Abstract
BMP and Wnt signaling pathways play a crucial role in organogenesis, including tooth development. Despite extensive studies, the exact functions, as well as if and how these two pathways act coordinately in regulating early tooth development, remain elusive. In this study, we dissected regulatory functions of BMP and Wnt pathways in early tooth development using a transgenic noggin (Nog) overexpression model (K14Cre;pNog). It exhibits early arrested tooth development, accompanied by reduced cell proliferation and loss of odontogenic fate marker Pitx2 expression in the dental epithelium. We demonstrated that overexpression of Nog disrupted BMP non-canonical activity, which led to a dramatic reduction of cell proliferation rate but did not affect Pitx2 expression. We further identified a novel function of Nog by inhibiting Wnt/β-catenin signaling, causing loss of Pitx2 expression. Co-immunoprecipitation and TOPflash assays revealed direct binding of Nog to Wnts to functionally prevent Wnt/β-catenin signaling. In situ PLA and immunohistochemistry on Nog mutants confirmed in vivo interaction between endogenous Nog and Wnts and modulation of Wnt signaling by Nog in tooth germs. Genetic rescue experiments presented evidence that both BMP and Wnt signaling pathways contribute to cell proliferation regulation in the dental epithelium, with Wnt signaling also controlling the odontogenic fate. Reactivation of both BMP and Wnt signaling pathways, but not of only one of them, rescued tooth developmental defects in K14Cre;pNog mice, in which Wnt signaling can be substituted by transgenic activation of Pitx2. Our results reveal the orchestration of non-canonical BMP and Wnt/β-catenin signaling pathways in the regulation of early tooth development.
Keywords: BMP, Wnt, Noggin, Tooth, Development, Mouse
Summary: The direct binding of Noggin to Wnt modulates non-canonical BMP and Wnt/β-catenin signalling to regulate cell proliferation and cell fate during early tooth development.
INTRODUCTION
In mice, tooth development begins at embryonic day (E) 11.5 as a local thickening of the dental epithelium to form the dental placode (the lamina stage), which subsequently proliferates and invaginates into the subjacent mesenchyme, forming the epithelial bud at E12.5 and E13.5 (the bud stage). At E14.5, tooth development progresses to the cap stage, with enamel knot formation in the dental epithelium to control the patterning of tooth cusps. The cap stage is followed by the bell stage, which begins around E16.5, when ameloblasts and odontoblasts start to differentiate. All these developmental processes are under elaborate control of multiple families of signaling molecules, including bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), Shh and Wnt proteins (Tummers and Thesleff, 2009).
The importance of canonical Wnt signaling in odontogenesis has been well illustrated (Liu and Millar, 2010), as evidenced by the expression of multiple Wnt ligands predominantly in the dental epithelium. It is also highlighted by the arrest of tooth development at the bud stage in mice carrying either epithelial or mesenchymal inactivation of Catnb (Ctnnb1 – Mouse Genome Informatics), the gene encoding β-catenin, the master transducer of the canonical Wnt signaling (Sarkar and Sharpe, 1999; Liu et al., 2008; Chen et al., 2009). On the other hand, constitutive activation of Wnt/β-catenin signaling in the oral epithelium induces ectopic tooth formation (Järvinen et al., 2006; Liu et al., 2008; Wang et al., 2009), suggesting that Wnt is probably one of the earliest signaling molecules that regulate initiation of tooth development. However, the exact biological function of Wnt signaling in early dental epithelium development remains unknown.
BMP signaling is transduced into the cell via a heteromeric receptor complex of a type II transmembrane serine-threonine kinase receptor with each of the three type I receptors (BMPR-IA/Bmpr1a – Mouse Genome Informatics, BMPR-IB/Bmpr1b – Mouse Genome Informatics and Alk2/Acvr1 – Mouse Genome Informatics), whereas Bmpr1a and Bmpr1b have been shown to be expressed and have limited redundant function in the developing tooth (Li et al., 2011). Binding of ligand to the receptor elicits a Smad-dependent canonical pathway and/or Smad-independent mitogen-activated protein kinase (MAPK) pathways, including p38, ERK1/2 and JNK, known as non-canonical pathways (Sieber et al., 2009). Several Bmp genes are expressed in either epithelial or mesenchymal components of developing tooth germs, and BMP activity has been implicated in multiple steps of odontogenesis (Vainio et al., 1993; Chen et al., 1996; Zhang et al., 2000; Tummers and Thesleff, 2009; Jia et al., 2013). Whereas Bmpr1b null mice develop normal teeth (Yi et al., 2000), inactivation of Bmpr1a in either epithelium or mesenchyme or in both results in arrest of tooth development at the bud or early cap stages (Andl et al., 2004; Liu et al., 2005; Li et al., 2011). However, it remains to be identified which BMP-mediated signaling pathways are utilized and what biological functions these pathways exert during early tooth development.
