Abstract
The pathophysiology of type 1 diabetes mellitus (T1DM) is largely related to an innate defect in the immune system culminating in a loss of self-tolerance and destruction of the insulin-producing β-cells. Currently, there is no definitive cure for T1DM. Insulin injection does not mimic the precise regulation of β-cells on glucose homeostasis, leading long term to the development of complications. Stem cell therapy is a promising approach and specifically mesenchymal stem cells (MSCs) offer a promising possibility that deserves to be explored further. MSCs are multipotent, nonhematopoietic progenitors. They have been explored as an treatment option in tissue regeneration as well as potential of in vitro transdifferentiation into insulin-secreting cells. Thus, the major therapeutic goals for T1DM have been achieved in this way. The regenerative capabilities of MSCs have been a driving force to initiate studies testing their therapeutic effectiveness; their immunomodulatory properties have been equally exciting; which would appear capable of disabling immune dysregulation that leads to β-cell destruction in T1DM. Furthermore, MSCs can be cultured under specially defined conditions, their transdifferentiation can be directed toward the β-cell phenotype, and the formation of insulin-producing cells (IPCs) can be targeted. To date, the role of MSCs-derived IPC in T1DM–a unique approach with some positive findings–have been unexplored, but it is still in its very early phase. In this study, a new approach of MSCs-derived IPCs, as a potential therapeutic benefit for T1DM in experimental animal models as well as in humans has been summarized.
Keywords: Insulin-producing cells, mesenchymal stem cells, transdifferentiation, type 1 diabetes mellitus
INTRODUCTION
Type 1 diabetes mellitus (T1DM) is a T-cell mediated, organ-specific autoimmune disorder leading to β-cell destruction and reduced insulin production and has no definitive cure currently. Standard treatment strategies for T1DM are based on different insulin replacements. However, as exogenous insulin cannot mimic exactly the physiology of insulin secretion, good metabolic control is difficult to reach and frequently associated with severe hypoglycemic episodes.[1] At present, pancreas transplantation or islet transplantation as a treatment of T1DM is here to stay until something better comes along. The first efforts focused on whole pancreas transplants, which have been performed now for over 50 years. Although they have been shown to lead to insulin independence for several years, pancreas transplants to treat T1DM are not widespread for a number of reasons. Being a major surgery, the accompanying risk of mortality is 1-3% and the complications that ensue include cardiac death and systemic infections. In addition, to prevent the body from rejecting the transplanted pancreas, recipients must take powerful immunosupressions for the rest of their lives, leaving them susceptible to infections and a range of other diseases. Many feel that the immunosupressions therapy could be a greater health threat than the diabetes itself.[2]
In an effort to more tightly control blood glucose levels, researchers began to explore the possibilities of using cell-based therapies that would replace lost β-cells. Within recent years, stem cell research has become a very important part of the scientific understanding of T1DM. Research has demonstrated that stem cells can be grown in the lab and could lead to a better availability of β-cells for future research purposes to treat T1DM. Many types of stem cells are candidates for the treatment of T1DM.[3] The ideal stem cells would be the one with strong immunomodulatory and regenerative properties which would have to deal with active process of autoimmunity that probably target the newly formed insulin-producing cells (IPCs). Mesenchymal stem cells (MSCs) have been a driving force to initiate studies testing their therapeutic effectiveness with their regenerative capabilities and their immunomodulatory properties. Generation of IPCs from MSCs represents an attractive alternative.[2] Although the sources of stem cells used in the generation of IPC have been previously reviewed, the literature describing MSCs alone as a source for in vitro transdifferentiation of IPC, further clinical use of IPC as a therapeutic agent in experimental animal models and in humans, and its outcome are limited, which encourage the author to carry out this review.
REVIEW
Mesenchymal stem cells therapeutic potential: In vitro and in vivo evidence
Friedenstein et al., in a ground-breaking study, isolated clonogenic fibroblast precursor cells from whole bone marrow (BM) and showed that they were capable of forming bone- and cartilage-like colonies.[4] Since then BM-derived stem cells are still the most frequently investigated cell type and often designated as the gold standard. Till date isolation of multipotent MSCs from different sources has been reported. There is no universally agreed upon set or a specific singular marker to identify these cells. As a result, a battery of negative and positive markers is generally used to phenotypically characterize these cells. As MSCs are a nonhematopoietic cell lineage, they generally lack specific cell surface markers of HSC and do not express hematopoietic markers such as CD34, CD14 and CD45, CD11a/LFA-1, erythrocytes (glycophorin A), and platelet and endothelial cell adhesion molecules (CD31), but they express several other cell surface antigens, such as CD73 (SH3/4), CD90, CD105 (SH2), CD146, and CD200. They also express variable levels of CD44, stromal antigen 1, and a group of other adhesion molecules and receptors including CD166 (vascular cell adhesion molecule), CD54/CD102 (intracellular adhesion molecule), and CD49.[5] BM aspirate is considered to be the most accessible and enriched source of MSCs. Bone marrow-derived MSCs (BM-MSCs) represent a rare population of cells that make up only 0.001-0.01% of total nucleated cells and are 10-fold less abundant than hematopoietic stem cells (HSCs), but they can be readily grown and expanded in culture.[6] However, MSCs derived from adipose tissue (AD-MSCs),[7,8] peripheral blood,[9,10] and umbilical cord blood (UCB)[11,12,13] have also shown promising potential for proliferation and differentiation into different cell types including IPCs. Moreover, protein transduction technology also offers a novel approach for generating IPCs from stem cells including MSCs.[14,15]
Why MSC?
