Abstract
The past 15 years of allergic disease research have produced extraordinary improvements in our understanding of the pathogenesis of airway allergic diseases such as asthma. Whereas it was previously viewed as largely an immunoglobulin E-mediated process, the gradual recognition that T cells, especially Type 2 T helper (Th2) cells and Th17 cells, play a major role in asthma and related afflictions has inspired clinical trials targeting cytokine-based inflammatory pathways that show great promise. What has yet to be clarified about the pathogenesis of allergic inflammatory disorders, however, are the fundamental initiating factors, both exogenous and endogenous, that drive and sustain B- and T-cell responses that underlie the expression of chronic disease. Here we review how proteinases derived from diverse sources drive allergic responses. A central discovery supporting the proteinase hypothesis of allergic disease pathophysiology is the role played by airway fibrinogen, which in part appears to serve as a sensor of unregulated proteinase activity and which, when cleaved, both participates in a novel allergic signaling pathway through Toll-like receptor 4 and forms fibrin clots that contribute to airway obstruction. Unresolved at present is the ultimate source of airway allergenic proteinases. From among many potential candidates, perhaps the most intriguing is the possibility such enzymes derive from airway fungi. Together, these new findings expand both our knowledge of allergic disease pathophysiology and options for therapeutic intervention.
Keywords: asthma, sinusitis, proteolysis, fibrinogen, Th2 cells
Since its discovery in 1966 and the elucidation of the crucial molecular and cellular details underlying Type 1 immediate hypersensitivity reactions soon thereafter, IgE has figured heavily in our perceptions of the pathophysiology of allergic diseases of all types (1–3). The central putative role of IgE indeed drove the majority of allergic disease research and provided the basis for many human clinical trials until recently, leading, directly or indirectly, to modern pharmaceuticals that include leukotriene modifiers and the monoclonal anti-IgE antibody omalizumab (4).
For most of IgE’s history, however, the molecular and biological tools needed to formally prove its importance in allergic diseases did not exist. That situation began to change approximately 20 years ago with the advent of gene-deficient mice that were ideal for testing the importance of IgE and the factors required for IgE production in experimental systems. Initial studies of IgE-deficient mice, far from confirming what for many were foregone conclusions, provided the first evidence that allergic responses were more complex than originally envisaged. Anaphylaxis, the prototypical Type 1 hypersensitivity reaction, was found to be possible in IgE-deficient mice, as was asthma-like disease in the same mice (5, 6). Unlike anaphylaxis, in which disease expression requires antibody (because it appears that IgG isotypes are capable of substituting for IgE in some instances), all antibody isotypes, and even B cells, in models of asthma are entirely dispensable for the major disease phenotypes that include airway hyperresponsiveness to cholinergic challenge, allergic airway inflammation, and airway goblet cell metaplasia with mucus hypersecretion (7). Thus, these early studies provided the first evidence of the existence of two types of allergic diseases: those mediated primarily by antibodies, especially IgE, and those, such as asthma, in which antibodies are not the major mediator of disease.
Earlier studies further demonstrated the central role played by CD4+ T cells in the pathophysiology of experimental asthma (8–10). Central to the creation of a credible T-cell model of allergic disease pathophysiology was elucidating the mechanisms by which T cells mediate airway obstruction, the sine qua non of asthma and related diseases. In large part, T helper cells mediate inflammation and disease (or resolution of infection or cancer) through the cytokines that they secrete. Of the many cytokines that Type 2 T helper (Th2) cells secrete, two emerged with particular relevance to the expression of asthma-like disease, especially airway obstruction: interleukin 4 (IL-4) and IL-13 (10–12). These interesting cytokines, which almost certainly descend from the same ancestral gene, signal through a multimeric receptor complex that varies in composition, but must include for both cytokines the α-chain of the IL-4 receptor (IL-4Rα) (13, 14). Signals through both IL-4 and IL-13 activate the allergic disease-related transcription factor signal transducer and activator of transcription 6 (STAT6) to induce allergy-related genes with two discrete effects (15). First, the effects of IL-4 are largely confined to lymphoid cells and consist of promotion of Th2 cell differentiation and effector function as well as IgE class switching and secretion. IL-4 further promotes Th2 and IgE+ B-cell stability, but is not the only cytokine contributing to this function during established inflammation. Second, IL-13, for reasons that are still not fully understood, is less important for lymphoid cell differentiation and stability and much more important for activating innate immune cells such as macrophages and nonimmune cells such as airway epithelia, smooth muscle, and endothelia (16).
