose medium containing 10% FBS, 1% Pen/Strep, and 500 µg/ml Geneticin. BT-474 and CAMA-1 (ATCC, Wesel, Germany) were grown in DMEM and T-47D was grown in RPMI-1640, respectively supplemented with 10% FBS and 1% Pen/Strep. Medium without antibiotics was used for transfection. MCF-7 WT and TamR cells were cultured between passages 100 and 120, as it takes 1 year to develop the resistant cells. Other cell lines were cultured between passages 5 and 20. Multiplex human cell authentication as well as contamination tests of the cell lines were routinely performed at the DKFZ Core Facility.
Transfections with miRNA mimics, inhibitors, miRNA expression vectors, and siRNAs
Cells were seeded in full growth medium. After 24 h, medium was replaced with medium without antibiotics. miRNA mimics (Dharmacon, Lafayette, CO, USA), miRCURRY LNA microRNA inhibitors (Exiqon, Vedbaek, Denmark), miRNA-overexpression vectors pCMV-MIRs (Origene, Oxon, UK), and siRNAs (Dharmacon, Lafayette, CO, USA) were transfected to a final concentration of 25 nm, 50 nm, 25 ng/ml, and 20 nm, respectively, using Lipofectamine 2000 transfection reagent (Invitrogen). Combination siRNA knockdowns were transfected to a final concentration of 20 nm. For silencing of RB1, PTEN, and CDKN1A, pools of four small interfering RNAs (siRNAs) were used (for sequences, see Supplementary Table 3).
Quantitative RT-PCR for protein-coding genes and miRNAs
qRT-PCR for mRNA and miRNAs was carried out as previously described 16. Sequences of primers and the respective UPL probe numbers (Roche, Penzberg, Germany) are given in Supplementary Table 4. ACTB, GAPDH, and HPRT were used as mRNA housekeeping genes, while small RNAs RNU44 and RNU48 were used as miRNA housekeeping genes. Data were analysed using the Delta-Delta-Ct algorithm 17 (Bioconductor ddCt package).
Cell viability and cell cycle assays
Cell viability assays were carried out as previously described 12 using the Cell Titer Glo Luminescent Cell Viability assay (Promega, Madison, WI, USA) following the manufacturer's instructions 18. 7-AAD and BrdU cell cycle assays were carried out as previously described 15 according to the manufacturer's protocol (BD Pharmingen San Diego, CA, USA). Stained cells were measured by flow cytometry (FACS Calibur; BD Biosciences, Heidelberg, Germany) using Cell Quest Pro software (BD Biosciences).
Apoptosis assay and PI staining
Apoptosis assays were carried out using the caspase 3/7 activity assay (Promega) following the manufacturer's instructions. For propidium iodide (PI) staining, cells and medium were harvested into FACS tubes and washed with PBS. Cells were re-suspended in 500 µl of Nicoletti buffer containing 50 µg/ml PI (Sigma Aldrich) and incubated for 15 min 19. Stained cells were measured by flow cytometry using Cell Quest Pro software (BD Biosciences).
miRNA target prediction
The miRWalk database 20 was used to identify predicted targets of miRNA-519a. 3′UTRs with a seed match of at least 7 bases and a p value less than 0.05 were searched for using three database algorithms: TargetScan, PITA, and DIANA-mT.
Results
The microRNA cluster, C19MC, is up-regulated in tamoxifen-resistant cells and one of its members, miRNA-519a, confers tamoxifen resistance
In order to identify miRNAs which are up-regulated upon tamoxifen resistance, we performed a miRNA microarray and found 67 miRNAs to be significantly up-regulated in TamR versus WT cells. C19MC, the largest known cluster of miRNAs in the human genome 21 encoding around 50 mature miRNAs, was mostly up-regulated (Figures 1a and 1b). Until now, few reports have suggested a role for this cluster in breast cancer or drug resistance; however, studies are emerging describing both the tumour suppressor and oncogenic functions of these miRNAs in different cancer entities 22. In total, 18 members of C19MC were co-up-regulated in TamR cells (Figure 1b). We selected four of the top up-regulated miRNAs from the cluster and validated their overexpression in TamR cells with qRT-PCR (Figure 1c). Since miRNA-519a expression and miRNA-522 expression were both strongly induced in TamR cells, we were interested to know if this deregulation had an impact on tamoxifen sensitivity. We first overexpressed miRNA-519a and miRNA-522 in WT cells using miRNA mimics. Control transfected cells had around a 25% reduction in growth after tamoxifen treatment (p < 0.001), while the growth of cells transfected with miRNA-519a was not affected (Figure 1d), indicating that they are no longer sensitive to tamoxifen treatment after 72 h. However, growth of cells transfected with miRNA-522 was reduced to a similar extent to that of the control (Supplementary Figure 1), indicating that miRNA-522 may not play a role in the resistant phenotype observed. Because miRNA mimics introduce an unphysiologically high expression level (Supplementary Figure 2a), we employed a miRNA-overexpressing vector system to recapitulate our results (Supplementary Figure 2b). Using the same experimental set-up described above, pCMV–MIR-519a was also able to confer resistance to tamoxifen (Figure 1e). Conversely, when we inhibited miRNA-519a in TamR cells using a synthetic inhibitor, only the growth of miRNA-519a inhibitor-transfected cells was reduced by around 20%, showing that TamR cells were re-sensitized to tamoxifen treatment (p < 0.01) (Figure 1f). Overall, these results verified that overexpression of miRNA-519a in WT cells confers resistance to tamoxifen, which could be abrogated by inhibiting the miRNA in TamR cells.
Figure 1.