The frequency of T1DM has been steadily increasing worldwide and T1DM prevention studies using immunosuppressants, self-antigens, and dietary interventions have demonstrated largely disappointing results.[1] In T1DM, selective and irreversible destruction of the insulin-secreting β-cells in the pancreatic islets of Langerhans occurs by an autoimmune attack. While insulin replacement remains the cornerstone treatment for T1DM, the transplantation of pancreatic islets of Langerhans provides a cure for this disorder and yet, islet transplantation is limited by the lack of donor pancreas. The challenge involves the development of safe and effective means affording the prevention or reversal of T1DM. MSCs have the capacity to differentiate into multiple mesodermal and nonmesodermal cell lineages including IPCs in vitro.[2] MSCs that have been precommitted to one mesenchyme cell lineage can differentiate into other cell types in response to inductive extracellular cues by process known as transdifferentiation. These precommitted cells proliferate and are able to dedifferentiate into a primitive stem cell stage through genome reprogramming.[3] On the one hand, MSCs have the potential to transdifferentiate into IPCs by genetic modification and/or defined culture conditions in vitro. On the other hand, MSCs are able to serve as a cellular vehicle for the expression of human insulin gene and has a promising role as therapeutic agents in the treatment, the complications of DM such as cardiac function and in treatment of diabetic cardiomyopathy, nephropathy, diabetic polyneuropathy, and wounds in diabetic patients.[16,17,18,19,20,21,22] MSCs have generated marked interest and attention for their capacity to elicit tissue regeneration[6,23,24,25] and one of the most remarkable and least understood findings is the ability of MSC to migrate to sites of tissue injury.[26,27,28,29] Besides, MSCs also possess immunosuppressive effects by modulating the immune function of the major cell populations involved in alloantigen recognition and elimination[30,31,32] and they have also shown promising results in the treatment of other autoimmune disorders (e.g., experimental autoimmune encephalomyelitis and rheumatoid arthritis).[33,34] Adult human MSCs express intermediate levels of major histocompatibility complex (MHC) class I molecules on their cell surface, but not MHC class II, properties that allow their transplantation across MHC barriers. Because of the lack in expression of MHC class II and most of the classical costimulatory molecules on MSCs, these cells have historically been regarded as hypoimmunogenic cells. The immunomodulatory properties of MSCs were initially reported in T-cell proliferation assays using one of a variety of stimuli including mitogens, CD3/CD28, and alloantigen settings where the ability of MSCs to suppress T-cell proliferation was readily determined. Such suppression occurs irrespective of a donor source, including settings in which one uses “third-party” MSCs and suppress proliferation of both CD4+ and CD8+ lymphocytes; and are able to abrogate the response of memory T-cells and naïve T cells to their antigen.[35] MSCs could regulate diabetes through a direct effect by presenting differential levels of negative costimulatory molecules and secreting regulatory cytokines such as transforming growth factor-β and IL-10 that control regulatory T-cells/auto-reactive T-cells. It is also possible that MSCs could correct the dysregulation observed at the level of B-cells and natural killer cells as well.[36,37,38,39] MSCs also exert antiinflammatory effects that could be important in maintaining peripheral tolerance.[40,41,42] It has been shown that AD-MSCs are capable of producing anti-inflammatory cytokines and angiogenic factors, which could potentially improve the diabetes-associated inflammatory and ischemic conditions.[2] According to one hypothesis, MSC transplantation into diabetic animals can prevent apoptosis of injured pancreatic β-cells and enhance regeneration of endogenous progenitor cells through paracrine actions such as angiogenic, cytoprotective, anti-inflammatory, mitogenic, and antiapoptotic effects.[43] MSCs have been also shown to provide cytokine and growth factor support for expansion of HSCs and human embryonic stem cells (ESCs), which in turn during cotransplantation of IPCs and HSCs promote IPC engraftment and survival. Here HSCs act as “feeder” cells for the IPCs, supporting its protection, tissue revascularization, and immune acceptance.[25,44,45,46]
In vitro directed transdifferentiation of mscs into IPCs
Assady et al. (2001) reported that IPCs can be generated from spontaneous differentiation using ESCs. Although the number of IPCs and the insulin content in these cells was low, it was the first proof-of-principle experiment showing that stem cells-ESCs were a potential source for generating β-like cells.[47]
MSCs have the advantage over ESCs in that they usually do not form teratomas and are free from the ethical issues of ESCs. MSCs can be easily obtained and are easily expanded and cultured in the laboratory. Different types of stem cells require different culture and induction media for transdifferentiation of IPCs to take place. The capacity of MSCs to undergo functional transdifferentiation has been questioned over the years. Nonetheless, recent studies support that gene-therapy or factor-based transdifferentiation of MSCs are two distinctive pathways to be considered as means of obtaining functional β-cells. Gene-therapy refers to the in vivo or in vitro transfer of a foreign gene into MSCs, allowing it to produce insulin. The foreign gene in turn activates or represses on demand the insulin gene. The factor-based approach involves exposing MSCs to a cocktail of insulin-promoting factors and cytokines over an extended period of in vitro culture, followed by transplantation of the transdifferentiated IPC into the receiving diabetic patient.
