Abstract
In this study, we examined arbuscular mycorrhizal fungal (AMF) community structure colonizing field-cultivated ginseng roots according of different ages, such as 1- to 5-year-old plant, collected from Geumsan-gun, Korea. A total of seven AMF species namely, Funnelliformis caledonium, F. moseae, Gigaspora margarita, Paraglomus laccatum, P. occultum, Rhizophagus irregularis, and Scutellospora heterogama were identified from the roots using cloning, PCR-restriction fragment length polymorphism and sequence analysis of the large subunit region in rDNA. AMF species diversity in the ginseng roots decreased with the increase in root age because of the decreased species evenness. In addition, the community structures of AMF in the roots became more uniform. These results suggest that the age of ginseng affects mycorrhizal colonization and its community structure.
Keywords: AMF, Korean ginseng, LSU, Species diversity
Arubuscular mycorrhizal fungi (AMF) belong to phylum Glomeromycota and symbiosis with the roots of most terrestrial plants [1]. AMF improve the nutrient uptake efficiency of the host plant and also enhance drought resistance, improving resistance to leaf herbivory by insects, increasing heavy metal and salt tolerance, and increasing soil pathogen resistance [2, 3, 4, 5]. Thus, we thought AMF play important roles in plant physiology, plant health and nutrient cycling of the ecosystem with basis of commensalism [6].
Korean ginseng (Panax ginseng C. A. Mey.) is a slow growing perennial herb with thick roots and palmate leaves. The herb belongs to the genus Panax of family Araliaceae. Ginseng roots have been used as a medicine for thousands of years in Asian countries, especially in Korea, China, and Japan. Medicinal effects of ginseng are attributed to ginsenosides found mainly in their roots [7]. The biochemical properties of the ginsenosides and their abundance are influenced by the genetic composition of ginseng as well as by the environmental conditions, including chemical composition of the soil, climate and interaction with other organisms including soil microorganisms [8].
AMF can increase the survival of American ginseng, Panax quinquefolius and inoculation with AMF promote growth and increases root ginsenoside content [9]. Each AMF species affects plant growth and nutrient uptake differently [10] as has been described previously for different species of AMF that colonize ginseng roots [11]. Thus, understanding AMF diversity in ecosystems is an important factor for developing sustainable agriculture.
There are previous reports on the detection of AMF in the rhizosphere and roots of ginseng [12, 13]. Recently, Kil et al. [14] reported that AMF spore community structures in the soil of field-cultivated ginseng changed and AMF species diversity decreased with the increase in the age of ginseng roots. However, it is not clear whether AMF species found in the soils colonized the ginseng roots. Previous studies showed that the positive relationship between AMF spore communities in the soil and active AMF colonizing within roots was low [15, 16] and there is no reports on AMF community in the ginseng roots with different ages. In this study, we examined community compositions of AMF colonizing the interior of the ginseng roots collected from Geumsan-gun, Korea and investigated the relationship of AMF colonization rate and community structure according to the ginseng root age.
Root samples and staining
Field-cultivated ginseng roots were collected 20 different plots growing ginsengs from 1 to 5 year-old plants in Geumsan-gun, Korea (N 36°03'~05', E 127°32'~33'), with 4 replicate plots for each age. A root sample was used for each plot in this study, making a total of 20 samples. To examine AMF colonization rates, ginseng roots were washed, and stained with trypan blue [15]. The roots were observed under the microscope (AXIO Imager; Carl Zeiss, Jena, Germany) and root colonization rates were measured by the previously described magnified intersects technique [16].
Cloning, restriction fragment length polymorphism (RFLP), and sequencing
DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Venlo, Netherlands) from 20 field-cultivated ginseng root samples. The nested PCR was performed to amplify the 28S rDNA using primers LR1 and FLR2 for first PCR, and LR1 and FLR4 for second PCR as previously described [17, 18, 19]. The PCR product was purified by using the QIAquick Gel Extraction kit (Qiagen) and cloned using the pGEM-T Easy Vector System following the manufacturer's instructions (Promega Co., Madison, WI, USA). Typically, twenty clones from each root sample were selected randomly for RFLP in order to group for sequencing. Restriction enzymes HinfI and Hsp92II (both from Promega Co.) were used for PCR-RFLP analysis. The nucleotide sequence of one clone from each RFLP type was determined using an automated sequencer (SolGent Co., Daejeon, Korea), and submitted to the GenBank at NCBI (accession Nos. JX975296~JX975326).