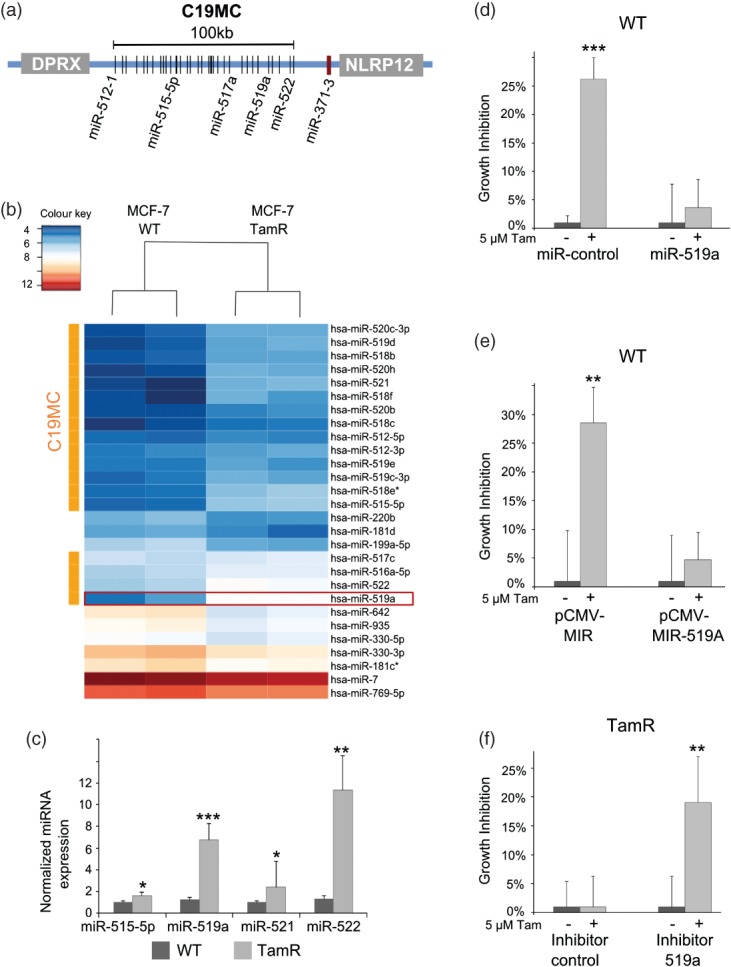
C19MC is up-regulated in TamR cells and miRNA-519a regulates tamoxifen resistance. (a) The C19MC locus comprises approximately 50 mature miRNAs and spans 100 kb on chromosome 19q13.42 bordered by genes DPRX distal and NLRP12, as well as the unrelated miR-371-3 cluster, proximal. (b) Heat map showing 18 members of the miRNA cluster C19MC, depicted with an orange bar, which are up-regulated in MCF-7 TamR cells. miR-519a is boxed in red. (c) qRT-PCR validation of the up-regulation of selected C19MC members in TamR cells compared with WT cells, normalized to RNU44 and RNU48. (d) Overexpressing miRNA-519a in WT cells using mimics or an overexpressing vector (e) confers resistance to tamoxifen after 72 h, while (f) inhibition of miRNA-519a in TamR cells sensitizes cells to tamoxifen treatment. All results are shown as an average of three biological and three technical replicates. One asterisk (*) denotes a p value of less than 0.05, two (**) denote p < 0.01, and three (***) denote p < 0.001, determined by a two-sided t-test, hereafter.
miRNA-519a targets several tumour-suppressor genes involved in cell cycle control
As we found miRNA-519a to be an important modulator of tamoxifen resistance, we were next interested to identify target genes of the miRNA which may have been involved in the acquisition of resistance. Using a combination of miRNA target prediction algorithms: Target Scan, PITA, DIANA-mT and miRWalk 20, we generated a list of 1704 predicted target genes. Next, we used the functional enrichment tool DAVID 23 to identify common deregulated pathways within these 1704 potential target genes, by KEGG (Kyoto Encyclopedia of Genes and Genomes) analysis, within the DAVID tool (Supplementary Table 1). We found ‘Pathways in Cancer’ to be the most enriched pathway (Supplementary Table 1), containing 49 of the predicted target genes. From these 49 genes, five were part of PI3K–Akt signalling and seven belonged to the cell cycle pathway, within the ‘Pathways in Cancer’ module (Supplementary Table 2). We focused on these two pathways because both have previously been strongly implicated in tamoxifen resistance in separate contexts 24,25. Furthermore, because we identified miRNA-519a as a potential oncomir in TamR cells, we hypothesized that its target genes may have a tumour-suppressive function. Thus, we chose the well-known tumour-suppressor gene (TSG), PTEN, within the PI3K–Akt pathway and the negative regulators of the cell cycle, CDKN1A/p21 and retinoblastoma protein (RB1), to validate as direct targets of miRNA-519a.
Initially, we validated that all three predicted target genes were down-regulated in TamR cells compared with WT cells (Figure 2a). Next, we analysed the expression of the target genes after overexpressing miRNA-519a in WT cells and inhibiting miRNA-519a in TamR cells, respectively. In WT cells, mRNA and protein levels were reduced for all three genes 48 h after transfection (Figure 2b). Conversely, inhibition of miRNA-519a in TamR cells rescued the expression of the target genes (Figure 2c), although we did not observe a significant increase in p21 protein level. We then analysed the 3′UTRs of each target gene (Figure 2d) and found that PTEN contained two target sites of miRNA-519a in its 3′UTR; one site was conserved (413–419) and the other one was not (1148–1155). For CDKN1A and RB1, there was one predicted target site of the miRNA (Figure 2d). To determine whether the reduced expression was due to direct targeting by miRNA-519a, we cloned reporter constructs containing the 3′UTR of each gene downstream of a luciferase reporter gene (Supplementary Table 5). Overexpression of miRNA-519a in both WT and TamR cells significantly reduced luciferase activity for all three genes, which was rescued by mutating the respective interaction sites of miRNA-519a by site-directed mutagenesis (Figures 2e and 2f). Furthermore, in order to show that these miRNA–target interactions are not breast cancer-specific, we transfected HEK 293FT cells (human embryonic kidney cells) and repeated the luciferase reporter assays. We obtained very similar results to the case of MCF-7 WT and TamR cells (Figure 2 g), except for RB1. Conversely, we observed an increase in luciferase activity when miR-519a was inhibited in TamR cells (Figure 2 h). Finally, in order to examine the pathological relevance of miRNA–target interactions, we carried out a correlation analysis of miRNA-519a and its target genes in patients and cell lines using publically available data sets from the Gene Expression Omnibus (GEO) (see the Materials and methods section) and the NCI-60 cancer cell line panel, representing nine different tumour types (http://dtp.nci.nih.gov/index.html; experiment ID: 372685). In the dataset GSE22220 26, comprising 208 breast cancer patients, the expression of PTEN significantly correlated inversely with that of miRNA-519a (p = 0.0389) (Figure 3a). In GSE19783 27, miRNA-519a significantly correlated inversely with RB1 in 101 patients (p = 0.027, Figure 3b). Finally, in a panel of 60 cell lines of nine different cancer origins, RB1 and CDKN1A had significant inverse correlations (p = 0.05 and p = 0.009, respectively) with miRNA-519a expression (Figure 3c).