MSCs can be differentiated into IPCs by using a specific culture medium enriched with extrinsic insulin-promoting factors. The IPC transdifferentiation period varies greatly with the use of different protocols, it may last from several days to several months. Addition and withdrawal of a combination of extrinsic insulin-promoting factors in a stage-wise manner are required. Many extrinsic insulin-promoting factors, which are biologically active compounds, that have been used in endocrine pancreas differentiation have been shown to promote β-cell proliferation and differentiation and increase insulin content of IPC. A number of these factors have been commonly observed in protocols for IPC transdifferentiation. Different types of stem cells require different culture and induction media for differentiation of IPCs to take place. However, some common themes seem to appear in various induction methods. Commonly known insulin-promoting factors include epidermal growth factor, activin A, betacellulin, nicotinamide, exendin-4, hepatocyte growth factor, fibroblast growth factors, and pentagastrin. Careful use of serum and glucose in the induction media has also been indicated for successful transdifferentiation of IPCs. Signaling by these factors in MSCs allows the induction of the transcription factors, which are prerequisites for pancreas development [Table 1].
Table 1.
Extrinsic factors and their role in promoting IPC differentiation and insulin production
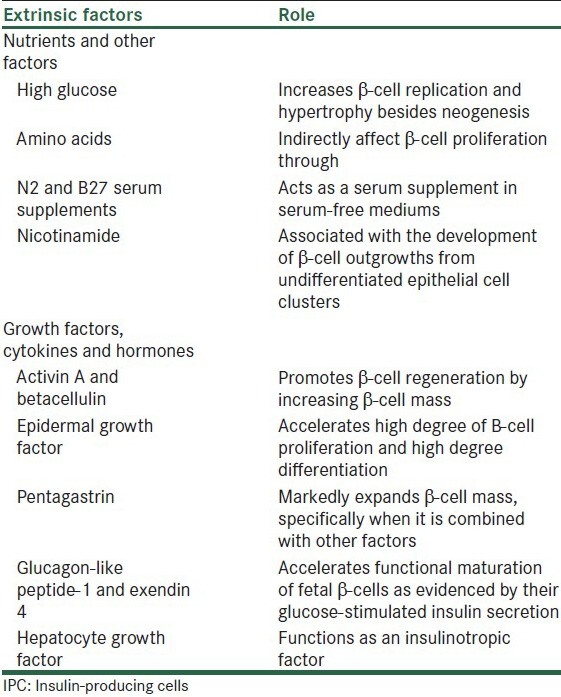
IPC identification is then based on the ability to express genes related to pancreatic development and function, such as IslI and IslII, GLUT2, glucose kinase, islet amyloid polypeptide, nestin, and Pdx 1 and Pax 6, and to synthesize C-peptide and insulin, which have been shown to play a role in the development of the pancreas and/or the differentiation of insulin-producing β-cells. Although pancreas development has been partly deciphered by identification and characterization of many transcription factors little is known about their function and molecular mechanism of action. Most of these transcription factors are sequentially and transiently expressed during pancreas development. However, for successful transdifferentiation of MSCs into IPCs the in vitro culture procedure should follow at least the major part of transcriptional program.
