Abstract
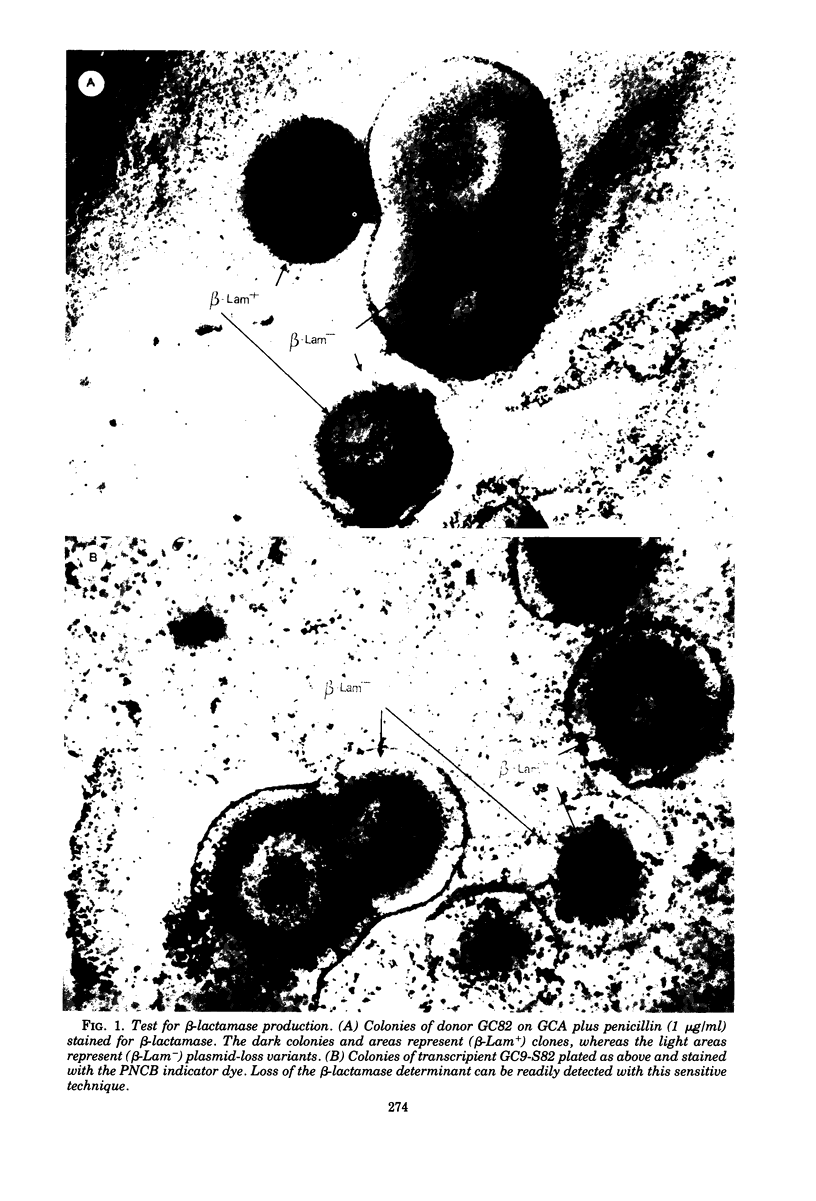
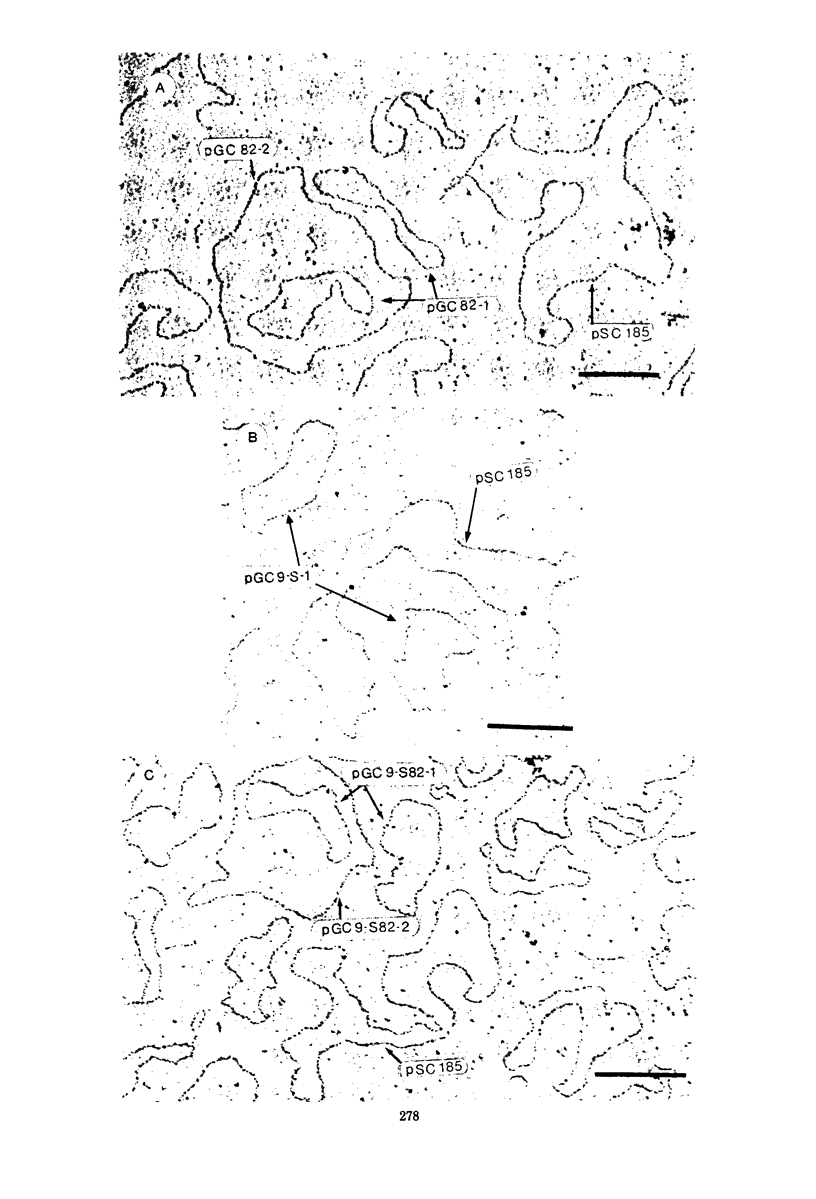
Neisseria gonorrhoeae strain GC82 contains a plasmid specifying a β-lactamase (β-Lam+). Mixed incubation of strain GC82 with a penicillin-susceptible (β-Lam−), streptomycin-resistant mutant of strain GC9 results in the expression of β-lactamase activity and streptomycin resistance in the transcipients. The frequency of transfer of the plasmid-specified resistance to penicillin seems to be proportional to the initial input ratio of the mating mixture of donor to recipient and to correlate positively with bacterial density. Cell-to-cell transmission of the deoxyribonucleic acid (DNA) appears to be by a conjugal mechanism or, alternatively, by an as yet undescribed transducing phage. Additionally, whole-cell DNA from a β-lactamase-producing strain could be used to transform streptomycin-resistant recipients, resulting in the expression of both β-lactamase activity and streptomycin resistance in the transformants, and purified gonococcal plasmid DNA transformed Escherichia coli but not the gonococcus. Circular DNA extracted from donor GC82 comprised three molecular species (approximately 2.7, 4.8, and 25 megadaltons [Mdal]), whereas the recipients GC9-S (Strr) contained only the 2.7-Mdal cryptic DNA species. DNA from the GC9-S82 (Strr, β-Lam+) transcipient contained a 4.8-Mdal species in addition to the cryptic molecular species (2.7 Mdal). The finding that the transcipient will not retransfer β-lactamase is consistent with the hypothesis that the 25-Mdal plasmid promotes mobilization of the smaller 4.8-Mdal R plasmid.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asheshov E. H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J Gen Microbiol. 1966 Mar;42(3):403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- Ashford W. A., Golash R. G., Hemming V. G. Penicillinase-producing Neisseria gonorrhoeae. Lancet. 1976 Sep 25;2(7987):657–658. doi: 10.1016/s0140-6736(76)92467-3. [DOI] [PubMed] [Google Scholar]