IL-13-STAT6–dependent changes collectively result in the clinically significant parameter of airway obstruction (11, 12, 17). Although there are likely many cell types and additional secreted factors that contribute to this complex phenomenon, two that have been studied in detail are airway hyperresponsiveness (AHR) and goblet cell metaplasia with mucus hypersecretion. Presumably mediated by airway smooth muscle, AHR accounts for the exaggerated tendency of the airways to transiently constrict in response to diverse and often unexpected stimuli, including particulate pollution, allergens, hot, cold or warm air, various odors, and heightened emotional states. Ultimately, the airway constriction is mediated by muscarinic cholinergic impulses from the parasympathetic nervous system via the vagus nerve. As a mediator of AHR, but not immediate bronchoconstriction, IL-13, and, to a lesser extent IL-4, thus participate indirectly in airway constriction (18, 19).
IL-13 (and, again, to a lesser extent, IL-4) also promotes the induction of airway mucus, especially Muc5AC, that is secreted into the airway and thereby directly contributes to airway obstruction. AHR-related airway obstruction is quickly reversed by commonly used asthma drugs that promote airway dilation, including cholinergic receptor antagonists (e.g., ipratropium, tiotropium) and agonists of the β2-adrenergic receptor (β2-AR; e.g., albuterol, salmeterol). In contrast, the physical blockade of the airways due to mucus hypersecretion is likely to be much less responsive to the same pharmacologic interventions. Indeed, severe airway obstruction that is largely unresponsive to medical management and linked to asthma-related death is invariably due to physical obstruction of the airways and asphyxiation, presumably due to mucus plugging, a poorly understood phenomenon (20). To further understand this process, we turned to the less well-understood innate immune mechanisms that most likely precede and strongly influence subsequent Th2 and IgE responses.
Innate Immunity in Asthma Airway Proteolysis
To gain insight into the earliest, innate immune mechanisms that contribute to allergic airway diseases such as asthma, we first sought to determine the exogenous factor(s) that initiate allergic inflammation in mice. A key observation in this regard is that some agents are capable of inducing allergic airway inflammation in mice if inhaled, whereas others are not. Of the latter, nonintrinsically allergenic agents, also termed Type 1 respiratory allergens (21), chicken egg ovalbumin is most representative. Ovalbumin will induce allergic airway inflammation and asthma-like disease, but, curiously, only if mice are first immunized intraperitoneally or subcutaneously, usually with the ovalbumin precipitated in an aluminum salt, after which airway administration of the antigen alone will elicit robust allergic disease, albeit only temporarily. In contrast, many intrinsically allergenic or Type 2 respiratory allergens exist. They include complex extracts of pollen, dust mites, and fungi. These agents can all elicit severe, asthma-like disease in mice merely through airway application. To understand the biochemical and immunological bases of Types 1 and 2 respiratory allergens, we previously compared their biochemical profiles and discovered that proteinase activity was consistently found in Type 2 allergens, but absent in Type 1 allergens (21). It could be argued that, owing to the great complexity of Type 2 allergens that are derived from whole organisms, there are many biochemical properties that distinguish these from the much simpler ovalbumin. However, the importance of industrially used proteinases, such as subtilisins (22), bromelain (23), and papain (24), as causes of occupational asthma strongly suggest that the proteinases of Type 2 respiratory allergens are functionally important. Indeed, we previously demonstrated that a fungal proteinase, the aspergillopepsin derived from Aspergillus oryzae, functioned as a Type 2 respiratory allergen, inducing profound asthma-like disease that was equal to, and even far exceeded, that elicited by other Type 2 allergens (21). However, it is not the proteinase itself that induces allergic inflammation and disease, but rather the proteinase activity that is required. Moreover, we discovered that proteinases make poor allergens, in the sense that we could not detect specific T- and B-cell responses against the proteinase itself. Rather, the proteinase serves as an allergy-inducing adjuvant factor that promotes Th2 and IgE responses against other antigens, including Type 1 respiratory allergens such as ovalbumin, but without the need for peripheral immunization. These essential observations suggest that exogenous proteinases initiate allergic disease, but do so indirectly, by cleaving an endogenous substrate. This possibility further implies that an immune receptor that detects the cleaved substrate must also exist (25). The problem, however, was finding the putative proteinase-activated substrate(s) and receptor(s).