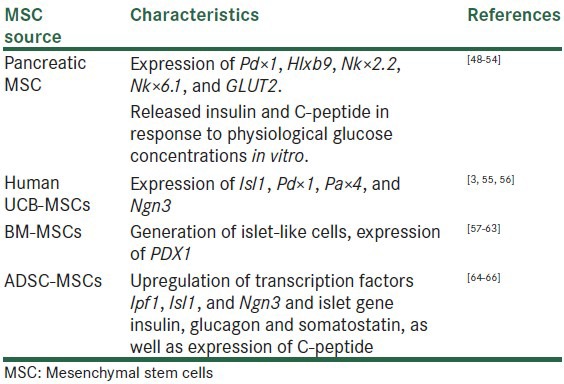
There are evidence to suggest that pancreatic stem or progenitor cells derived MSCs.[48,49,50,51,52,53,54] UCB derived MSCs,[13,55,56] BM-MSCs,[57,58,59,60,61,62,63] and AD-MSC[52,64,65,66] can be isolated from rodents and/or human and extensively expanded in vitro, where they differentiate to form new IPCs and exhibited with the characteristics genes and a panel of markers considered essential for differentiation into pancreatic endocrine tissue (Isl1, Pdx 1, Pax 4, and Ngn3, Pax 6) [Table 2].
Table 2.
In vitro approaches to generate IPCs from different MSC sources
The majority of researchers have described a multistep protocol for generation of insulin-producing islet-like clusters from MSCs derived from different sources. The glucose challenge tests revealed the production of insulin, and such production was regulated through physiological signaling pathways, i.e. in a glucose responsible manner and they believed that IPCs derived from MSCs could be potentially used for cell therapy of T1DM in human models followed by trials in experimental animal models [Figure 1].
Figure 1.
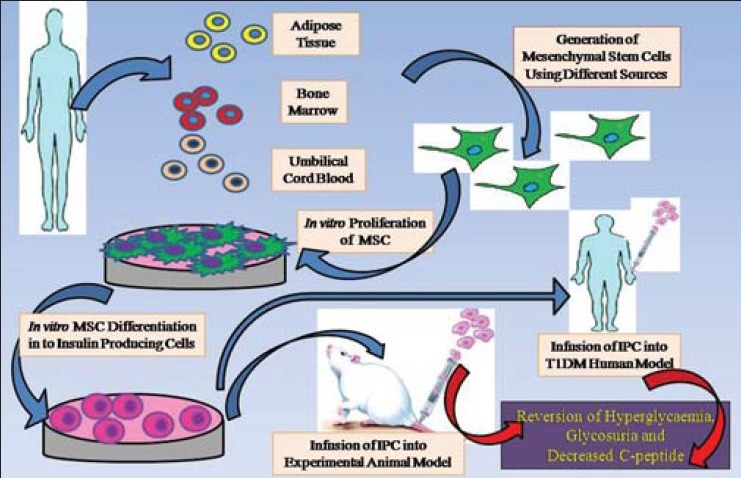
Schematic representation showing in vitro transdifferentiation of insulin-producing cells from mesenchymal stem cells; IPC in vivo infusion in an experimental animal model, T1DM human model, and therapeutic effect
Success story: MSCs derived IPCs-based therapy in autoimmune animal models of T1DM
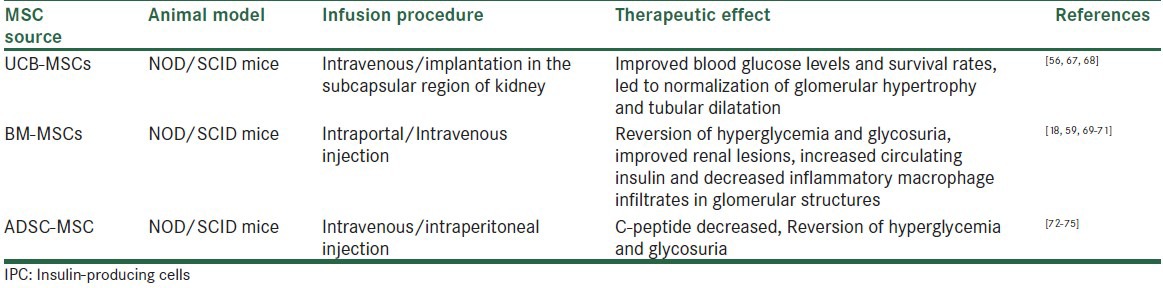
Experimental data for the therapeutic effects of MSCs-derived IPCs in animal models of T1DM are important tools for analyzing results before considering further human clinical applications. NOD/SCID mice, which are severely deficient in T and B lymphocytes, represent an invaluable diabetic model for experimental research. Use of IPCs derived from UCB-MSCs,[56,67,68] BM-MSCs alone or in association with HSCs,[18,59,69,70,71] and from AD-MSC[72,73,74,75] represent a potential source of diabetic cell replacement. AD-MSCs because of their availability, low risk for immune rejection and increased capacity for expansion with encouraging results in terms of improving glycaemia, enhanced islet regeneration, lowered blood sugar and increased circulating blood insulin levels, increased production of endogenous β-cells, reversion of glycosuria, with increased morphologically normal β-pancreatic islets, acquired functional β-cell phenotype, partially restored pancreatic function in comparison with nontransplanted diabetic mice. Histological examination of IPCs transplanted organ showed the presence of transplanted cells with formation of tissue-like structure, which stained positive for insulin Table 3.