- Baron L. S., Carey W. F., Spilman W. M. GENETIC RECOMBINATION BETWEEN ESCHERICHIA COLI AND SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1959 Jul;45(7):976–984. doi: 10.1073/pnas.45.7.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- CITRI N. DETERMINATION OF PENICILLINASE ACTIVITY. Methods Med Res. 1964;10:221–232. [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Clewell D. B. Transmissible toxin (hemolysin) plasmid in Streptococcus faecalis and its mobilization of a noninfectious drug resistance plasmid. J Bacteriol. 1975 Nov;124(2):784–790. doi: 10.1128/jb.124.2.784-790.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein B. I., Sox T., Biswas G., Blackman E., Sparling P. F. Conjugal transfer of the gonococcal penicillinase plasmid. Science. 1977 Mar 11;195(4282):998–1000. doi: 10.1126/science.402693. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., Roberts M., Mayer L. W., Falkow S. Plasmid-mediated beta-lactamase production in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1977 Mar;11(3):528–533. doi: 10.1128/aac.11.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelkirk P. G., Schoenhard D. E. Physical evidence of a plasmid in Neisseria gonorrhoeae. J Infect Dis. 1973 Feb;127(2):197–200. doi: 10.1093/infdis/127.2.197. [DOI] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1141–1146. doi: 10.1128/iai.11.5.1141-1146.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. S., Foster G. C. Electrophoretic comparison of endonuclease-digested plasmids from Neisseria gonorrhoeae. J Bacteriol. 1976 Jun;126(3):1297–1304. doi: 10.1128/jb.126.3.1297-1304.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebeler B. H., Young F. E. Autolysis of Neisseria gonorrhoeae. J Bacteriol. 1975 May;122(2):385–392. doi: 10.1128/jb.122.2.385-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecko D. J., Brevet J., Cohen S. N. Involvement of multiple translocating DNA segments and recombinational hotspots in the structural evolution of bacterial plasmids. J Mol Biol. 1976 Dec;108(2):333–360. doi: 10.1016/s0022-2836(76)80124-6. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Transfer of tetracycline-resistance between strains of Staphylococcus aureus in mixed cultures. J Gen Microbiol. 1971 Dec;69(2):229–237. doi: 10.1099/00221287-69-2-229. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P., RICHMOND M. H. NATURE AND INTERACTIONS OF THE GENETIC ELEMENTS GOVERNING PENICILLINASE SYNTHESIS IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1965 Aug;90:467–480. doi: 10.1128/jb.90.2.467-480.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard K., Solberg O. Isolation of a penicillinase producing strain of Neisseria gonorrhoeae. Acta Pathol Microbiol Scand B. 1976 Dec;84B(6):458–460. doi: 10.1111/j.1699-0463.1976.tb01967.x. [DOI] [PubMed] [Google Scholar]
