Abstract
Fruiting bodies similar to those of the ascomycete fungi Podostroma cornu-damae and Cordyceps militaris were collected from Mt. Seunghak in Busan, Korea on August 21, 2012. The fruiting bodies were cylindrical, with tapered ends and golden red in color. The fruiting bodies contained abundant conidiophores bearing single-celled conidia, but no perithecia or asci. Pure culture of the fungal isolates was obtained through single-spore isolation. Analyses of morphological characteristics, including conidia shape, and phylogenetic traits, using internal transcribed spacer sequences, showed that these isolates belonged to the species Simplicillium lanosoniveum. Although this fungal species is known to be mycoparasitic, the isolates obtained in this study were unable to infect fungi. However, silkworms (Bombyx mori) inoculated with the fungal isolates died during the larval or pupal stages, as has been shown for the strongly entomopathogenic fungus Beauveria bassiana. This study is the first report of the entomopathogenicity of S. lanosoniveum and indicates its potential for use in biological control of insects.
Keywords: Ascomata, Entomoparasite, Fruiting body, Mycoparasite, Simplicillium lanosoniveum
Fungal species belonging to the genus Simplicillium have been isolated from soils, diseased plant tissues, fungi, nematodes, and human nails [1, 2, 3, 4, 5, 6]. This genus is phylogenetically related to Cordyceps [7], but its morphological characteristics resemble those of Lecanicillium, resulting in frequent misclassification. However, using a combination of morphological characteristics and phylogenetic traits, this genus can be clearly distinguished from related fungal genera [7, 8].
Some species of the genus Simplicillium have been reported to be mycoparasites [4, 5, 6, 9]. Strains of S. lanosoniveum isolated from diseased soybean leaves infected with the soybean-rust pathogen Phakopsora pachyrhizi suppress the development of uredinia of the rust fungus [5]. S. lanosoniveum strains isolated in India show hyperparasitism on Aecidium elaneagni-latifoliae, which causes rust on Elaeagnus latifolia [10]. S. lamellicola causes gill mildew and brown spots on Agaricus bisporus [11]. S. lanosoniveum is known to be a causal agent of brown spot on Salvinia spp. plants [1], but most species of the genus Simplicillium do not appear to be plant pathogens.
Species of Simplicillium have also been described as entomopathogens. S. lamellicola decreases the average survival time of several species of tick; however, the virulence of this fungus against insects, including ticks, is much less than that of the well-known entomopathogenic fungus Beauveria bassiana [9]. S. lamellicola also parasitizes the cysts and eggs of nematodes [12], and recently, it was reported that a S. lamellicola isolate has both antibacterial activity and antifungal activity. This species suppresses the development of bacterial diseases in plants by the production of antibacterial metabolites such as mannosyl lipids [13].
Although the genus Simplicillium occurs in a broad range of ecological niches, and the importance of biological control agents against plant pathogens and insects has been growing worldwide, reports on this genus in Korea are very limited. The only published report is on the use of S. lamellicola to develop biological control agents against plant pathogenic fungi and bacteria [13]. In this study, we identified S. lanosoniveum from fruiting bodies generated on a pine cone and reported its entomopathogenicity. S. lanosoniveum showed virulence against silkworms as strong as that of the well-known entomopathogenic fungus B. bassiana, indicating its potential as a biological control agent against insects.
MATERIALS AND METHODS
Isolation of fungal strains
A specimen collected from Mt. Seunghak in Busan, Korea, on August 21, 2012 was used in this study. For single spore isolation, a small cubeshaped piece (approximately 5 mm × 5 mm × 5 mm) of the fruiting body was soaked in 1 mL of distilled water. After vigorous vortexing for 30 sec, 0.1 mL was surface-spread on 2% water agar containing 50 mg/L kanamycin, and the plates were incubated for 8 hr at 25℃. Two to four germinating conidia per plate were isolated under a dissecting microscope. Each isolate was transferred into fresh potato dextrose agar (PDA) [14] containing 50 mg/L kanamycin and incubated for 5 days at 25℃. For isolation from the mycelium of the fruiting body, a whole fruiting body was detached from the pine cone and soaked in 1% (w/v) sodium hypochlorite for 2 min. After rinsing the fruiting body in sterile water for 2 min, it was diced, and the internal portion of the stipe (a cube, with dimensions 3mm× 3 mm × 3 mm) was placed on PDA containing 50 mg/L kanamycin. The plates were then incubated at 25℃ for 4~7 days, at which point fungal isolates were transferred to fresh PDA. Single spore isolation from the PDA culture was also performed, as previously described [14].