Table 3.
Approaches to treat experimental diabetic model using IPCs generated from different MSC sources
MSCs or MSCs derived IPC-based therapy in T1DM humans
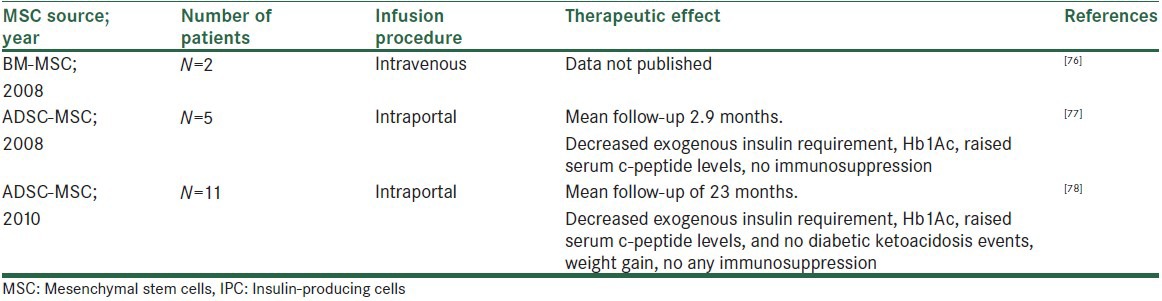
The most effective protocols till date have produced cells that express insulin and have molecular characteristics that closely resemble bonafide IPC; however, these cells are often unresponsive to glucose, which is also a most vital characteristic concern that needs to be solved before finding a definite clinical application. There are very few reports noted till now using MSCs-derived IPC as a treatment of T1DM in humans. In 2008, researchers studied the role of MSCs in humans with T1DM. The protocol includes BM biopsy under general anesthesia in the first-degree relatives for the collection of MSC. These cells were sent to a laboratory to be stimulated to proliferate for a month and were later infused into the patient through a gelatinous solution of approximately 100 mL without chemotherapy. After 1 month, the patients were given another infusion. So far, they are not sure about how many infusions will be necessary. Two patients had been included in this protocol but still results are not published.[76]
In the same year, in 2008, Trivedi et al. published results using AD-MSCs transdifferentiated IPCs in combination with HSCs to treat T1DM in five patients having disease duration of 0.6-10 years. Intraportal administration of in vitro generated IPCs and HSCs were carried out. We decided to infuse the cells in portal circulation since liver is the most tolerogenic organ.[77] The results showed 30% to 50% decreased insulin requirements with 4 to 26-fold increased serum c-peptide levels, with a mean follow-up of 2.9 months without any untoward effects and without use of any immunosuppression.[78]
In 2010, again vanikar et al. published results of insulin replacement therapy using AD-MSCs-derived IPC in combination with HSCs to treat T1DM in another 11 patients having disease duration of 1-24 years. Cells were administered intraportally. In vitro generated IPCs showed the presence of pancreatic transcriptional factors Pax 6, Isl1, and Pdx 1 (by immunoflurescence assay). Chemiluminescence assay detected secretion of glucose and C-peptide in response to glucose concentration in vitro. Over a mean follow-up of 23 months, treated patients showed a decreased mean exogenous insulin requirement, Hb1Ac, raised serum c-peptide levels, and became free of diabetic ketoacidosis events with weight gain on normal diet and physical activities without any untoward effects, without use of any immunosuppression[79] [Table 4].
Table 4.
Insulin replacement therapy using MSC-derived IPCs in humans
CONCLUSIONS AND FUTURE PERSPECTIVES
Taken altogether, these in vitro and in vivo experiments demonstrate that the beneficial effects of MSCs in T1DM may be related to both their immunosuppressant activity and subsequent protective effects on damaged tissue, and their capacity to transdifferentiate into IPCs [Figure 2].
Figure 2.