Figure 2.
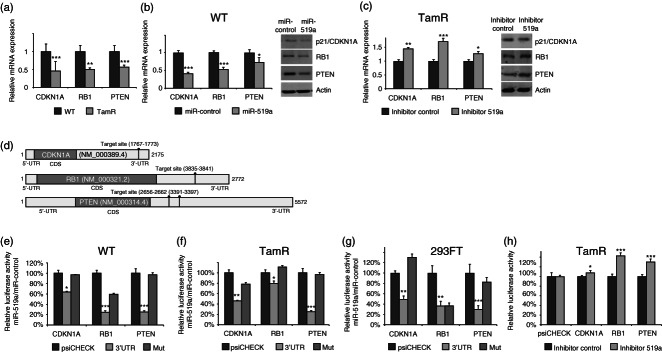
Validation of CDKN1A, RB1, and PTEN as direct targets of miRNA-519a. (a) qRT-PCR reveals that predicted targets of miRNA-519a are down-regulated in TamR cells. (b) Predicted targets are down-regulated 48 h after transfection with mimic in WT cells at RNA as well as protein levels and (c) up-regulated after 48 h transfection with inhibitor in TamR cells. (d) mRNA structure of target genes showing the predicted target sites of miRNA-519a in the respective 3′UTRs. (e) Luciferase assays show that the activity of each 3′UTR is reduced when WT (e), TamR (f), and HEK 293FT (g) cells are transfected with miRNA-519a compared with control. The signal is rescued after mutating the binding site for miRNA-519a. (h) Luciferase signal when TamR cells are transfected with miR-519a inhibitor. Luciferase signal was normalized to empty vector. All results are shown as an average of three biological and three technical replicates, with significance levels explained in Figure 1.
Figure 3.
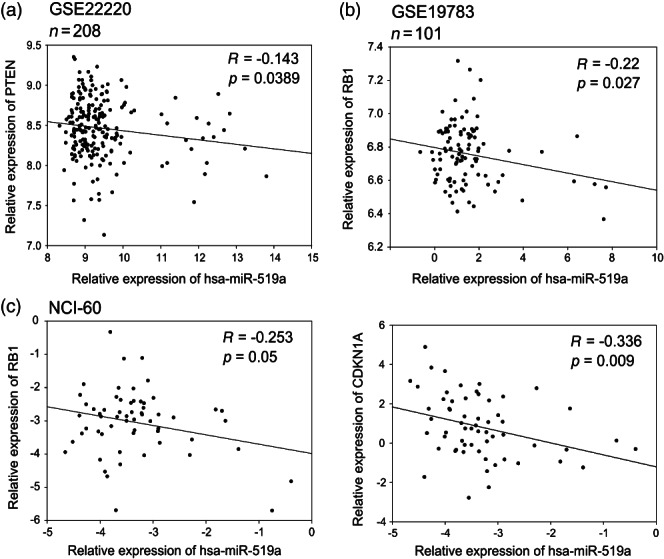
Correlation analysis of miRNA-519a and its target genes in patient datasets and NCI-60 cell line panel presented as scatter plots. miRNA-519a expression is inversely correlated with expression of target genes in two patient datasets consisting of 208 patients 26 (a) and 101 patients 27 (b), as well as in the NCI-60 cell line panel (http://dtp.nci.nih.gov/index.html; experiment ID: 372685) (c).
miRNA-519a co-targets genes that are involved in tamoxifen response both in vitro and in tamoxifen-treated patients
Little is known about how concerted actions of miRNAs targeting biological networks contribute to drug resistance. We have shown that overexpressing miRNA-519a confers resistance to tamoxifen in WT cells. The direct targeting of three TSGs by miRNA-519a led us to hypothesize that down-regulating these genes in WT cells could also confer resistance to tamoxifen. For this purpose, we silenced CDKN1A, RB1, and PTEN in WT cells using siRNAs (Supplementary Figure 4). Individual knockdown of either CDKN1A or RB1 or PTEN could only partially phenocopy the effects observed on resistance to tamoxifen after overexpression of miRNA-519a (Figure 4a). This suggested that regulation of tamoxifen resistance is likely multifactorial. To investigate this hypothesis, we used a double knockdown approach 28. Combinatorial knockdown of either CDKN1A and PTEN or CDKN1A and RB1 was indeed more efficient in phenocopying the effect of miRNA-519a on tamoxifen sensitivity (Figure 4b). However, the combination of PTEN and RB1 knockdown did not confer resistance to tamoxifen, indicating that CDKN1A is an essential mediator of this process. Since we identified important regulators of the cell cycle and PI3K signalling as direct targets, we hypothesized that miRNA-519a could be able to co-regulate a network of proteins in TamR cells, supporting the concept of combinatorial targeting of several genes, as we have previously demonstrated for the EGFR-driven cell cycle 15. Indeed, when we created a network from the predicted target genes in the KEGG ‘Pathways in Cancer’ (Supplementary Table 1), we found the three direct target genes, CDKN1A, RB1, and PTEN, to form a central core within this network (Supplementary Figure 3).