Crabs and Clots Provide a Clue
The role of proteinases in coordinating allergic responses is best understood in relation to arthropod-based innate immunity. Arthropods such as the fruit fly Drosophila melanogaster and the horseshoe crab Limulus polyphemus have only innate immune responses. In the absence of T and B cells, these organisms mount a limited set of responses against a wide variety of infectious organisms. In response to gram-negative bacterial invasion, lipopolysaccharide (LPS) activates a series of proteolytic events within the circulating hemolymph of these organisms to activate two defensive outcomes: (1) the hemolymph coagulates, preventing further dissemination of the infection, and (2) a fragment of the main coagulant protein (pro-Späetzele in Drosophila and coagulogen in horseshoe crabs [26, 27]) signals through the main arthropod immune receptor, Toll, to induce additional antibacterial defensive mechanisms (28). The identical pathway is triggered upon invasion by fungi, but instead of LPS, fungal proteinases directly activate the proteolytic cascade, leading to hemolymph coagulation and activation of Toll through the endogenous proteinase Persephone (29). This fundamental pathway provides the basis of the limulus amoebocyte lysate assay, a widely used test for detecting LPS. More importantly, however, this extremely primitive but highly successful innate immune response has remained evolutionarily intact, much like Toll itself.
Inspired by the remarkably efficient arthropod immune response, we first sought to determine whether mammalian Toll-like receptors (TLRs) are relevant to proteinase-dependent allergic responses. Unlike arthropods, which have only a single Toll, mammals have at least 10 (30). To determine if one or more TLR serves as an essential proteinase-activated receptor in allergic responses, we took advantage of the limited number of adapter signaling molecules that are differentially used by TLRs to signal in response to a wide variety of potential ligands, most of which are organism-derived, pathogen-associated molecular patterns (PAMPs), although some endogenous molecules may also activate TLRs. MyD88 is an adaptor protein obligatorily used by TLRs 1, 2, 5, 6, 7, 8, 9, and 10. In contrast, Toll/IL-1 receptor domain-containing, adapter-inducing interferon β protein (TRIF) is the exclusive adaptor protein used by TLR3. TLR4 is a special case, utilizing both MyD88 and TRIF to signal in response to LPS stimulation (30).
We challenged both MyD88- and TRIF-deficient mice with fungal proteinase to determine the effect of LPS stimulation on the asthma phenotype as a means of determining which TLRs, if any, can serve as proteinase-activated receptors in allergic airway disease. To our surprise, mice lacking MyD88 showed exaggeration of all aspects of their disease that we measured, including airway hyperresponsiveness and airway eosinophilia, Th2, and Th17 responses (31). This result was particularly intriguing because MyD88 is required not just for signaling by many TLRs but also for signaling by several cytokines, including IL-1, IL-18, and IL-33 (30). Thus, this experiment not only ruled out a requisite role for most TLRs as proteinase-activated receptors but also indicated that one or more TLRs or cytokines might actually inhibit allergic responses.
TRIF-deficient mice showed essentially no difference from proteinase-challenged wild-type mice, indicating that TLR3 is also not a proteinase-activated receptor (31). This result left only TLR4 to consider further. We conducted two additional experiments to determine if TLR4 was the proteinase-activated receptor that we had long sought. First, we studied mice deficient in TLR4. Regardless of the allergen used (proteinase, live Aspergillus niger, or ovalbumin), these mice were incapable of developing asthma-like disease after a suitable period of allergen challenge. A close examination of the TLR4−/− phenotype after allergen challenge disclosed some surprises, however. Although the overall asthma phenotype was either strongly attenuated (e.g., markedly reduced airway eosinophilia, goblet cell metaplasia) or abrogated (airway hyperresponsiveness), lung IL-4 and IL-17A responses were entirely preserved. We further examined the ability of Th2, Th17, and IgE responses to develop in TLR4-deficient mice during ovalbumin immunization and found no defect. Thus, TLR4 was not, paradoxically for us at the time, required for development of adaptive immune T- and B-cell responses (31).
The second TLR4-related experiment we performed was to challenge mice simultaneously deficient in both MyD88 and TRIF with allergen. These mice were highly immunodeficient, as indicated by their almost complete insensitivity to proteinase challenge, producing no eosinophilia, airway hyperresponsiveness, or Th2 or Th17 responses. Together, these results indicated that TLR4 was the only proteinase-activated receptor from the TLR family that was required for allergic inflammation and disease, but again was not required for adaptive immune responses (31). To understand how TLR4 controlled allergic responses independent of effects on T and B cells, we turned to innate immune responses that are likely operative in allergic diseases such as asthma.
To study innate immune responses during proteinase exposure, we examined in isolation the two innate immune cells most likely to encounter inhaled proteinase: airway macrophages and epithelial cells. We began our studies with bone marrow-derived macrophages. Macrophages exposed to LPS, interferon γ (IFN-γ), IL-4, or fungal proteinase all appear to be physically identical. However, we determined transcriptional changes occurring within these distinct macrophage populations and found that each challenge condition led to a unique gene expression signature. Most interestingly, fungal proteinase enhanced the expression of several genes linked to antifungal activity, including lysozyme, secretory leukoproteinase inhibitor (SLPI), and a scavenger receptor, macrophage receptor with collagenous structure (MARCO) (31). Lysozyme and SLPI appear to shed antimicrobial peptides having direct antifungal activity, whereas MARCO enhances phagocytosis of fungal cells (32–35).