MSC transdifferentiation into IPC and its use in clinic learning points
With sporadic reports of use of MSCs-derived IPCs and their further use in clinical trials to treat T1DM, it can be concluded that (i) this study is only a first step toward using MSCs-derived IPCs as a cell-based treatment, which appears to be suitable for treatment of for T1DM, (ii) MSCs-derived IPCs are an ideal population of personal stem cells for cell replacement therapy and also MSCs could be induced to differentiate into physiologically competent functional islet-like cell aggregates, which may provide as a source of alternative islets for cell replacement therapy in T1DM. Generation of MSCs into IPCs is feasible and promising making the transplantation of IPC a promising approach for the treatment of T1DM. However, there is no standard method for IPC generation and the wide variations in induction techniques used may be a challenge to researchers. As most of these stem cells are being tested in preclinical T1DM with rare clinical use in humans, further exploration is necessary for the in vitro generation of sufficient IPCs that can produce sufficient insulin for wide clinical use. The biggest challenge for the future trials using this approach is to prevent or treat relapses and maintain complete or very good partial responses for very long time.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Schatz D, Gale EA, Atkinson MA. Why can’t we prevent type 1 diabetes? Maybe it's time to try a different combination. Diabetes Care. 2003;26:3326–8. doi: 10.2337/diacare.26.12.3326. [DOI] [PubMed] [Google Scholar]
- 2.Liu M, Han ZC. Mesenchymal stem cells: Biology and clinical potential in type 1 diabetes therapy. J Cell Mol Med. 2008;12:1155–68. doi: 10.1111/j.1582-4934.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song L, Tuan RS. Transdifferentiation potential of human mesenchymal stem cells derived from bone marrow. FASEB J. 2004;18:980–2. doi: 10.1096/fj.03-1100fje. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–4. [PubMed] [Google Scholar]
- 5.Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–5. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Fraser J, Wulur I, Alfonso Z, Hedrick M. Fat tissue: An underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–4. doi: 10.1016/j.tibtech.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Cao C, Dong Y. Study on culture and in vitro osteogenesis of blood-derived human mesenchymal stem cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2005;19:642–7. [PubMed] [Google Scholar]
- 10.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, et al. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–88. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu LL, Liu YJ, Yang SG, Zhao QJ, Wang X, Gong W, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006;91:1017–26. [PubMed] [Google Scholar]
- 12.Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 13.Pessina A, Eletti B, Croera C, Savalli N, Diodovich C, Gribaldo L. Pancreas developing markers expressed on human mononucleated umbilical cord blood cells: Biochem Biophys. Res Commun. 2004;323:315–22. doi: 10.1016/j.bbrc.2004.08.088. [DOI] [PubMed] [Google Scholar]
- 14.Vija L, Farge D, Gautier JF, Vexiau P, Dumitrache C, Bourgarit A, et al. Mesenchymal stem cells: Stem cell therapy perspectives for type 1 diabetes. Diabetes Metab. 2009;35:85–93. doi: 10.1016/j.diabet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Davani B, Ariely S, Ikonomou L, Oron Y, Gerhengon M. Human islet derived cells can cycle between epithelial clusters and mesenchymal phenotypes. J Cell Mol Med. 2009;13:2570–6. doi: 10.1111/j.1582-4934.2008.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, Li J, Luo R, Jiang J, Wang JA. Bone marrow mesenchymal stem cells induce angiogenesis and attenuate the remodeling of diabetic cardiomyopathy. Exp Clin Endocrinol Diabetes. 2008;116:104–11. doi: 10.1055/s-2007-985154. [DOI] [PubMed] [Google Scholar]
- 17.Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yañez AJ, Conget PA. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–40. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, et al. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci USA. 2006;103:17438–43. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera MB, Bussolati B, Bruno S, Fonsato V, Romanazzi GM, Camussi G. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–41. [PubMed] [Google Scholar]
- 20.Yang Z, Li K, Yan X, Dong F, Zhao C. Amelioration of diabetic retinopathy by engrafted human adipose-derived mesenchymal stem cells in streptozotocin diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2010;248:1415–22. doi: 10.1007/s00417-010-1384-z. [DOI] [PubMed] [Google Scholar]
- 21.Kwon DS, Gao X, Liu YB, Dulchavsky DS, Danyluk AL, Bansal M, et al. Treatment with bone marrow-derived stromal cells accelerates wound healing in diabetic rats. Int Wound J. 2008;5:453–63. doi: 10.1111/j.1742-481X.2007.00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007;25:2648–59. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- 23.Caplan A, Dennis J. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, et al. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+cells. Exp Hematol. 2004;32:657–64. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841–8. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger M, Martin B. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 27.Bittira B, Shum-Tim D, Al-Khaldi A, Chiu R. Mobilization and homing of bone marrow stromal cells in myocardial infarction. Eur J Cardiothorac Surg. 2003;24:393–8. doi: 10.1016/s1010-7940(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 28.Mackenzie T, Flake A. Human mesenchymal stem cells persist, demonstrate site-specific multipotential differentiation, and are present in sites of wound healing and tissue regeneration after transplantation into fetal sheep. Blood Cells Mol Dis. 2001;27:1–4. doi: 10.1006/bcmd.2001.0424. [DOI] [PubMed] [Google Scholar]