Figure 4.
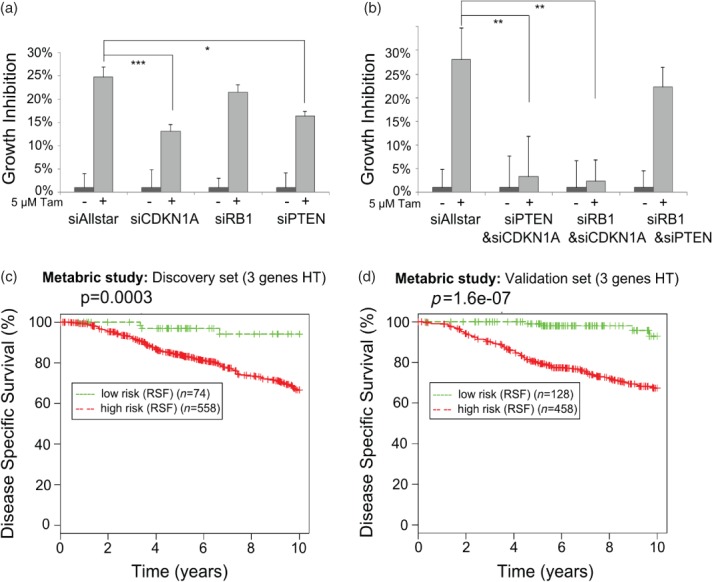
Target genes of miRNA-519a co-operatively regulate tamoxifen resistance. Inhibiting individual (a) and combinations (b) of the TSGs using siRNAs in WT cells promotes resistance to tamoxifen after 72 h. Results are shown as an average of three biological and three technical replicates, with significance levels explained in Figure 1. (c) Random survival forests (RSF) analysis of 632 (d) and 586 breast cancer patients who had received hormone therapy (HT) 30 reveals a significant correlation between survival and expression of target genes.
To test the potential clinical relevance of such a co-regulation in breast cancer patients, we carried out a random survival forests (RSF) analysis 29 on a large cohort of tamoxifen-treated patients, combining the three target genes of miRNA-519a. We extracted gene expression data from the METABRIC study on around 2000 primary breast tumours with long-term clinical follow-up data 30. Next, we selected only patients who had received hormonal therapy (HT), ie tamoxifen. In both the discovery (n = 632) and the validation sets (n = 586) from this study, we found that the patients in the poor outcome group had significantly lower expression levels of RB1, CDKN1A, and PTEN compared with the patients in the group of good survival outcome (Figures 4c and 4d), demonstrating the impact of the co-regulation of these target genes in ER + tamoxifen-treated patients.
miRNA-519a increases viability and S-phase population of the cell cycle, but does not affect EMT or invasion
After identifying key TSGs co-regulated by miRNA-519a in breast cancer, we hypothesized that overexpressing the miRNA would functionally affect cell proliferation. To this end, we measured cell viability after transfection with the miRNA-519a mimic in WT MCF-7 cells. Indeed, in miRNA-519a-transfected cells, there was a significant increase in viability after 48 (p < 0.01) and 72 h (p < 0.01) (Figure 5a) which was accompanied by a significant reduction of cells in the G1 phase, as measured by 7AAD (Figure 5b), and an increase of cells in the S phase, measured by BrdU staining after 72 h (Figure 5c). These results were validated using the overexpressing vector, pCMV–MIR-519a (Figures 5d–5f). In order to show that this effect was not restricted to MCF-7 cells, we reproduced the effects of miR-519a overexpression in three additional ER + cell lines, T-47D, BT-474, and CAMA-1 (Supplementary Figure 5).
Figure 5.
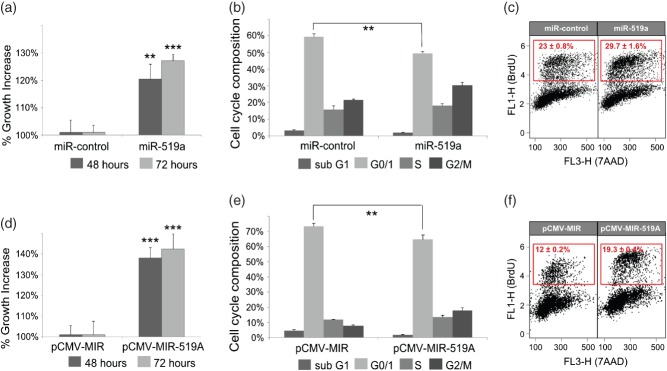
miRNA-519a effects on viability and cell cycle. (a) Viability of MCF-7 WT cells was measured 48 and 72 h after transfection with miR-control or miRNA-519a. 7-AAD (b) and BrdU (c) staining was measured 72 h after transfection with miR-control or miRNA-519a to assay cell cycle phases. The S-phase population is gated in the red box for BrdU-positive cells. (d–f) Cell viability and cell cycle assays similar to a–c except that empty vector, pCMV-MIR or pCMV–MIR-519a overexpressing miRNA-519a were transfected as indicated. The results are presented as an average of two biological and three technical replicates, with significance levels explained in Figure 1.