Data analysis
Using MEGA 5, rDNA sequences were aligned with other Glomeromycota sequences as reference sequences from GenBank to test the topological appropriation. From this analysis, a phylogenetic tree was constructed by the neighbor-joining method, with a bootstrap value of 1,000 based on Tamura-Nei model using MEGA 5 [20]; Geosiphon pyriformis was used as an outgroup.
Statistical analysis
Relative abundances of each fungal phylotypes were estimated based on clone counts for each RFLP type. Shannon diversity index were calculated for AMF community analysis [21]. Univariate analysis of variance (ANOVA) was used to evaluate the fixed effect of root ages on percent mycorrhizal root colonization rates and species diversity of AMF communities, and principle component analysis was performed to compare the compositions of AMF communities using a statistical package SPSS-WIN ver. 12 (SPSS Inc., Chicago, IL, USA).
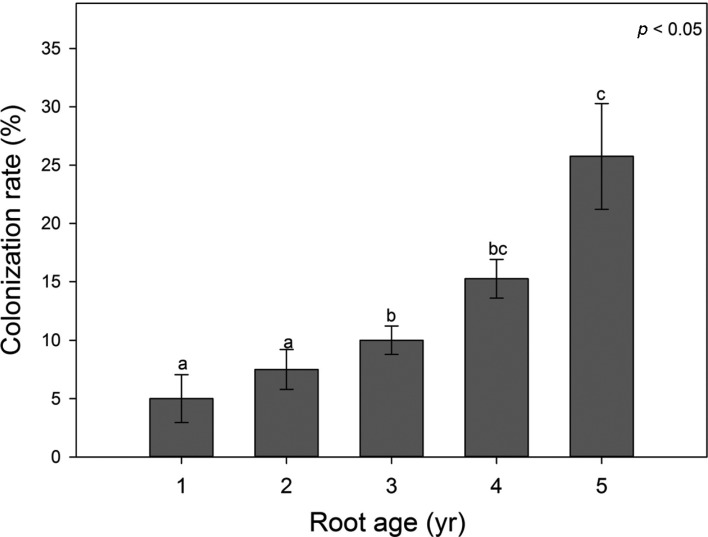
Mycorrhizal colonization rates significantly increased with the age of the root (p < 0.05) (Fig. 1). The mean colonization rate increased gradually from 5% in the 1-year-old ginseng roots to over 25% in the 5-year-old roots. Root colonization rate is influenced by numerous biotic or abiotic factors in the field. Moreover, the overuse of soil fertilizer containing phosphorus and limited light availability has been shown to reduce the rate of colonization by AMF [22, 23]. In addition, root growth at a faster rate than that of the AM fungal hyphae, can also decrease the colonization rate [24]. Even if some of the possible causes described above or other complex environmental factors result in low AMF colonization rates, this neither implies a low AMFdependence of the host plant nor is it a reflection of the effects of AMF on the host plant [1]. Here, we found that the AMF colonization rate in ginseng roots increased while the species diversity simultaneously decreased with age. Therefore, we found no positive relationship between the colonization rate and species diversity of AMF.
Fig. 1.
Mycorrhizal root colonization rates of Panax ginseng with cultivation periods. Means and standard errors are shown. Different letters on each bar indicate significantly different mean at p < 0.05 according to least significant difference of one-way ANOVA.