The consistent theme of antifungal activity suggested by the microarray study convinced us to test the antifungal ability of both proteinase-activated macrophages and epithelial cells. We therefore developed an in vitro assay that would allow us to detect the inhibition of fungal growth in vitro when A. niger was cultured in the presence of either macrophages or epithelial cells activated under a variety of conditions. With this assay, we could both directly visualize the effect of these cells on fungal growth by inverted microscopy and indirectly quantify fungal growth through the use of a dye (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide, or XTT) that changes color in proportion to the number of mitochondria (i.e., live cells) present. These complementary approaches confirmed that both human and mouse proteinase-activated macrophages and epithelial cells efficiently controlled fungal growth in vitro (31).
Thus, simply by following the natural progression of our studies, we had discovered that allergic airway inflammation is linked to the control of fungal growth through TLR4. At this point, we turned our attention to determining the proteinase-activated endogenous substrate that was the natural ligand for TLR4. Two observations greatly assisted us in this search, without which our studies might have continued for many fruitless years. Most importantly, the endogenous proteinase-activated substrate in horseshoe crabs that activates arthropod Toll was previously shown to be coagulogen, the functional, albeit not structural, equivalent of mammalian fibrinogen (26). Second, we discovered that, in order for fungal proteinase to induce antifungal responses from cultured macrophages, serum was required (31). Although serum is technically depleted of fibrinogen, fibrinogen in fact remains in serum after one round of clotting, such that a second, and even a third, round of clotting reactions is possible with the same sample. Indeed, exogenous fibrinogen activated by proteinase was alone able to replace serum entirely in our fungistasis assay. Although fibrinogen was previously reported to serve as a TLR4 ligand (36), our studies indicate that it is not fibrinogen per se that is the ligand, but rather a fibrinogen cleavage product (FCP).
Other serum proteins that can serve as TLR4 ligands, such as fibronectin, had no effect on macrophage-dependent fungistasis, whether activated by proteinase or not (31). To determine if fibrinogen was required in vivo for allergic responses, we first immunized mice intraperitoneally with ovalbumin and then intranasally challenged them simultaneously with the thrombin antagonists hirudin and ovalbumin. An enduring mystery of the mouse allergic response is why ovalbumin is so effective at eliciting IgE responses and asthma-like lung disease when it is devoid of all proteinase activity. Because ovalbumin becomes allergenic only when mice are immunized with aluminum salts such as alum (i.e., aluminum potassium sulfate), we reasoned that the immunization procedure in this artificial context elicits an endogenous proteolytic response that allows allergic responses against ovalbumin to proceed. Conversely, we reasoned that this proteinase is not invoked when ovalbumin is administered only in the nonimmunogenic, intranasal context. Alum has long been known as an activator of coagulation.
The anticoagulant and antithrombin agent hirudin blocked the ability of ovalbumin to induce allergic lung disease normally induced by ovalbumin; in fact, hirudin- and ovalbumin-challenged mice strongly resembled TLR4-deficient mice challenged with any allergen (31). We later discovered that hirudin also blocked the proteinase activity of our fungal proteinase, such that hirudin could inhibit induction of the macrophage fungistasis response and allergic airway disease induced by either ovalbumin or fungal proteinase. These findings firmly link the coagulation cascade to the induction of allergic inflammation and help to explain the many reports of the salutary effects of aerosolized anticoagulants in asthma that have accumulated over the past half-century (37).
A lingering question, however, is how innate antifungal immunity occurring at the cellular level is linked to the more macroscopic world of allergic inflammation and asthma-like disease. Perhaps the best way to view the activity of FCPs acting through TLR4 is that they induce activation and differentiation of innate immune cells such as macrophages and epithelial cells, but almost certainly other innate immune cells are involved as well, including potentially Type 2 innate lymphoid cells (ILC-2) that secrete IL-5 and IL-13 during allergen challenge (38). Part of the FCP-TLR4 activation cascade involves induction of innate antifungal responses, but an equally important component of this response is induction of cytokine receptors, especially the IL-13 receptor chain IL-13Rα1. Thus, FCP-TLR4 signaling induces both antifungal responses and responsiveness to Th2 cytokines such as IL-13. A curious result of this biology is that if FCP-TLR4 signaling is blocked (e.g., through hirudin or lack of TLR4), airway obstruction is relieved but Th2 and Th17 responses are not attenuated (31).