Microscopy
The fungal isolate was inoculated on PDA and incubated for 10 days at 25℃, and the mycelial plugs from the PDA plates were fixed for scanning electron microscopy (SEM), as previously described [15]. The culture plugs were immersed overnight at 4℃ in modified Karnovsky's fixative (2% paraformaldehyde and 2% glutaraldehyde in 50 mM sodium cacodylate buffer, pH 7.2) and washed with the same buffer three times for 10 min each. Postfixation with 1% osmium tetroxide in 0.05 M sodium cacodylate buffer (pH 7.2) was performed at 4℃ for 2 hr, and the samples were washed twice with distilled water. The fixed samples were dehydrated in a graded ethanol series (30%, 50%, 70%, 80%, 90%, and 100%, and three times in 100% for 10 min each) and then transferred to 100% isoamyl acetate. The samples were dried with liquid carbon dioxide and then mounted on metal stubs. Samples were coated with gold and observed with a field emission scanning electron microscope JSM-6700F (JEOL, Tokyo, Japan) at the Joint Instrumentation Center of Dong-A University (Busan, Korea).
DNA extraction, PCR amplification, and sequencing
Each fungal isolate was also identified based on internal transcribed spacer (ITS) sequences. Fungal genomic DNA was extracted from mycelia cultured in complete medium (CM) by using the cetyltrimethyl ammonium bromide procedure [14]. The rDNA ITS region was amplified using primers ITS1 and ITS4 [16]. The primers were synthesized at an oligonucleotide synthesis facility (Macrogen, Seoul, Korea) and diluted to 20 µM in sterilized water. PCR products were purified using a QIAquick PCR purification kit (Qiagen, Valencia, CA, USA), according to the manufacturer's instructions, and were directly sequenced at the National Instrumentation Center for Environmental Management (Seoul National University, Seoul, Korea). The sequences were compared to the NCBI database with standard nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi/).
Antifungal activity assays
The biological activity of the isolates was tested on three different media: PDA, CM agar, and minimal medium agar [14]. A small piece of the isolate (5 mm in diameter) was inoculated on one side of a Petri dish and another fungal strain, either Fusarium graminearum, Colletotrichum gloeosporioides, C. acutatum, A. nidulans, or Magnaporthe oryzae, was inoculated on the other side. The plates were incubated for 10 days at 25℃.
To test the mycoparasitic activity of the isolates, 1 mL of conidial suspension (105/mL) was spread on PDA. When the fungal mycelia covered the whole plate, a piece of mycelial block (7 mm in diameter) from the isolates was placed in the center of culture and the plates were incubated for 10 days at 25℃. Each experiment was repeated twice, with three replicates.
Entomopathogenicity assay
The entomopathogenicity of the isolates obtained in this study was also compared with that of the entomopathogenic fungus B. bassiana and the phytopathogenic fungus F. graminearum. Each fungal isolate was incubated on PDA or synthetic nutrient agar (SNA) [14] for 4~10 days at 25℃ to induce production of conidia. Conidia were harvested and adjusted to a concentration of 105/mL with distilled water. Conidial suspensions (100 µL) were injected into silkworm pupae (Bombyx mori), as previously described [17]. The inoculated pupae were placed on a wet cotton cloth and incubated at 25℃.
To test the entomopathogenicity of the isolates against silkworm larvae, the technique developed by Shimazu [18] was employed with slight modifications. One milliliter of conidial suspension (105/mL) of each fungal isolate was spread on PDA or SNA to allow both mycelial growth and conidial production, and the plates were incubated for 4~10 days at 25℃. Fifth-instar silkworm larvae were allowed to walk on the plates for 12 hr and then transferred to fresh Petri dishes containing only silkworm feed. Nine pupae and nine larvae were inoculated with each fungal isolate, and the experiments were repeated twice.