We previously determined that TamR cells underwent EMT and gained invasive properties 12. We thus wanted to test whether miRNA-519a could also regulate these processes in tamoxifen resistance. To this end, we transfected WT MCF-7 cells with miRNA-519a and tested their invasive capacity. However, miRNA-519a had no significant impact on invasion as similar numbers of invading cells were counted in the control (Supplementary Figure 6). In addition, there was no difference in miRNA-519a expression when comparing epithelial and mesenchymal cell lines from the NCI-60 panel (not shown). Taken together, these data suggest that miRNA-519a regulates resistance to tamoxifen through cell proliferation; however, it is not involved in the establishment of mesenchymal-like properties of the cells.
miRNA-519a-expressing cells evade tamoxifen-induced apoptosis
As well as inhibiting proliferation and cell cycle progression, tamoxifen exerts its effects through the induction of apoptosis. Reports have suggested that in vitro models of tamoxifen resistance show increased resistance to apoptosis induction, even at high concentrations 31. When we treated WT and TamR cells with 5 µm tamoxifen, little apoptosis was induced in either cell type, as measured by the activity of caspase-3 and -7 (Figure 6a). However, when we treated them with a higher dose of tamoxifen (10 µm), in line with other studies 32,33, TamR cells remained resistant, while WT cells displayed a significant induction of apoptosis (Figure 6a). Since miRNA-519a strongly induces cell proliferation, we hypothesized that this would correlate with a reduction in apoptosis. To test this, we transfected WT cells with miRNA-519a and again treated the cells with 5 and 10 µm tamoxifen. Caspase activity was significantly increased by around 40% (p < 0.001) in control transfected cells treated with 10 µm tamoxifen, while miRNA-519a-transfected cells were only slightly affected (Figure 6b). To confirm these results after 72 h of treatment, we stained the cells with propidium iodide. In line with the caspase activity, cells that had been transfected with miRNA-519a had a significantly lower apoptotic population (10% and 18%) compared with control cells (14% and 24%) after treatment with 5 and 10 µm tamoxifen, respectively (Figure 6c). Conversely, when we inhibited miR-519a expression in TamR cells, caspase 3/7 activity was significantly increased after treatment with 10 µm tamoxifen (p < 0.01; Figure 6d). Taken together, in addition to enhancing cell cycle and viability via the targeting of key TSGs, miRNA-519a is capable of evading tamoxifen-induced apoptosis. Both processes confirm the oncogenic potential of this miRNA in breast cancer and additionally elucidate part of the mechanism of resistance to tamoxifen observed.
Figure 6.
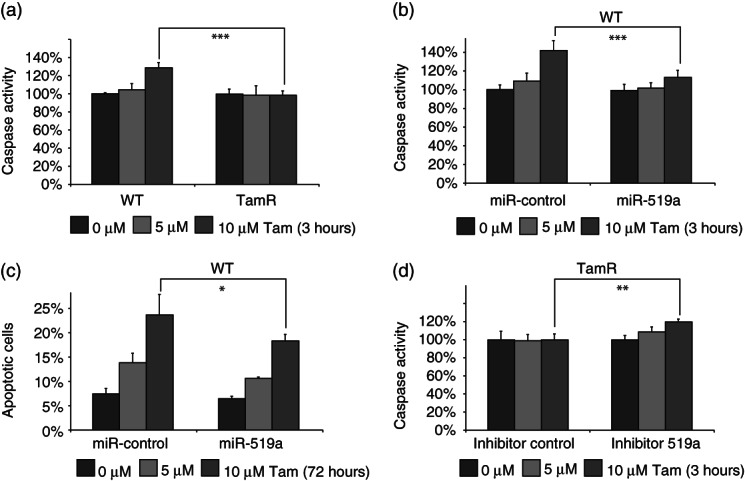
miRNA-519a reduces tamoxifen-induced apoptosis. (a) Caspase activity is elevated only in WT cells after 3 h treatment with 10 µm tamoxifen. (b) Caspase activity is increased only in miR-control transfected cells and is abrogated by transfection with a miRNA-519a mimic. (c) PI staining reveals that more apoptotic cells are present in miR-control than in miRNA-519a mimic-transfected cells after 72 h treatment with tamoxifen. (d) Caspase activity is increased only in inhibitor 519a-transfected TamR cells. The results are presented as an average of two biological and three technical replicates each, with significance levels explained in Figure 1.
miRNA-519a expression correlates with poor outcome and clinicopathological features in breast cancer patients
Since miRNA-519a was highly up-regulated in TamR cells and little was known about its role in breast cancer, we analysed breast cancer patients' datasets with respect to the potential involvement of miRNA-519a in pathogenesis. We found that high expression of miRNA-519a correlated significantly with poorer survival in two independent datasets (Figure 7a and Supplementary Figure 7a, p = 0.03). Next, we divided patients according to ER status. In GSE19783, miRNA-519a expression correlated even more significantly with the outcome exclusively in ER + patients (p = 0.0071, Figure 7b) but not in ER − patients (p = 0.94, Figure 7c). A similar trend was observed in the second dataset, GSE22220, although it did not reach statistical significance (p = 0.064, Supplementary Figures 7b and 7c). Because miRNA-519a expression is associated more significantly with survival in ER + patients, this gives the miRNA prognostic potential for those patients who will potentially develop resistance to tamoxifen and regress more quickly.
Figure 7.
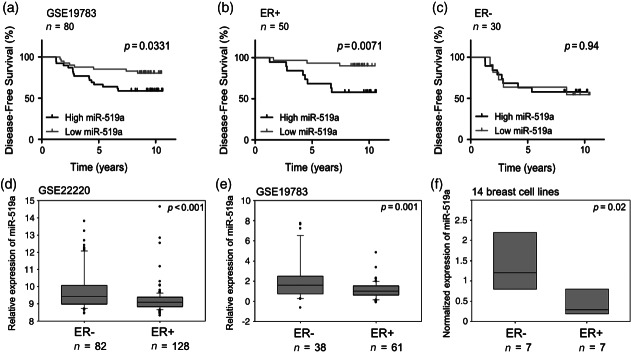
Increased expression of miRNA-519a is significantly correlated with poorer disease-free survival in ER+ breast cancer patients and poor clinicopathological features in breast cancer. Patients were separated according to miRNA-519a expression (median cut-off) in a cohort of breast cancer patients 27 and miRNA-519a expression was significantly correlated with survival (a). When patients were further sub-classified according to ER status, miRNA-519a only correlated with survival in ER+ patients (b), but not in ER− patients (c). miRNA-519a was found to be significantly more highly expressed in ER− patients in two patient datasets (GEO Accession GSE19783 27 and GSE22220 26) (d, e) and in 14 breast cell lines, analysed by qRT-PCR (f).