However, the importance of airway fibrinogenolysis in allergic responses extends well beyond this novel biochemical pathway. The phenomenon of mucus plugging, discussed earlier, is more appropriately termed plastic bronchitis. Plastic bronchitis is a phenomenon that occurs in three principal settings: cystic fibrosis, asthma, and children undergoing surgical repair of complex congenital heart malformations. Although mucus is clearly present in the airways of affected patients, the more important component is fibrin. Fibrinolytics have proven useful in treating children affected by plastic bronchitis, suggesting that it is the fibrin component that is largely responsible for the obstruction (39–41). These and other studies (42) were the first to document fibrinogenolysis occurring on a large scale in the airways of asthma patients, but their results still provide little insight into why this harmful phenomenon occurs. Our studies nonetheless indicate that airway fibrinogenolysis is the natural consequence of unregulated airway proteinase activity and has two inevitable consequences: the initiation of allergic inflammation and the deposition of airway fibrin, the latter of which can be life-threatening in severe cases.
Sources of Proteinase in Typical Allergic Disease and Future Therapies
A final challenge raised by our findings is to understand the sources of proteinase activity that are presumably relevant to human allergic diseases. We previously showed that fungal proteinases are to a large extent responsible for the proteinase activity found in household dust (43). The discovery that allergic airway inflammation is, in fact, a complex antifungal response further suggests that fungal proteinases are potentially linked to many allergic diseases. Whether this association involves direct inhalation of fungal proteinases or inhalation of fungal spores that then lead to airway colonization or infection and in situ production of allergenic proteinases is a critically important, unresolved issue. Our previous imperfect analysis of household dust suggested that fungal proteinases were present at only trace levels, most likely existing at levels that are insufficient to cause allergic disease by themselves (43). Conversely, published studies are gradually building the case for a fungal infectious basis for asthma and chronic rhinosinusitis, including the observations that (1) many fungi found in human environments can produce asthma-like disease in mice (43, 44), (2) cultures of lower airway samples of patients with severe asthma exacerbations consistently yield fungi (45), and (3) many patients with allergic upper-airway diseases, such as chronic rhinosinusitis with nasal polyps, show strong Th2 responses to fungal antigens upon restimulation of peripheral blood T cells (46). Moreover, although the data are not consistent across all studies, several reports have documented the efficacy of antifungal antibiotics in allergic upper and lower respiratory tract disease (47–49).
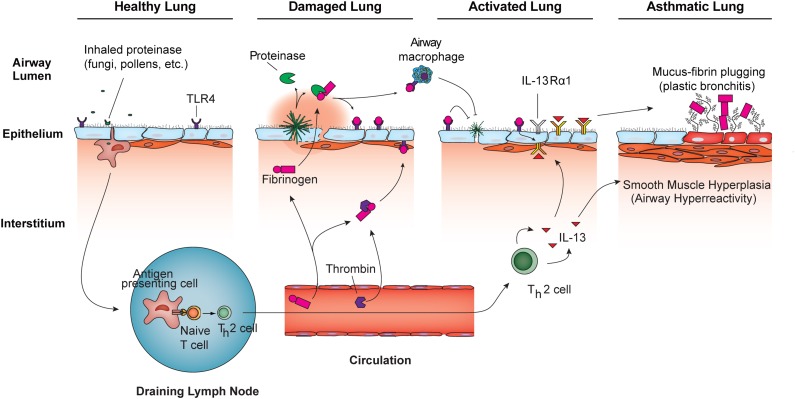
These diverse observations do not yet add up to a wholly compelling indictment of fungi in the pathogenesis of severe asthma and other allergic syndromes, but they could help to explain plastic bronchitis, which rarely presents as a unique complication of asthma. Much like hemolymph coagulation in arthropods, airway fibrin deposition could serve to contain proteinase-secreting infectious organisms such as fungi to prevent their invasion and further dissemination. This would then bring us full circle with arthropod immunity: unregulated proteinases trigger both the physical containment of infectious organisms while initiating biochemical events that elicit more complex protective immunity. Nonetheless, in addition to industrial exposures, there are abundant natural sources of proteinases from noninfectious sources that could spuriously contribute to the expression of allergic diseases, including house dust mites and pollens. An integrated view of innate and adaptive immune responses as coordinated by airway proteinases in allergic airway disease and antifungal immunity is presented in Figure 1.
Figure 1.