- 29.Wu GD, Nolta JA, Jin YS, Barr ML, Yu H, Starnes VA, et al. Migration of mesenchymal stem cells to heart allografts during chronic rejection. Transplantation. 2003;75:679–85. doi: 10.1097/01.TP.0000048488.35010.95. [DOI] [PubMed] [Google Scholar]
- 30.Tse W, Pendleton J, Beyer W, Egalka M, Guinan E. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–97. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 31.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 32.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+regulatory T cells. Stem Cells. 2008;26:212–22. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 33.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 34.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immuno. 2003;l57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 36.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit the formation of cytotoxic T lymphocytes, but not activated cytotoxic T lymphocytes or natural killer cells. Transplantation. 2003;76:1208–13. doi: 10.1097/01.TP.0000082540.43730.80. [DOI] [PubMed] [Google Scholar]
- 37.Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–98. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 38.Poggi A, Prevosto C, Massaro AM, Negrini S, Urbani S, Pierri I, et al. Interaction between human NK cells and bone marrow stromal cells induces NK cell triggering: Role of NKp30 and NKG2D receptors. J Immunol. 2005;175:6352–60. doi: 10.4049/jimmunol.175.10.6352. [DOI] [PubMed] [Google Scholar]
- 39.Spaggiari G, Capobianco A, Abdelrazik H, Becchetti F, Mingari M, Moretta L. Mesenchymal stem cells inhibit natural killer cell proliferation, cytotoxicity and cytokine production: Role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327–33. doi: 10.1182/blood-2007-02-074997. [DOI] [PubMed] [Google Scholar]
- 40.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, et al. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 41.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–25. doi: 10.1111/j.1365-2796.2007.01844.x. [DOI] [PubMed] [Google Scholar]
- 42.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–6. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 43.Xu YX, Chen L, Wang R, Hou WK, Lin P, Sun L, et al. Mesenchymal stem cell therapy for diabetes through paracrine mechanisms. Med Hypotheses. 2008;71:390–3. doi: 10.1016/j.mehy.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 44.Haynesworth S, Baber M, Caplan A. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: Effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–92. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 45.Cheng L, Qasba P, Vanguri P, Thiede M. Human mesenchymal stem cells support megakaryocyte and pro-platelet formation from CD34+hematopoietic progenitor cells. J Cell Physiol. 2000;184:58–69. doi: 10.1002/(SICI)1097-4652(200007)184:1<58::AID-JCP6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 46.Cheng L, Hammond H, Ye Z, Zhan X, Dravid G. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–42. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- 47.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–7. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 48.Ramiya V, Maraist M, Arfors K, Schatz D, Peck A, Cornelius J. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med. 2000;6:278–82. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 49.Lü P, Liu F, Yan L, Peng T, Liu T, Yao Z, et al. Stem cells therapy for type 1 diabetes. Diabetes Res Clin Pract. 2007;78:1–7. doi: 10.1016/j.diabres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Seeberger K, Dufour J, Shapiro A, Lakey J, Rajotte R, Korbutt G. Expansion of mesenchymal stem cells from human pancreatic ductal epithelium. Lab Invest. 2006;86:141–53. doi: 10.1038/labinvest.3700377. [DOI] [PubMed] [Google Scholar]
- 51.Gershengorn MC, Hardikar AA, Wei C, Geras-Raaka E, Marcus-Samuels B, Raaka BM. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306:2261–4. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 52.Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagons-expressing cells. Biochem Biophys Res Commun. 2006;341:1135–40. doi: 10.1016/j.bbrc.2006.01.072. [DOI] [PubMed] [Google Scholar]
- 53.Eberhardt M, Salmon P, von Mach MA, Hengstler JG, Brulport M, Linscheid P, et al. Multipotential nestin and Isl-1 positive mesenchymal stem cells isolated from human pancreatic islets. Biochem Biophys Res Commun. 2006;345:1167–76. doi: 10.1016/j.bbrc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 54.Chou Y, Khoun S, Hermann H, Goldman R. Nestin promotes the phosphorylation-dependent dissasembly of vimentin intermediate filaments during mitosis. Mol Biol Cell. 2003;14:1468–78. doi: 10.1091/mbc.E02-08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu YH, Wu DQ, Gao F, Li GD, Yao L, Zhang XC. A secretory function of human insulin-producing cells in vivo. Hepatobiliary Pancreat Dis Int. 2009;8:255–60. [PubMed] [Google Scholar]