As miRNA-519a is associated with poorer survival in breast cancer and targets several TSGs, we finally assessed whether it was associated with other clinicopathological features. In line with the survival data and our model of tamoxifen resistance, miRNA-519a was found to be significantly more highly expressed in the more aggressive ER − tumours in the same two datasets (Figures 7d and 7e). We observed the same trend in a panel of 14 breast cell lines (Figure 7f). We had previously shown that TamR cells lose their expression of ER 12 and it is known that ER − patients have a poorer prognosis and outcome 34. This may further suggest a link between ER status and miR-519a expression level; however, there must be other factors affecting the poorer survival of ER − patients. Finally, we found significantly higher expression of the miRNA in high-grade tumours (Supplementary Figure 8). Overall, these data show that miRNA-519a expression correlates with poor survival outcome as well as critical clinicopathological properties in breast cancer.
Discussion
In the present study, we identified a large cluster of miRNAs, C19MC, which were up-regulated in TamR cells compared with their parental WT cells. Overexpressing miRNA-519a in WT cells conferred resistance to tamoxifen, which was mediated by the direct co-targeting of several TSGs. Our findings indicate that tamoxifen-resistant cells express miRNA-519a at high levels, which directly represses the expression of PTEN, RB1, and CDKN1A, central nodes of a dense network, allowing the cells to proliferate, even in the presence of tamoxifen (Figure 8). Our results are supported by clinical data, where we found high expression of miRNA-519a or low co-expression of the target genes to correlate with poorer disease-free survival in ER + breast cancer patients.
Figure 8.
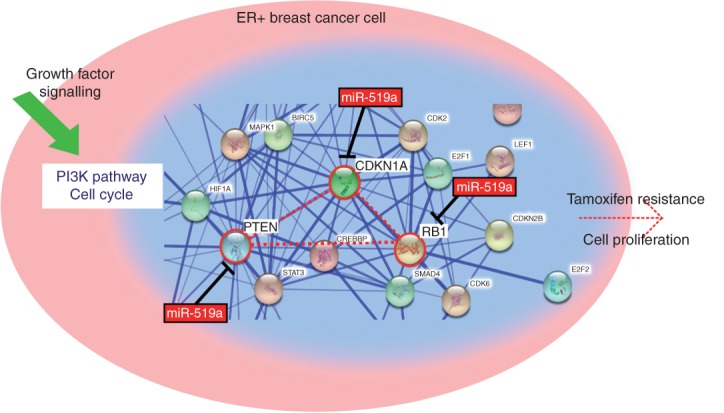
miR-519a targets central nodes (PTEN, CDKN1A, and RB1) from PI3K and cell cycle network leading to increased proliferation and tamoxifen resistance. A snapshot from STRING (http://string-db.org) analysis depicts how the three validated direct target genes of miR-519a form a central connection in the network, highlighted in red.
Until now, there have been no reports indicating an oncogenic role of miRNA-519a in breast cancer. Other members of the C19MC family have been described in cancer; however, their roles seem to differ between cancer entities and even within subtypes 22. In contrast to our findings, Abdelmohsen et al reported miRNA-519a to reduce proliferation via the direct targeting of the HuR RNA binding protein in various cancer cell lines 35. On the other hand, the oncogenic role of C19MC miRNAs in cancer has been described. For example, miRNA-519a was found to be up-regulated and associated with poorer survival and other clinicopathological features in ovarian carcinoma 36. Fornari et al recently discovered miR-519d to be up-regulated in hepatocellular carcinoma (HCC). In line with our findings, they described the miRNA to promote proliferation, invasion, and evade apoptosis 37. In ER + breast cancer, Foekens et al identified miR-516-3p as part of a signature of four up-regulated miRNAs linked with tumour aggressiveness and cell cycle progression 38. Another study identified C19MC members (miR-520c-3p, miR-520 g, and miR-520 h) in a screen for biomarkers which predict outcome in tamoxifen-treated patients and found them to be associated with the probability of recurrence 39. These findings support our results, where we found miRNA-519a to be involved in tumourigenesis and tamoxifen resistance.
Using a functional gene enrichment tool, we identified target genes in PI3K-signalling and cell cycle pathways. We found the cyclin-dependent kinase inhibitor CDKN1A (p21) to be a direct target of miRNA-519a. CDKN1A had previously been identified in a genome-wide screen for tamoxifen resistance regulators 9, and loss of CDKN1A expression in tamoxifen-treated patients was shown to be a factor contributing to resistance 40. In line with our findings, using a screen-based approach, Wu et al identified seven members of C19MC to regulate CDKN1A, which in turn affected the cell cycle in choriocarcinoma cells 41. In addition, we identified RB1, the master regulator of the cell cycle and a prominent TSG, as a direct target. This finding is in line with other studies which have found RB1 inactivation to be associated with tamoxifen resistance and the deregulation of the RB1 pathway to be associated with recurrence in patients who were treated with tamoxifen 42. Finally, we showed PTEN, the negative regulator of PI3K signalling, to be a direct target. Aberrant PI3K signalling is a well-known factor contributing to tamoxifen resistance and has led to clinical trials testing PI3K inhibitors in combination with tamoxifen 24. Interestingly, others have reported PTEN to be inactivated by DNA methylation in tamoxifen resistance 18. In our model, we have shown a novel epigenetic mechanism: inactivation through miRNA-519a.
