Abstract
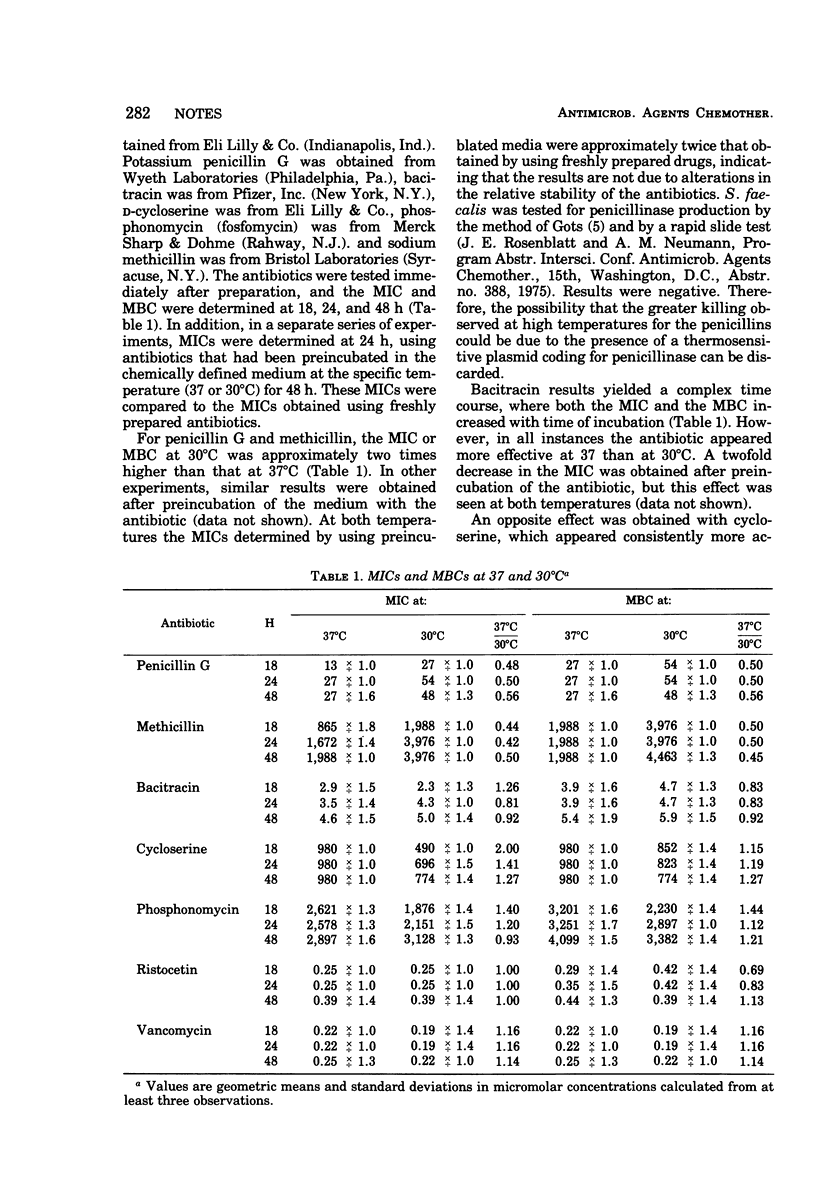
The minimal inhibitory and bactericidal concentrations were determined at 30 and 37°C for several antibiotics affecting cell wall biosynthesis. The test organism, Streptococcus faecalis ATCC 9790, possesses a single autolytic enzyme and does not produce penicillinase. Penicillin, methicillin, and, possibly, bacitracin appeared more effective at 37 than at 30°C. Vancomycin, ristocetin, and phosphonomycin appeared equally effective at both temperatures, and cycloserine appeared consistently more active at 30 than at 37°C.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annear D. I. The effect of temperature on resistance of Staphylococcus aureus to methicillin and some other antibioics. Med J Aust. 1968 Mar 16;1(11):444–446. [PubMed] [Google Scholar]
- Asheshov E. H. Loss of antibiotic resistance in Staphylococcus aureus resulting from growth at high temperature. J Gen Microbiol. 1966 Mar;42(3):403–410. doi: 10.1099/00221287-42-3-403. [DOI] [PubMed] [Google Scholar]
- DiJoseph C. G., Bayer M. E., Kaji A. Host cell growth in the presence of the thermosensitive drug resistance factor, Rts1. J Bacteriol. 1973 Jul;115(1):399–410. doi: 10.1128/jb.115.1.399-410.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gots J. S. THE DETECTION OF PENICILLINASE-PRODUCING PROPERTIES OF MICROORGANISMS. Science. 1945 Sep 21;102(2647):309–309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. On the mechanism of action of vancomycin: inhibition of peptidoglycan synthesis in Gaffkya homari. Antimicrob Agents Chemother. 1974 Dec;6(6):722–728. doi: 10.1128/aac.6.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan F. M., Kahan J. S., Cassidy P. J., Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974 May 10;235(0):364–386. doi: 10.1111/j.1749-6632.1974.tb43277.x. [DOI] [PubMed] [Google Scholar]
- MAY J. W., HOUGHTON R. H., PERRET C. J. THE EFFECT OF GROWTH AT ELEVATED TEMPERATURES ON SOME HERITABLE PROPERTIES OF STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1964 Nov;37:157–169. doi: 10.1099/00221287-37-2-157. [DOI] [PubMed] [Google Scholar]
- Perkins H. R., Nieto M. The chemical basis for the action of the vancomycin group of antibiotics. Ann N Y Acad Sci. 1974 May 10;235(0):348–363. doi: 10.1111/j.1749-6632.1974.tb43276.x. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D. Reversal of cycloserine inhibition by D-alanine. Proc Soc Exp Biol Med. 1959 Aug-Sep;101:693–695. doi: 10.3181/00379727-101-25064. [DOI] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Takayasu H., Akiba T. Thermosensitive replication of a kanamycin resistance factor. J Bacteriol. 1967 Sep;94(3):687–690. doi: 10.1128/jb.94.3.687-690.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