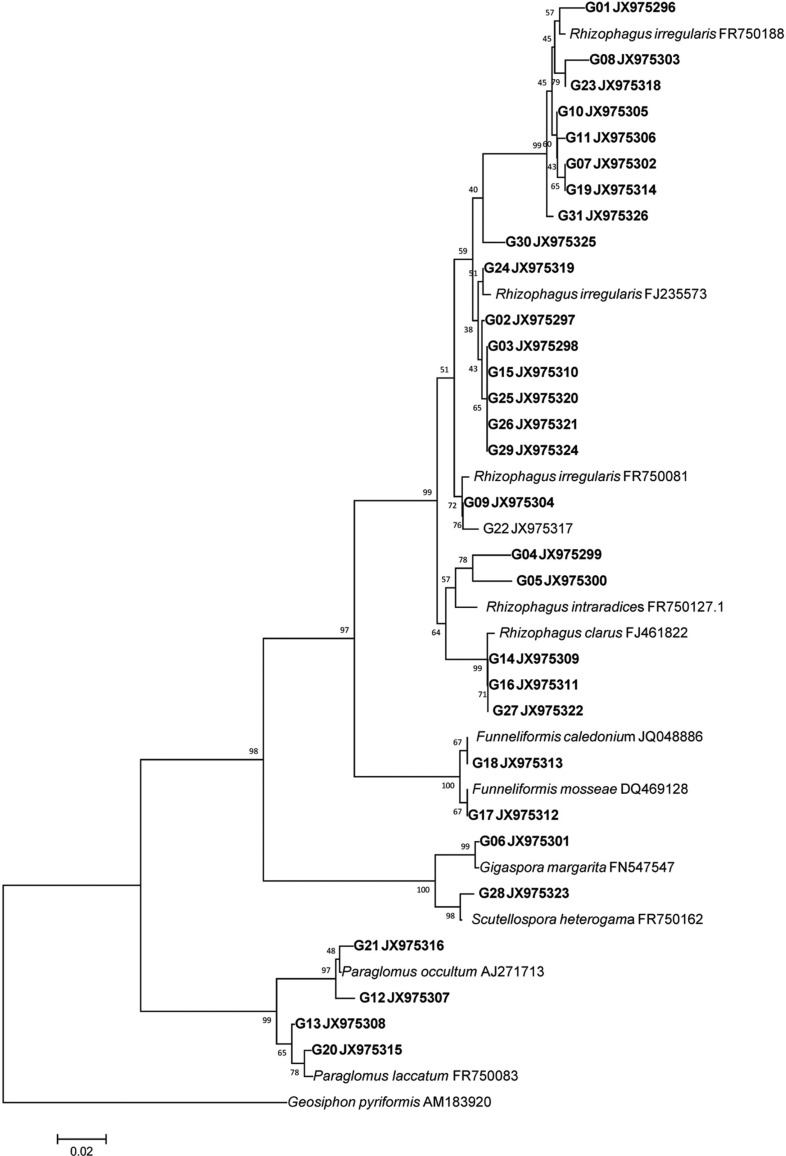
In total, 31 RFLP types were obtained from the 200 clones and one clone representing each RFLP type was sequenced from each group. These sequences were used to construct a phylogenetic tree based on the large subunit (LSU) regions of rDNA (Fig. 2). Based on the phylogeny, all AMF clones were divided into 7 AMF clades: Funnelliformis caledonium, F. mosseae, Gigaspora margarita, Paraglomus laccatum, P. occultum, Rhizophagus irregularis, and Scutellospora heterogama (Table 1, Fig. 2).
Fig. 2.
Neighbor joining phylogenetic tree of arubuscular mycorrhizal fungi colonizing on field-cultivated Panax ginseng. Large subunit of rDNA region was sequenced. Geosiphon pyriformis was used as an outgroup and bootstrap values are shown at the branches (1,000 replicates). Bold letters indicates clone sequences from this study.
Table 1.
Relative abundance (%) of phylotypes of arbuscular mycorrhizal fungi colonizing in the roots of Panax ginseng with different ages
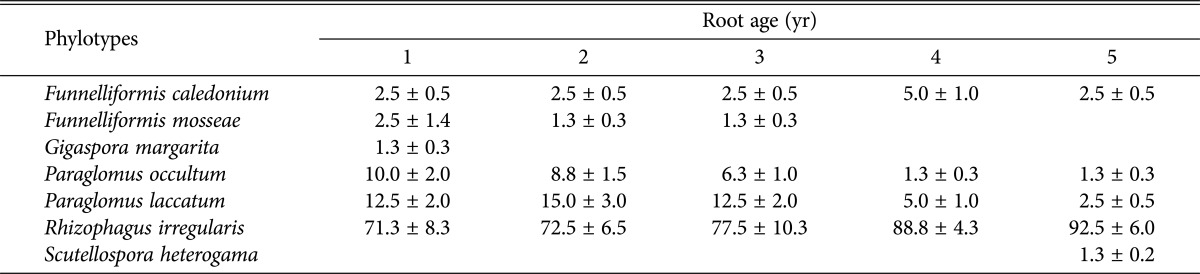
Values are presented as mean ± standard errors.