Proteinase model of allergic airway disease pathogenesis. The healthy lung routinely encounters low-grade proteinase activity through inhalation of fungi, pollens, and other proteinase sources. Enhanced proteinase exposure, possibly through airway fungal infections, causes lung damage that may include enhanced airway epithelial and vascular endothelial cell permeability, which promotes the release of fibrinogen into the airway interstitium and lumen. Airway epithelial cells may also secrete fibrinogen directly into the airway. Exogenous proteinases and endogenous thrombin promote fibrinogen cleavage and the generation of fibrinogen cleavage products (FCPs) that function as alternate ligands for TLR4, triggering the activation of distinct innate immune cells, including airway epithelium and macrophages. Enhanced antifungal immunity induced in FCP-activated innate immune cells promotes resolution of fungal infections and increases responsiveness to the cytokines of Th2 cells (e.g., IL-13) through increased expression of the IL-13 receptor subunit IL-13Rα1. Simultaneously, through a non-TLR4–dependent mechanism, airway proteinases initiate the differentiation of Th2 cells (52), which also promote antifungal immunity (44). Both FCP-dependent innate and Th2 responses (7, 11) are required for the full expression of allergic airway diseases such as asthma (adapted from Reference 31).
Understanding which sources of allergenic proteinases are the most important, whether infectious or not, is essential in planning the next generation of therapies for allergic diseases. For example, anticoagulant or antiproteinase approaches to allergic disease management might lead to a reduction in disease severity, at least in the short term, but interrupting airway fibrinogenolysis might also lead to less effective antimicrobial immunity and would be expected to impair clearance of fungi and perhaps other proteinase-secreting organisms that potentially initiate and sustain the disease for chronic periods. Overreliance on antifibrinogenolysis strategies alone, though they might not lead to invasive fungal disease, could, over time, lead to gradual progression of airway fungal colonization and/or infection and thus to progression of what would otherwise remain a mild, self-limited condition to severe, life-threatening disease. Indeed, this is precisely the concern with regard to the chronic use of corticosteroids in asthma. Specifically, severe, steroid-resistant asthma may in fact represent an iatrogenic, evolved state in which steroid efficacy is not compromised, but rather one in which airway fungal burden has progressed to the point that generic anti-inflammatory therapy can no longer mask the fungus-induced airway obstruction.
Carefully designed future clinical studies may clarify the role of fungi and antifungal therapy in allergic airway diseases. Moreover, the discovery of the central importance of the innate immune FCP-TLR4 signaling pathway in allergic disease provides a compelling rationale for designing future targeted therapies. Combined with strategies that interrupt the IL-4/IL-13/STAT6 signaling pathway, which early studies suggest also hold much promise (50, 51), the day may be dawning in which our hard-won battle to understand allergic diseases will finally translate into the preventative and diagnostic strategies and, yes, even cures, that our patients deserve.
Footnotes
Supported by National Institutes of Health (NIH) Grants HL75243, AI057696, and AI070973 (D.B.C.); NIH Grants T32GM088129 and R25GM56929 (V.O.M.); and the C.N. and Mary V. Papadopoulos Charitable Fund from the Biology of Inflammation Center.
Author contributions: V.O.M. designed experiments, performed the work described, and co-wrote the manuscript. G.M., J.M.K., P.P., W.L., and X.Y. performed experiments and co-wrote the manuscript. F.K. and D.B.C. conceived the studies, designed experiments, and co-wrote the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org
References
- 1.Ishizaka K, Ishizaka T. Physicochemical properties of reaginic antibody: 1. Association of reaginic activity with an immunoglobulin other than γA- or γG-globulin. J Allergy. 1966;37:169–185. doi: 10.1016/0021-8707(66)90091-8. [DOI] [PubMed] [Google Scholar]
- 2.Ishizaka K, Ishizaka T, Hornbrook MM. Physico-chemical properties of human reaginic antibody: IV. Presence of a unique immunoglobulin as a carrier of reaginic activity. J Immunol. 1966;97:75–85. [PubMed] [Google Scholar]
- 3.Ishizaka T, Tomioka H, Ishizaka K. Degranulation of human basophil leukocytes by anti-γE antibody. J Immunol. 1971;106:705–710. [PubMed] [Google Scholar]