- 56.Chao KC, Chao KF, Fu YS, Liu SH. Islet-like clusters derived from mesenchymal stem cells in Wharton's jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One. 2008;3:e1451. doi: 10.1371/journal.pone.0001451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oh S, Muzzonigro T, Bae S, LaPlante J, Hatch H, Petersen B. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for treatment of type I diabetes. Lab Invest. 2004;84:607–17. doi: 10.1038/labinvest.3700074. [DOI] [PubMed] [Google Scholar]
- 58.Chen L, Jiang X, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta cells. World J Gastroenterol. 2004;10:3016–20. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu XH, Liu CP, Xu KF, Mao XD, Zhu J, Jiang JJ, et al. Reversal of hyperglycaemia in diabetic rats by portal vein transplantation of islet-like cells generated from bone marrow mesenchymal stem cells. World J Gastroenterol. 2007;13:3342–9. doi: 10.3748/wjg.v13.i24.3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J (Engl) 2007;120:771–6. [PubMed] [Google Scholar]
- 61.Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells. 2007;25:2837–44. doi: 10.1634/stemcells.2007-0164. [DOI] [PubMed] [Google Scholar]
- 62.Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes. 2004;53:1721–32. doi: 10.2337/diabetes.53.7.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ianus A, Holz G, Theise N, Hussain M. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–50. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Okura H, Komoda H, Fumimoto Y, Lee CM, Nishida T, Sawa Y, et al. Transdifferentiation of human adipose tissue-derived stromal cells into insulin-producing clusters. J Artif Organs. 2009;12:123–30. doi: 10.1007/s10047-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 66.Dave SD, Vanikar AV, Trivedi HL. Ex vivo generation of glucose sensitive insulin secreting mesenchymal stem cells derived from human adipose tissue. Indian J Endocr Metab. 2012;16:S65–9. doi: 10.4103/2230-8210.94264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ende N, Chen R, Reddi A. Transplantation of human human umbilical cord blood cells improves glycemia and glomerular hypertrophy in type 2 diabetec mice. Biochem Biophys Res Commun. 2004;321:168–71. doi: 10.1016/j.bbrc.2004.06.121. [DOI] [PubMed] [Google Scholar]
- 68.Ende N, Chen R, Reddi A. Effects of human human umbilical cord blood cells on glycemia and insulitis in type 1 diabetec mice. Biochem Biophys Res Commun. 2004;325:665–9. doi: 10.1016/j.bbrc.2004.10.091. [DOI] [PubMed] [Google Scholar]
- 69.Urbán VS, Kiss J, Kovács J, Gócza E, Vas V, Monostori E, et al. Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells. 2008;26:244–53. doi: 10.1634/stemcells.2007-0267. [DOI] [PubMed] [Google Scholar]
- 70.Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;2:763–70. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 71.Ezquer F, Ezquer M, Parrau D, Carpio D, Yahez A, Conget P. Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycaemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant. 2008;14:631–40. doi: 10.1016/j.bbmt.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 72.Mahmoud A, Zuhair AB, Khaled RA, Sami AA, Abdelkarim SA, Iyad AA, et al. A preliminary study of the use of human adipose tissue-derived stem cells for the treatment of streptozotocin-induced diabetes mellitus in a rat model. Comp Clin Pathol. 2010;19:1–4. [Google Scholar]
- 73.Lin G, Wang G, Liu G, Yang LJ, Chang LJ, Lue TF, et al. Treatment of Type 1 Diabetes with Adipose Tissue-Derived Stem Cells Expressing Pancreatic Duodenal Homeobox1. Stem Cells Dev. 2009;18:1399–406. doi: 10.1089/scd.2009.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chandra V, Muthyala S, Jaiswal A, Bellare J, Nair P, Bhonde R. Islet-Like Cell Aggregates Generated from Human Adipose Tissue Derived Stem Cells Ameliorate Experimental Diabetes in Mice. PLoS one. 2011;6:e20615. doi: 10.1371/journal.pone.0020615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kajiyama H, Hamazaki TS, Tokuhara M, Masui S, Okabayashi K, Ohnuma K, et al. Pd×1-transfected adipose tissue-derived stem cells differentiate into insulin-producing cells in vivo and reduce hyperglycemia in diabetic mice. Int J Dev Biol. 2010;54:699–705. doi: 10.1387/ijdb.092953hk. [DOI] [PubMed] [Google Scholar]
- 76.Couri CE, Voltarelli JC. Stem cell therapy for type 1 diabetes mellitus: A review of recent clinical trials. Diabetol Metab Syndr. 2009;1:19. doi: 10.1186/1758-5996-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Starzl TE. The “Privileged” Liver and Hepatic Tolerogenicity. Liver Transpl. 2001;7:918–20. doi: 10.1053/jlts.2001.0070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trivedi HL, Vanikar AV, Thakker U, Firoze A, Dave SD, Patel CN, et al. Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc. 2008;40:1135–9. doi: 10.1016/j.transproceed.2008.03.113. [DOI] [PubMed] [Google Scholar]
- 79.Vanikar AV, Dave SD, Thakkar UG, Trivedi HL. Co-transplantation of adipose tissue-derived insulin-secreting mesenchymal stem cells and hematopoietic stem cells: A novel therapy for insulin-dependent diabetes mellitus. Stem Cells Int. 2010;2010:582382. doi: 10.4061/2010/582382. [DOI] [PMC free article] [PubMed] [Google Scholar]