AMF community composition significantly changed with age of the roots (Table 1). R. irregularis was the most dominant species in ginseng roots of all ages used in this study. On the other hand, G. margarita was observed only in the 1-year-old roots. The relative abundance of R. irregularis increased from 71.3% in 1-year-old ginseng roots to 92.5% in 5-year-old roots. However, abundance of other species, including F. mosseae, P. laccatum decreased despite widespread presence in cultivated soil; F. mosseae, which seems to have adapted successfully to agricultural soil conditions did not colonize substantially with the roots of P. ginseng in this study [25, 26, 27]. The field management for ginseng cultivation differs from other crops. It is therefore likely that the AM fungal community composition changed as result of this difference in the agricultural system. In our study, S. heterogama was observed to colonize the roots of only 5- year-old ginseng at a relatively lower rate (1.3 ± 0.2%). Overall, the mean colonization rate increased with an increase in the age of ginseng roots. We observed a higher rate of colonization for R. irregularis. This species has not been detected in cultivated soil and ginseng roots previously [28]. In our future work, we will study how symbiosis between R. irregularis and P. ginseng affects ginsenoside content of perennial herbaceous plants.
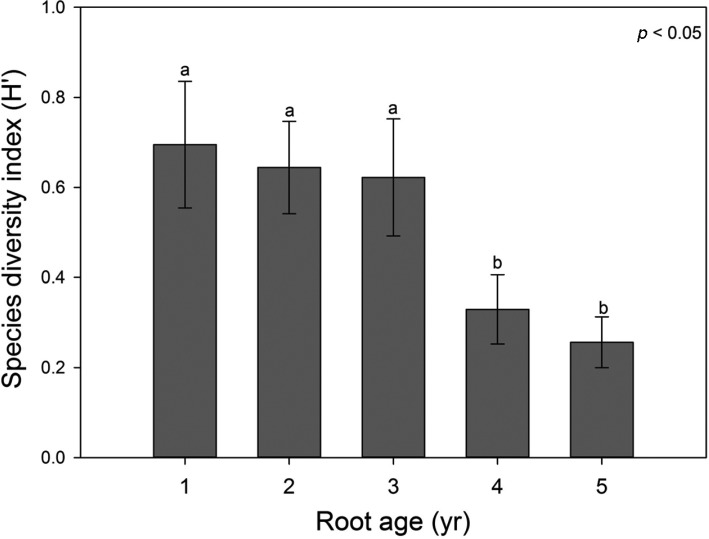
Eom et al. [13] reported two species of AMF, P. brasilianum and Glomus spurcum from the roots of 2-year-old fieldcultivated ginseng using primers AML1/AML2. Other AMF species were not observed to colonize ginseng in this study. Several other studies have reported that G. intraradices dominates the AMF community in other plant roots, while fungi from the Acaulosporaceae or Gigasporaceae family are lower in abundance. Although several studies have identified G. intraradices or other species that belong to the Acaulosporaceae family, these species were not detected in our study. However, species diversity of AMF colonizing the roots significantly decreased with the root age (Fig. 3). The diversity of AMF in 1- to 3-year-old ginseng roots was higher than in 4- to 5-year-old ginseng roots. This was reflected primarily through the decreased species evenness because the richness of AMF was not significantly affected by the age of the ginseng roots. Our results are consistent with those of previous studies because the observed increase in the colonization by R. irregularis with the age of the roots and the absence of other species from more mature roots.
Fig. 3.
Shannon's diversity index of arbuscular mycorrhizal fungi colonizing different ages of ginseng roots. Means and standard error are shown. Different letter on each bar indicates significantly different mean at p < 0.05 according to least significant difference test of one-way ANOVA.