- 4.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, van As A, Gupta N. Omalizumab, anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–190. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 5.Oettgen HC, Martin TR, Wynshaw-Boris A, Deng C, Drazen JM, Leder P. Active anaphylaxis in IgE-deficient mice. Nature. 1994;370:367–370. doi: 10.1038/370367a0. [DOI] [PubMed] [Google Scholar]
- 6.Mehlhop PD, van de Rijn M, Goldberg AB, Brewer JP, Kurup VP, Martin TR, Oettgen HC. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc Natl Acad Sci USA. 1997;94:1344–1349. doi: 10.1073/pnas.94.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corry DB, Grünig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, Rennick DM, Locksley RM. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 8.Van Loveren H, Garssen J, Nijkamp FP. T cell-mediated airway hyperreactivity in mice. Eur Respir J Suppl. 1991;13:16s–26s. [PubMed] [Google Scholar]
- 9.Müller KM, Jaunin F, Masouyé I, Saurat JH, Hauser C. Th2 cells mediate IL-4-dependent local tissue inflammation. J Immunol. 1993;150:5576–5584. [PubMed] [Google Scholar]
- 10.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grünig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 13.Gauchat JF, Schlagenhauf E, Feng NP, Moser R, Yamage M, Jeannin P, Alouani S, Elson G, Notarangelo LD, Wells T, et al. A novel 4-kb interleukin-13 receptor α mRNA expressed in human B, T, and endothelial cells encoding an alternate type-II interleukin-4/interleukin-13 receptor. Eur J Immunol. 1997;27:971–978. doi: 10.1002/eji.1830270425. [DOI] [PubMed] [Google Scholar]
- 14.Zurawski SM, Vega F, Jr, Huyghe B, Zurawski G. Receptors for interleukin-13 and interleukin-4 are complex and share a novel component that functions in signal transduction. EMBO J. 1993;12:2663–2670. doi: 10.1002/j.1460-2075.1993.tb05927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 16.Corry DB. IL-13 in allergy: home at last. Curr Opin Immunol. 1999;11:610–614. doi: 10.1016/s0952-7915(99)00025-4. [DOI] [PubMed] [Google Scholar]
- 17.Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corry DB, Irvin CG. Promise and pitfalls in animal-based asthma research: building a better mousetrap. Immunol Res. 2006;35:279–294. doi: 10.1385/IR:35:3:279. [DOI] [PubMed] [Google Scholar]
- 19.Corry DB, Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am J Respir Med. 2002;1:185–193. doi: 10.1007/BF03256608. [DOI] [PubMed] [Google Scholar]
- 20.Fahy JV. Goblet cell and mucin gene abnormalities in asthma. Chest. 2002;122(6 Suppl):320S–326S. doi: 10.1378/chest.122.6_suppl.320s. [DOI] [PubMed] [Google Scholar]
- 21.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–5911. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 22.Pepys J, Wells ID, D’Souza MF, Greenberg M. Clinical and immunological responses to enzymes of Bacillus subtilis in factory workers and consumers. Clin Allergy. 1973;3:143–160. doi: 10.1111/j.1365-2222.1973.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 23.Gailhofer G, Wilders-Truschnig M, Smolle J, Ludvan M. Asthma caused by bromelain: an occupational allergy. Clin Allergy. 1988;18:445–450. doi: 10.1111/j.1365-2222.1988.tb02894.x. [DOI] [PubMed] [Google Scholar]
- 24.Milne J, Brand S. Occupational asthma after inhalation of dust of the proteolytic enzyme, papain. Br J Ind Med. 1975;32:302–307. doi: 10.1136/oem.32.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter PC, Yang T, Luong A, Delclos GL, Abramson SL, Kheradmand F, Corry DB. Proteinases as molecular adjuvants in allergic airway disease. Biochim Biophys Acta. 2011;1810:1059–1065. doi: 10.1016/j.bbagen.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gay NJ, Keith FJ. Regulation of translation and proteolysis during the development of embryonic dorso-ventral polarity in Drosophila: homology of Easter proteinase with Limulus proclotting enzyme and translational activation of Toll receptor synthesis. Biochim Biophys Acta. 1992;1132:290–296. doi: 10.1016/0167-4781(92)90163-t. [DOI] [PubMed] [Google Scholar]
- 27.Arnot CJ, Gay NJ, Gangloff M. Molecular mechanism that induces activation of Spätzle, the ligand for the Drosophila Toll receptor. J Biol Chem. 2010;285:19502–19509. doi: 10.1074/jbc.M109.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- 29.Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart J-M. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- 30.Casanova JL, Abel L, Quintana-Murci L. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol. 2011;29:447–491. doi: 10.1146/annurev-immunol-030409-101335. [DOI] [PubMed] [Google Scholar]