Intriguingly, we found the three TSGs co-targeted by miRNA-519a to form the core of a dense biological network (Figure 8). Our findings highlight the potential of miRNAs to modulate phenotypes in cancer cells by synergistically targeting genes within such networks. The combined expression of the validated target genes was significantly associated with disease risk, highlighting their prognostic potential in tamoxifen-treated patients. To our knowledge, this is the first study implicating the co-targeting effect of miRNAs in tamoxifen resistance. Using a similar approach to our study, Cittelly et al found miR-342 to be down-regulated in tamoxifen resistance. They also identified a number of potential target genes in biological pathways such as cell cycle and apoptosis but did not validate potential co-targeting effects 43. We have previously highlighted the global effects that miRNAs have in the EGFR-driven cell cycle network, where we could show that miRNAs can directly and indirectly co-regulate networks of proteins 15. Interestingly, miR-519a did not alter the invasive phenotype of TamR cells, as we have previously demonstrated for miR-375 12. This finding could be explained by the differential target spectrum of the two miRNAs. While miR-519a acts as an oncomir, conferring tamoxifen resistance through the down-regulation of a network of TSGs, miR-375 acts as a tumour suppressor, sensitizing TamR cells to tamoxifen through the down-regulation of MTDH. It is likely that both miRNAs may cooperate to modulate tamoxifen resistance, which was beyond the scope of this study.
Taken together, we have shown that co-regulation of several TSGs by miRNA-519a confers tamoxifen resistance in WT MCF-7 cells through regulation of the cell cycle and apoptosis. Furthermore, our study defined a novel role for miRNA-519a in ER + breast cancer and tamoxifen resistance. Our in vitro findings are supported by clinical data, where we found a statistically significant correlation between miRNA-519a expression and its target genes with patient survival. miRNA-519a or its target genes could serve as potential predictive markers for tamoxifen resistance or might be inhibited using synthetic antagomirs as an approach to overcome tamoxifen resistance.
Acknowledgments
We thank Christian Breunig and Jitao D Zhang for their valuable discussions. We are grateful to Angelika Wörner, Heike Wilhelm, and Ewald Münstermann for their excellent technical assistance. We thank the DKFZ Genomics and Proteomics Core Facility for performing cell line authentication and contamination control services. We acknowledge research funding from the German Federal Ministry of Education and Research (NGFN grant 01GS0816) and the Deutsche Forschungsgemeinschaft (DIP project WI3499/1-1). This work was supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1002 and the eBio Initiative, SYSMET-BC 0316168D.
Author contribution statement
AW, OS, and SW developed the concepts and designed the experiments. AW carried out most of the experiments and data analysis. KS and AB contributed to some of the experiments. ZS and RK provided the statistical support. AW, OS, and SW wrote the manuscript and all the authors contributed to the manuscript preparation.
Supporting Information
The following supporting information may be found in the online version of this article.
Supplementary Methods
Figure S1. Effect of miR-522 on tamoxifen resistance.
Figure S2. Verification of miRNA mimic, CMV vector, and inhibitor expression.
Figure S3. Predicted target genes of miR-519a form a dense network.
Figure S4. Knockdown efficiency of siRNAs.
Figure S5. miR-519a increases cell viability in three additional ER + cell lines.
Figure S6. Invasion assay.
Figure S7. Increased expression of miRNA-519a is significantly correlated with higher risk of relapse in breast cancer patients.
Figure S8. miRNA-519a expression is associated with tumour grade.
Table S1. Top ten significantly enriched KEGG pathways from DAVID functional enrichment analysis of predicted target genes.
Table S2. Seven predicted target genes of miR-519a were enriched in the cell cycle pathway and five were found in the PI3K–Akt signalling.
Table S3. siRNA sequences.
Table S4. Primers used for qRT-PCR.
Table S5. Primers used for 3′UTR cloning.
References
- Vargo-Gogola T, Rosen JM. Modelling breast cancer: one size does not fit all. Nature Rev Cancer. 2007;7:659–672. doi: 10.1038/nrc2193. [DOI] [PubMed] [Google Scholar]
- Jordan VC, Murphy CS. Endocrine pharmacology of antiestrogens as antitumor agents. Endocr Rev. 1990;11:578–610. doi: 10.1210/edrv-11-4-578. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen BS, Miller MA, Mullick A, et al. Antiestrogen action in breast cancer cells: modulation of proliferation and protein synthesis, and interaction with estrogen receptors and additional antiestrogen binding sites. Breast Cancer Res Treat. 1985;5:231–243. doi: 10.1007/BF01806018. [DOI] [PubMed] [Google Scholar]
- Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–937. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- Thiantanawat A, Long BJ, Brodie AM. Signaling pathways of apoptosis activated by aromatase inhibitors and antiestrogens. Cancer Res. 2003;63:8037–8050. [PubMed] [Google Scholar]
- Hui R, Finney GL, Carroll JS, et al. Constitutive overexpression of cyclin D1 but not cyclin E confers acute resistance to antiestrogens in T-47D breast cancer cells. Cancer Res. 2002;62:6916–6923. [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Malerva L, Park J, Zou L, et al. High-throughput ectopic expression screen for tamoxifen resistance identifies an atypical kinase that blocks autophagy. Proc Natl Acad Sci U S A. 2011;108:2058–2063. doi: 10.1073/pnas.1018157108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, et al. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Dong C, Ji C. MicroRNA and drug resistance. Cancer Gene Ther. 2010;17:523–531. doi: 10.1038/cgt.2010.18. [DOI] [PubMed] [Google Scholar]
- Ward A, Balwierz A, Zhang JD, et al. Re-expression of microRNA-375 reverses both tamoxifen resistance and accompanying EMT-like properties in breast cancer. Oncogene. 2013;32:1173–1182. doi: 10.1038/onc.2012.128. [DOI] [PubMed] [Google Scholar]