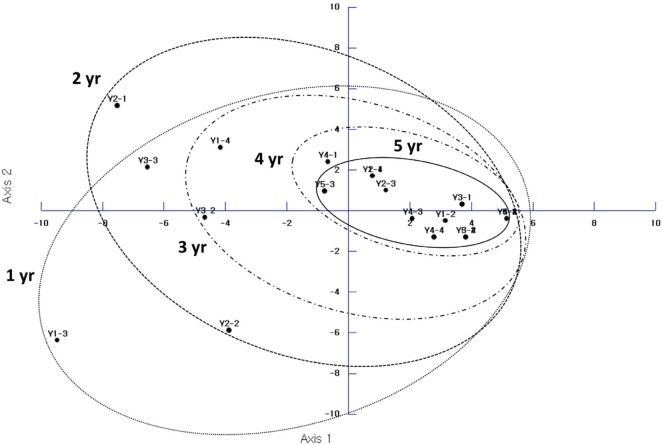
PCR analysis used to determine the influence of the age of ginseng roots on AMF communities and revealed that the AMF communities were able to discern among them (Fig. 4). This is similar to a previous study based on the data of AMF spores in soil from the field of cultivated ginseng [11]. In our study, AMF community composition changed with the age of ginseng roots. Based on the results of AMF spore study, the diversity of AMF species was found to be greater in the soil of field-cultivated ginseng than in the ginseng roots. However, not all the species identified from the soil were isolated from the roots. Six AMF species: Glomus sp., G. intraradices, G. etunicatum, Acaulospora longula, Archaeospora trappei, P. brasilianum were isolated only from the soil of cultivated ginseng and four AMF species: F. caledonium, F. mosseae, P. occultum, S. heterogama were observed both in the soil and the roots. Of these, P. brasilianum has also been detected in another study on ginseng and AMF symbiosis [13]. Hijri et al. [29] observed that there was a good agreement between the spores isolated from soil and the phylotypes amplified from roots in a survey of five agricultural fields. However, others have reported a poor species match between AMF spores in the soil and AMF in the roots of grassland plants [30].
Fig. 4.
Principle component analysis ordination of arbuscular mycorrhizal fungal communities colonizing different ages of field-cultivated Panax ginseng.
In this study, root colonization rates of AMF increased with the age of ginseng roots. However, with an increase in the root age, species diversity and evenness of AMF colonizing the roots decreased, whereas the dominance of R. irregularis increased. The AMF community colonizing ginseng roots became more uniform with an increase in root age indicating that changes in the preferences of AMF for ginseng roots were age-dependent.
References
- 1.Smith SE, Read D. Mycorrhizal symbiosis. 3rd ed. San Diego: Academic Press; 2008. [Google Scholar]
- 2.Auge RM, Duan X, Ebel RC, Stodola AJ. Nonhydraulic signalling of soil drying in mycorrhizal maize. Planta. 1994;193:74–82. [Google Scholar]
- 3.Gange AC, West HM. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 1994;128:79–87. doi: 10.1111/j.1469-8137.1994.tb03989.x. [DOI] [PubMed] [Google Scholar]
- 4.Kurle JE, Pfleger FL. Arbuscular mycorrhizal fungus spore populations respond to conversions between low-input and conventional management practices in a corn-soybean rotation. Agron J. 1994;86:467–475. [Google Scholar]
- 5.Leyval C, Turnau K, Haselwandter K. Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects. Mycorrhiza. 1997;7:139–153. [Google Scholar]
- 6.Gosling P, Hodge A, Goodlass G, Bending GD. Arbuscular mycorrhizal fungi and organic farming. Agric Ecosyst Environ. 2006;113:17–35. [Google Scholar]
- 7.Kim D. Reviews in ginseng research (I) Seoul: The Korea Society of Ginseng; 2007. [Google Scholar]
- 8.Lee MK, Park H, Lee CH. Effect of growth conditions on saponin content and ginsenoside pattern of Panax ginseng. Korea-Japan Panax ginseng Sym. 1987;11:89–107. [Google Scholar]
- 9.Li TS. Effect of vesicular-arbuscular mycorrhizae on the growth of American ginseng. Korean J Ginseng Sci. 1995;19:73–76. [Google Scholar]
- 10.Klironomos JN. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology. 2003;84:2292–2301. [Google Scholar]
- 11.Kil YJ, Eo JK, Eom AH. Effect of arbuscular mycorrhizal fungi on growth of Korean ginseng (Panax ginseng C. A. Mey.) seedlings. Kor J Mycol. 2013;41:81–84. [Google Scholar]
- 12.Cho EJ, Lee DJ, Wee CD, Kim HL, Cheong YH, Cho JS, Sohn BK. Effects of AMF inoculation on growth of Panax ginseng C.A. Meyer seedlings and on soil structures in mycorrhizosphere. Sci Hortic. 2009;122:633–637. [Google Scholar]