RESULTS AND DISCUSSION
Identification of fungal isolates from the fruiting bodies
The specimen was collected from Mt. Seunghak in Busan, Korea, on August 21, 2012. The fruiting bodies were generated on a cone of a pine tree (Pinus densiflora), which was covered with fallen leaves. The fruiting bodies were cylindrical with tapered ends and golden red in color (Fig. 1A). The morphology of the fruiting bodies was similar to that of the ascomycete fungi Podostroma cornu-damae and Cordyceps militaris. Sexual reproductive structures, such as perithecia or asci, were not observed on the fruiting bodies, but the surfaces of the fruiting bodies bore abundant conidiophores bearing single-celled conidia.
Fig. 1.

Fruiting bodies collected from Mt. Seunghak in Busan, Korea (A) and cultured on potato dextrose agar following single-spore isolation from the fruiting bodies (B).
Pure culture of 12 isolates was obtained by single spore isolation, and the ITS region was sequenced for identification of all the isolates. The ITS sequences of eight isolates showed 100% sequence identity with that of S. lanosoniveum. Two isolates were found to belong to Metarhizium sp., and the remaining isolates were found to belong to Penicillium sp. and Aspergillus sp., which could have contaminated the fruiting bodies. We also obtained five isolates from the internal portion of a fruiting body following surface sterilization; the ITS sequences of these isolates were also identical to that of S. lanosoniveum.
The teleomorph of S. lanosoniveum is known as Torrubiella sp., and it is known to produce immature ascomata when incubated on potato carrot agar (PCA) [11]. However, the fruiting bodies collected in this study were quite different in shape from the ascomata of Torrubiella sp., and no studies have reported ascomata production by S. lanosoniveum strains. We also tried to induce the isolates to produce ascomata on PCA, brown rice medium, or silkworm pupae, but fruiting body structures were not produced.
Microscopic observation of the isolates
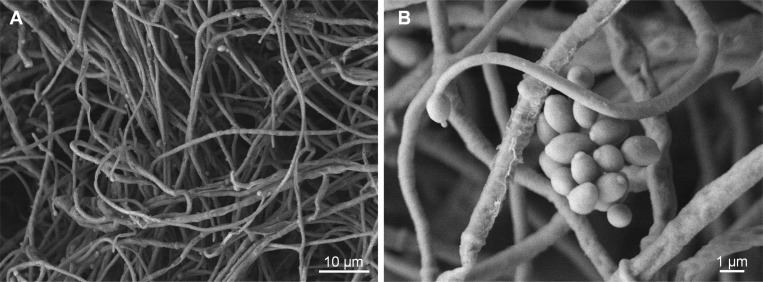
The mycelial growth of the isolates was approximately 15mm in diameter after 7 days of incubation on PDA at 25℃. The isolates produced white aerial mycelia and creamy yellow pigment at the bottom of the medium (Fig. 1B). SEM observation revealed that long, narrow, single phialides arose from aerial hyphae, and the conidia were oval or ellipsoidal with globose heads (Fig. 2). The conidia were, on average, 2.1-µm long and 1.1-µm wide. These morphological characteristics were also concordant with those previously reported for S. lanosoniveum [3, 4].
Fig. 2.
Scanning electron microscopic analysis of mycelia (A) and conidia (B). The fungal isolate was incubated on potato dextrose agar for 10 days at 25℃, and mycelial plugs from potato dextrose agar plates were fixed for microscopy.
Antifungal activity of S. lanosoniveum isolates
S. lanosoniveum has been well characterized as a mycoparasite [4, 5]. When S. lanosoniveum was grown in contraposition with other fungal species, including F. graminearum, C. acutatum, C. gloeosporioides, A. nidulans, and M. oryzae, the growth of the other fungal species remained unaffected. Antagonistic fungi can overgrow host colonies and cause lysis of host hyphae [13], but the present S. lanosoniveum isolates neither overgrew other fungal colonies nor caused lysis of the fungal isolates tested in this study. These results showed that the S. lanosoniveum isolates evaluated in this study did not exhibit antifungal activity or mycoparasitism.