- 31.Millien VO, Lu W, Shaw J, Yuan X, Mak G, Roberts L, Song LZ, Knight JM, Creighton CJ, Luong A, et al. Cleavage of fibrinogen by proteinases elicits allergic responses through Toll-like receptor 4. Science. 2013;341:792–796. doi: 10.1126/science.1240342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Józefowski S, Yang Z, Marcinkiewicz J, Kobzik L. Scavenger receptors and β-glucan receptors participate in the recognition of yeasts by murine macrophages. Inflamm Res. 2012;61:113–126. doi: 10.1007/s00011-011-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopera D, Aristizabal BH, Restrepo A, Cano LE, González A. Lysozyme plays a dual role against the dimorphic fungus Paracoccidioides brasiliensis. Rev Inst Med Trop Sao Paulo. 2008;50:169–175. doi: 10.1590/s0036-46652008000300008. [DOI] [PubMed] [Google Scholar]
- 34.Tomee JF, Hiemstra PS, Heinzel-Wieland R, Kauffman HF. Antileukoprotease: an endogenous protein in the innate mucosal defense against fungi. J Infect Dis. 1997;176:740–747. doi: 10.1086/514098. [DOI] [PubMed] [Google Scholar]
- 35.Woods CM, Hooper DN, Ooi EH, Tan LW, Carney AS. Human lysozyme has fungicidal activity against nasal fungi. Am J Rhinol Allergy. 2011;25:236–240. doi: 10.2500/ajra.2011.25.3631. [DOI] [PubMed] [Google Scholar]
- 36.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 37.de Boer JD, Majoor CJ, van ’t Veer C, Bel EH, van der Poll T. Asthma and coagulation. Blood. 2012;119:3236–3244. doi: 10.1182/blood-2011-11-391532. [DOI] [PubMed] [Google Scholar]
- 38.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. J Allergy Clin Immunol. 2012;129:191–198.e4. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 39.Wakeham MK, Van Bergen AH, Torero LE, Akhter J. Long-term treatment of plastic bronchitis with aerosolized tissue plasminogen activator in a Fontan patient. Pediatr Crit Care Med. 2005;6:76–78. doi: 10.1097/01.PCC.0000149320.06424.1D. [DOI] [PubMed] [Google Scholar]
- 40.Do TB, Chu JM, Berdjis F, Anas NG. Fontan patient with plastic bronchitis treated successfully using aerosolized tissue plasminogen activator: a case report and review of the literature. Pediatr Cardiol. 2009;30:352–355. doi: 10.1007/s00246-008-9312-2. [DOI] [PubMed] [Google Scholar]
- 41.Heath L, Ling S, Racz J, Mane G, Schmidt L, Myers JL, Tsai WC, Caruthers RL, Hirsch JC, Stringer KA. Prospective, longitudinal study of plastic bronchitis cast pathology and responsiveness to tissue plasminogen activator. Pediatr Cardiol. 2011;32:1182–1189. doi: 10.1007/s00246-011-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brims FJ, Chauhan AJ, Higgins B, Shute JK. Coagulation factors in the airways in moderate and severe asthma and the effect of inhaled steroids. Thorax. 2009;64:1037–1043. doi: 10.1136/thx.2009.114439. [DOI] [PubMed] [Google Scholar]
- 43.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter PC, Roberts L, Fields A, Knight M, Qian Y, Declos GL, Han S, Kheradmand F, Corry DB. Necessary and sufficient role for T helper cells to prevent fungal dissemination in allergic lung disease. Infect Immun. 2011;79:4459–4471. doi: 10.1128/IAI.05209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mak G, Porter PC, Bandi V, Kheradmand F, Corry DB. Tracheobronchial mycosis in a retrospective case-series study of five status asthmaticus patients. Clin Immunol. 2013;146:77–83. doi: 10.1016/j.clim.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luong A, Davis LS, Marple BF. Peripheral blood mononuclear cells from allergic fungal rhinosinusitis adults express a Th2 cytokine response to fungal antigens. Am J Rhinol Allergy. 2009;23:281–287. doi: 10.2500/ajra.2009.23.3311. [DOI] [PubMed] [Google Scholar]
- 47.Denning DW, O’Driscoll BR, Powell G, Chew F, Atherton GT, Vyas A, Miles J, Morris J, Niven RM. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: the Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–18. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 48.Chishimba L, Niven RM, Cooley J, Denning DW. Voriconazole and posaconazole improve asthma severity in allergic bronchopulmonary aspergillosis and severe asthma with fungal sensitization. J Asthma. 2012;49:423–433. doi: 10.3109/02770903.2012.662568. [DOI] [PubMed] [Google Scholar]
- 49.Thanasumpun T, Batra PS. Oral antifungal therapy for chronic rhinosinusitis and its subtypes: a systematic review. Int Forum Allergy Rhinol. 2011;1:382–389. doi: 10.1002/alr.20088. [DOI] [PubMed] [Google Scholar]
- 50.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, Harris JM, Scheerens H, Wu LC, Su Z, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011;365:1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 51.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, Wang L, Kirkesseli S, Rocklin R, Bock B, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/NEJMoa1304048. [DOI] [PubMed] [Google Scholar]
- 52.Lamhamedi-Cherradi S-E, Martin RE, Ito T, Kheradmand F, Corry DB, Liu Y-J, Moyle M. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]