- Miller TE, Ghoshal K, Ramaswamy B, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–29903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D, Villen J, Shin C, et al. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann S, Mannsperger H, Zhang JD, et al. Global microRNA level regulation of EGFR-driven cell-cycle protein network in breast cancer. Mol Syst Biol. 2012;8:570. doi: 10.1038/msb.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlmann S, Zhang JD, Schwager A, et al. miR-200bc/429 cluster targets PLCgamma1 and differentially regulates proliferation and EGF-driven invasion than miR-200a/141 in breast cancer. Oncogene. 2010;29:4297–4306. doi: 10.1038/onc.2010.201. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Phuong NT, Kim SK, Lim SC, et al. Role of PTEN promoter methylation in tamoxifen-resistant breast cancer cells. Breast Cancer Res Treat. 2011;130:73–83. doi: 10.1007/s10549-010-1304-2. [DOI] [PubMed] [Google Scholar]
- Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nature Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- Dweep H, Sticht C, Pandey P, et al. miRWalk – database: prediction of possible miRNA binding sites by ‘walking’ the genes of three genomes. J Biomed Inform. 2011;44:839–847. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152–D157. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor I, Bullerdiek J. The dark side of a success story: microRNAs of the C19MC cluster in human tumours. J Pathol. 2012;227:270–274. doi: 10.1002/path.4014. [DOI] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffa FM, Camps C, Winchester L, et al. MicroRNA-associated progression pathways and potential therapeutic targets identified by integrated mRNA and microRNA expression profiling in breast cancer. Cancer Res. 2011;71:5635–5645. doi: 10.1158/0008-5472.CAN-11-0489. [DOI] [PubMed] [Google Scholar]
- Enerly E, Steinfeld I, Kleivi K, et al. miRNA–mRNA integrated analysis reveals roles for miRNAs in primary breast tumors. PLoS One. 2011;6:e16915. doi: 10.1371/journal.pone.0016915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin O, Lobke C, Korf U, et al. Combinatorial RNAi for quantitative protein network analysis. Proc Natl Acad Sci U S A. 2007;104:6579–6584. doi: 10.1073/pnas.0606827104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishwaran H, Kogalur UB, Blackstone EH, et al. Random survival forests. Ann Appl Statistics. 2008;2:841–860. [Google Scholar]
- Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treeck O, Zhou R, Diedrich K, et al. Tamoxifen long-term treatment in vitro alters the apoptotic response of MCF-7 breast cancer cells. Anticancer Drugs. 2004;15:787–793. doi: 10.1097/00001813-200409000-00008. [DOI] [PubMed] [Google Scholar]
- Pfeiler G, Horn F, Lattrich C, et al. Apoptotic effects of signal transduction inhibitors on human tumor cells with different PTEN expression. Oncol Rep. 2007;18:1305–1309. [PubMed] [Google Scholar]
- Kumar R, Mandal M, Lipton A, et al. Overexpression of HER2 modulates bcl-2, bcl-XL, and tamoxifen-induced apoptosis in human MCF-7 breast cancer cells. Clin Cancer Res. 1996;2:1215–1219. [PubMed] [Google Scholar]
- Knight WA, Livingston RB, Gregory EJ, et al. Estrogen receptor as an independent prognostic factor for early recurrence in breast cancer. Cancer Res. 1977;37:4669–4671. [PubMed] [Google Scholar]
- Abdelmohsen K, Srikantan S, Kuwano Y, et al. miR-519 reduces cell proliferation by lowering RNA-binding protein HuR levels. Proc Natl Acad Sci U S A. 2008;105:20297–20302. doi: 10.1073/pnas.0809376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Kim YK, Kwon Y, et al. Deregulation of miR-519a, 153, and 485-5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology. 2010;57:734–743. doi: 10.1111/j.1365-2559.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- Fornari F, Milazzo M, Chieco P, et al. In hepatocellular carcinoma miR-519d is up-regulated by p53 and DNA hypomethylation and targets CDKN1A/p21, PTEN, AKT3 and TIMP2. J Pathol. 2012;227:275–285. doi: 10.1002/path.3995. [DOI] [PubMed] [Google Scholar]
- Foekens JA, Sieuwerts AM, Smid M, et al. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc Natl Acad Sci U S A. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng MB, Laenkholm AV, Sokilde R, et al. Global microRNA expression profiling of high-risk ER + breast cancers from patients receiving adjuvant tamoxifen mono-therapy: a DBCG study. PLoS One. 2012;7:e36170. doi: 10.1371/journal.pone.0036170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abukhdeir AM, Vitolo MI, Argani P, et al. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci U S A. 2008;105:288–293. doi: 10.1073/pnas.0710887105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang S, Ding J, et al. Multiple microRNAs modulate p21Cip1/Waf1 expression by directly targeting its 3′ untranslated region. Oncogene. 2010;29:2302–2308. doi: 10.1038/onc.2010.34. [DOI] [PubMed] [Google Scholar]
- Bosco EE, Wang Y, Xu H, et al. The retinoblastoma tumor suppressor modifies the therapeutic response of breast cancer. J Clin Invest. 2007;117:218–228. doi: 10.1172/JCI28803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cittelly DM, Das PM, Spoelstra NS, et al. Downregulation of miR-342 is associated with tamoxifen resistant breast tumors. Mol Cancer. 2010;9:317. doi: 10.1186/1476-4598-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin O, Frohlich H, Lobke C, et al. Modeling ERBB receptor-regulated G1/S transition to find novel targets for de novo trastuzumab resistance. BMC Syst Biol. 2009;3:1. doi: 10.1186/1752-0509-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Figure S1. Effect of miR-522 on tamoxifen resistance.
Figure S2. Verification of miRNA mimic, CMV vector, and inhibitor expression.
Figure S3. Predicted target genes of miR-519a form a dense network.
Figure S4. Knockdown efficiency of siRNAs.
Figure S5. miR-519a increases cell viability in three additional ER + cell lines.
Figure S6. Invasion assay.
Figure S7. Increased expression of miRNA-519a is significantly correlated with higher risk of relapse in breast cancer patients.
Figure S8. miRNA-519a expression is associated with tumour grade.
Table S1. Top ten significantly enriched KEGG pathways from DAVID functional enrichment analysis of predicted target genes.
Table S2. Seven predicted target genes of miR-519a were enriched in the cell cycle pathway and five were found in the PI3K–Akt signalling.
Table S3. siRNA sequences.
Table S4. Primers used for qRT-PCR.
Table S5. Primers used for 3′UTR cloning.