- Phillips I. Beta-lactamase-producing, penicillin-resistant gonococcus. Lancet. 1976 Sep 25;2(7987):656–657. doi: 10.1016/s0140-6736(76)92466-1. [DOI] [PubMed] [Google Scholar]
- Platt D. J. Prevalence of multiple antibiotic resistance in Neisseria gonorrhoeae. Br J Vener Dis. 1976 Dec;52(6):384–386. doi: 10.1136/sti.52.6.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Falkow S. Conjugal transfer of R plasmids in Neisseria gonorrhoeae. Nature. 1977 Apr 14;266(5603):630–631. doi: 10.1038/266630a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez W., Saz A. K. Possible mechanism of decreased susceptibility of Neisseria gonorrhoeae to penicillin. Antimicrob Agents Chemother. 1975 Jun;7(6):788–792. doi: 10.1128/aac.7.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders J. R., Sykes R. B. Transfer of a plasmid-specified beta-lactamase gene from Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Feb;11(2):339–344. doi: 10.1128/aac.11.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Lerner S. A., Bohnhoff M., Morello J. A. Plasmid deoxyribonucleic acid in clinical isolates of Neisseria gonorrhoeae. J Bacteriol. 1975 Jun;122(3):1293–1300. doi: 10.1128/jb.122.3.1293-1300.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura T., Hirano T., Ito T., Yoshioka M. Transmission of bacteriocinogenicity by conjugation in group D streptococci. Jpn J Microbiol. 1973 Nov;17(6):445–452. doi: 10.1111/j.1348-0421.1973.tb00930.x. [DOI] [PubMed] [Google Scholar]
- WATANABE T. Infective heredity of multiple drug resistance in bacteria. Bacteriol Rev. 1963 Mar;27:87–115. doi: 10.1128/br.27.1.87-115.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood D. O., Brownell G. H. Transformation of leucine and rifampin traits in Neisseria gonorrhoeae with deoxyribonucleic acid from homologous and heterologous origins. J Bacteriol. 1975 Feb;121(2):471–474. doi: 10.1128/jb.121.2.471-474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]