Entomopathogenicity of S. lanosoniveum isolates
When silkworm pupae were inoculated with the S. lanosoniveum spore suspension prepared in this study, the pupae showed melanization 3 days after inoculation. Melanization was more severe 5 days after inoculation, and mycelia were observed on the inter-segmental membranes and spiracles 8 days after inoculation (Fig. 3). The mycelial growth of the S. lanosoniveum isolates on the pupae was weaker than that of B. bassiana. Interestingly, the pupae inoculated with the plant pathogenic fungus F. graminearum were highly melanized, and fungal mycelia were observed to protrude from the inter-segmental membranes and spiracles of the pupae (Fig. 3). We also inoculated the pupae with other fungal species, including the saprophytic fungus A. nidulans and the plant pathogenic fungus M. oryzae, because F. graminearum produces mycotoxins such as trichothecens and zearalenone that might result in melanization and death of pupae. All pupae inoculated with each fungal species were also highly melanized. However, fungal mycelia appeared on approximately half of the pupae and the remaining pupae successfully underwent metamorphosis from pupae to imagines. This result suggested that the artificial injection method was not adequate to assay the entomopathogenicity of fungal species, although the different fungal species produced differing mortality rates.
Fig. 3.

Entomopathogenicity assay in silkworm pupae. Conidial suspensions of each fungal isolate (100 µL) were injected into silkworm (Bombyx mori) pupae, and the inoculated pupae were placed on a wet cotton cloth and incubated at 25℃. The numbers on the left indicate the days after inoculation. S. lanosoniveu, Simplicillium lanosoniveu; B. bassiana, Beauveria bassiana; F. graminearum, Fusarium graminearum.
To test infection under more natural conditions than the injection method, fifth-instar silkworm larva were allowed to walk on plates containing fungal mycelia and spores for 12 hr and were subsequently moved to fresh Petri dishes (Fig. 4). In the control silkworm larvae, which were not allowed contact with any fungal species, all larvae underwent metamorphosis from larvae to pupae. Although infection with F. graminearum by injection, as described above, caused the death of some pupae, infection by walking on Petri plates did not affect normal metamorphosis. Following infection with S. lanosoniveum and B. bassiana, approximately one-third of larvae failed to undergo normal metamorphosis from larvae to pupae. The remaining larvae progressed to the pupal stage but were not able to undergo the next phase of metamorphosis, from pupae to imagines (Fig. 4). These results suggested that the S. lanosoniveum isolates are as strongly entomopathogenic as is B. bassiana, and that they show potential as biological control agents for insects.
Fig. 4.

Entomopathogenicity assay in silkworm larvae. One milliliter of a conidial suspension (105/mL) of each fungal isolate was spread on potato dextrose agar or synthetic nutrient agar, and the plates were incubated for 4~10 days at 25℃. Fifth-instar silkworm larvae were allowed to walk on the plates for 12 hr (upper) and then moved to fresh Petri dishes. The photographs (lower) were taken 7 days after relocation. S. lanosoniveu, Simplicillium lanosoniveu; B. bassiana, Beauveria bassiana; F. graminearum, Fusarium graminearum.
In this study, we reported for the first time the entomopathogenicity of S. lanosoniveum against silkworms. Interestingly, the S. lanosoniveum isolates were obtained from fruiting bodies generated on a pine cone. Although the teleomorph of S. lanosoniveum was known (Torrubiella sp.), no studies had reported fruiting body formation of S. lanosoniveum in nature. Unlike previously reported isolates from other geographical regions, the S. lanosoniveum strains isolated in Korea did not show antifungal activity. However, the isolates were as strongly entomopathogenic as B. bassiana, indicating their potential as a biological control agent against insects.
ACKNOWLEDGEMENTS
This work was supported by the Dong-A University research fund.