- 13.Eom AH, Eo JK, Kim DH, Jeong HS. Identification of arbuscular mycorrhizal fungi colonizaing Panax ginseng using 18S rDNA sequence. J Korean Soc Appl Biol Chem. 2004;47:182–186. [Google Scholar]
- 14.Kil YJ, Eo JK, Eom AH. Diversities of arbuscular mycorrhizal fungi in cultivated field soils of Korean ginseng. Kor J Mycol. 2012;40:1–6. [Google Scholar]
- 15.Koske RE, Gemma JN. A modified procedure for staining roots to detect VA mycorrhizas. Mycol Res. 1989;92:486–488. [Google Scholar]
- 16.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Tuinen D, Jacquot E, Zhao B, Gollotte A, Gianinazzi-Pearson V. Characterization of root colonization profiles by a microcosm community of arbuscular mycorrhizal fungi using 25S rDNA-targeted nested PCR. Mol Ecol. 1998;7:879–887. doi: 10.1046/j.1365-294x.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 18.Trouvelot S, Van Tuinen D, Hijri M, Gianinazzi-Pearson V. Visualization of ribosomal DNA loci in spore interphasic nuclei of glomalean fungi by fluorescence in situ hybridization. Mycorrhiza. 1999;8:203–206. [Google Scholar]
- 19.Gollotte A, Van Tuinen D, Atkinson D. Diversity of arbuscular mycorrhizal fungi colonising roots of the grass species Agrostis capillaris and Lolium perenne in a field experiment. Mycorrhiza. 2004;14:111–117. doi: 10.1007/s00572-003-0244-7. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magurran AE. Ecological diversity and its measurement. Princeton: Princeton University Press; 1988. [Google Scholar]
- 22.Xie X, Weng B, Cai B, Dong Y, Yan C. Effects of arbuscular mycorrhizal inoculation and phosphorus supply on the growth and nutrient uptake of Kandelia obovata (Sheue, Liu & Yong) seedlings in autoclaved soil. Appl Soil Ecol. 2014;75:162–171. [Google Scholar]
- 23.Füzy A, Bothe H, Molnár E, Biró B. Mycorrhizal symbiosis effects on growth of chalk false-brome (Brachypodium pinnatum) are dependent on the environmental light regime. J Plant Physiol. 2014;171:1–6. doi: 10.1016/j.jplph.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Smith SE, Walker NA. A quantitative study of mycorrhizal infection in Trifolium: separate determination of the rates of infection and of mycelial growth. New Phytol. 1981;89:225–240. [Google Scholar]
- 25.Verbruggen E, van der Heijden MG, Rillig MC, Kiers ET. Mycorrhizal fungal establishment in agricultural soils: factors determining inoculation success. New Phytol. 2013;197:1104–1109. doi: 10.1111/j.1469-8137.2012.04348.x. [DOI] [PubMed] [Google Scholar]
- 26.Rosendahl S, McGee P, Morton JB. Lack of global population genetic differentiation in the arbuscular mycorrhizal fungus Glomus mosseae suggests a recent range expansion which may have coincided with the spread of agriculture. Mol Ecol. 2009;18:4316–4329. doi: 10.1111/j.1365-294X.2009.04359.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SW, Lee EH, Eom AH. Effects of organic farming on communities of arbuscular mycorrhizal fungi. Mycobiology. 2008;36:19–23. doi: 10.4489/MYCO.2008.36.1.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eo JK, Eom AH. The effect of benomyl treatments on ginsenosides and arbuscular mycorrhizal symbiosis in roots of Panax ginseng. J Ginseng Res. 2009;33:256–259. [Google Scholar]
- 29.Hijri I, Sýkorová Z, Oehl F, Ineichen K, Mäder P, Wiemken A, Redecker D. Communities of arbuscular mycorrhizal fungi in arable soils are not necessarily low in diversity. Mol Ecol. 2006;15:2277–2289. doi: 10.1111/j.1365-294X.2006.02921.x. [DOI] [PubMed] [Google Scholar]
- 30.Hempel S, Renker C, Buscot F. Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ Microbiol. 2007;9:1930–1938. doi: 10.1111/j.1462-2920.2007.01309.x. [DOI] [PubMed] [Google Scholar]