References
- 1.Chen RS, Huang CC, Li JC, Tsay JG. First report of Simplicillium lanosoniveum causing brown spot on Salvinia auriculata and S. molesta in Taiwan. Plant Dis. 2008;92:1589. doi: 10.1094/PDIS-92-11-1589C. [DOI] [PubMed] [Google Scholar]
- 2.Liu F, Cai L. Morphological and molecular characterization of a novel species of Simplicillium from China. Cryptogam Mycol. 2012;33:137–144. [Google Scholar]
- 3.Nonaka K, Kaifuchi S, Ōmura S, Masuma R. Five new Simplicillium species (Cordycipitaceae) from soils in Tokyo, Japan. Mycoscience. 2013;54:42–53. [Google Scholar]
- 4.Ward NA, Schneider RW, Aime MC. Colonization of soybean rust sori by Simplicillium lanosoniveum. Fungal Ecol. 2011;4:303–308. [Google Scholar]
- 5.Ward NA, Robertson CL, Chanda AK, Schneider RW. Effects of Simplicillium lanosoniveum on Phakopsora pachyrhizi, the soybean rust pathogen, and its use as a biological control agent. Phytopathology. 2012;102:749–760. doi: 10.1094/PHYTO-01-11-0031. [DOI] [PubMed] [Google Scholar]
- 6.Zhao D, Liu B, Li LY, Zhu XF, Wang YY, Wang JQ, Duan YX, Chen LJ. Simplicillium chinense: a biological control agent against plant parasitic nematodes. Biocontrol Sci Technol. 2013;23:980–986. [Google Scholar]
- 7.Sung GH, Hywel-Jones NL, Sung JM, Luangsa-ard JJ, Shrestha B, Spatafora JW. Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 2007;57:5–59. doi: 10.3114/sim.2007.57.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kouvelis VN, Sialakouma A, Typas MA. Mitochondrial gene sequences alone or combined with ITS region sequences provide firm molecular criteria for the classification of Lecanicillium species. Mycol Res. 2008;112(Pt 7):829–844. doi: 10.1016/j.mycres.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes EK, Bittencourt VR. Entomopathogenic fungi against South America tick species. Exp Appl Acarol. 2008;46:71–93. doi: 10.1007/s10493-008-9161-y. [DOI] [PubMed] [Google Scholar]
- 10.Baiswar P, Ngachan SV, Rymbai H, Chandra S. Simplicillium lanosoniveum, a hyperparasite on Aecidium elaeagni-latifoliae in India. Australas Plant Dis Notes. 2014;9:144. [Google Scholar]
- 11.Zare R, Gams W. A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia. 2001;73:1–50. [Google Scholar]
- 12.Gams W. A contribution to the knowledge of nematophagous species of Verticillium. Neth J Plant Pathol. 1988;94:123–148. [Google Scholar]
- 13.Le Dang Q, Shin TS, Park MS, Choi YH, Choi GJ, Jang KS, Kim IS, Kim JC. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J Agric Food Chem. 2014;62:3363–3370. doi: 10.1021/jf500361e. [DOI] [PubMed] [Google Scholar]
- 14.Leslie JF, Summerell BA. The Fusarium laboratory manual. Ames: Blackwell Publishing; 2006. [Google Scholar]
- 15.Kim JE, Lee HJ, Lee J, Kim KW, Yun SH, Shim WB, Lee YW. Gibberella zeae chitin synthase genes, GzCHS5 and GzCHS7, are required for hyphal growth, perithecia formation, and pathogenicity. Curr Genet. 2009;55:449–459. doi: 10.1007/s00294-009-0258-6. [DOI] [PubMed] [Google Scholar]
- 16.White TJ, Bruns T, Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. San Diego: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 17.Hong IP, Kang PD, Kim KY, Nam SH, Lee MY, Choi YS, Kim NS, Kim HK, Lee KG, Humber RA. Fruit body formation on silkworm by Cordyceps militaris. Mycobiology. 2010;38:128–132. doi: 10.4489/MYCO.2010.38.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimazu M. A novel technique to inoculated conidia of entomopathogenic fungi and its application for investigation of susceptibility of the Japanese pine sawyer, Monochamus alternatus, to Beauveria bassiana. Appl Entomol Zool. 2004;39:485–490. [Google Scholar]