In this study, we used a conditional transgenic Nog overexpression mouse model to dissect the biological function of BMP and Wnt signaling in early odontogenesis. We present evidence that transgenic Nog expression disrupts BMP-mediated non-canonical signaling pathways in the dental epithelium, leading to inhibition of cell proliferation. Meanwhile, overexpression of Nog also attenuates Wnt/β-catenin activity in the dental epithelium by directly binding to Wnt ligands, resulting in significantly reduced expression of Pitx2, an indication of loss of odontogenic fate. Together with genetic rescue results, our studies reveal an orchestration of non-canonical BMP and Wnt/β-catenin signaling pathways in regulating early tooth development.
RESULTS
Mesenchymal odontogenic program is unaltered in early tooth germs of K14Cre;pNog mice
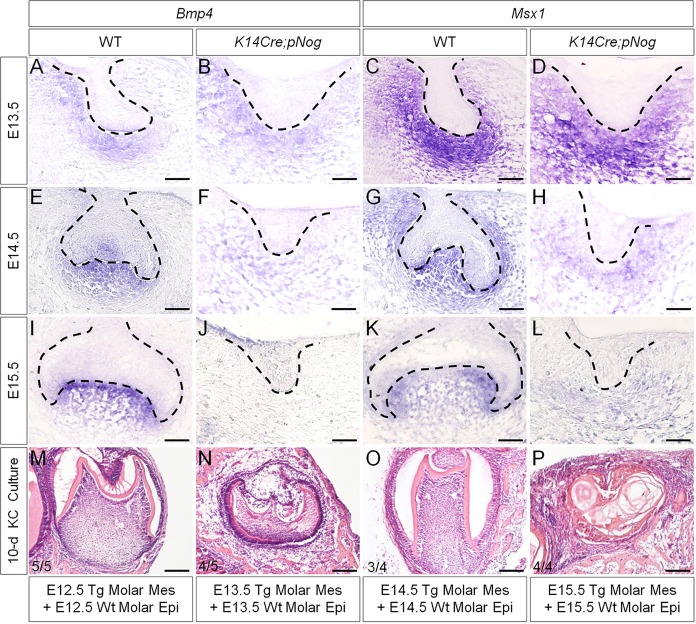
We reported previously that activation of a conditional transgenic Nog allele (pMes-Nog) by K14Cre leads to arrested tooth development at the lamina/early bud stage, accompanied by inhibition of cyclin D1 expression and significantly reduced cell proliferation rate in the dental epithelium (Wang et al., 2012). Meanwhile, the expression of Pitx2, a molecular marker for the dental epithelium (Mucchielli et al., 1997; St. Amand et al., 2000), is also downregulated, indicating a deviation of the odontogenic fate. By contrast, the mesenchymal odontogenic markers, including Bmp4 and Msx1, showed comparable expression levels in the binary transgenic mice (named as K14Cre;pNog) at E12.5, when the tooth phenotype became discernible (Wang et al., 2012). To determine whether the odontogenic program in the dental mesenchyme was affected and how long the program could be sustained in K14Cre;pNog mice, we examined Bmp4 and Msx1 expression. This was combined with a subrenal culture of tissue recombinants, consisting of dental mesenchyme of K14Cre;pNog mice with the same stage wild-type dental epithelium. In the transgenic animals, the expression levels of Bmp4 and Msx1 appeared unaltered at E13.5, became downregulated at E14.5 and were barely detectable at E15.5 compared with controls (Fig. 1A-L). Consistent with the expression of these odontogenic markers, grafts consisting of dental mesenchyme of E12.5-E14.5 K14Cre;pNog mice and wild-type dental epithelium did form teeth (E12.5, 5/5; E13.5, 4/5; E14.5, 3/4), whereas E15.5 transgenic dental mesenchyme failed to support tooth formation (0/4) (Fig. 1M-P). These observations indicate that, despite arrest of tooth development at or before E12.5, the odontogenic program in the dental mesenchyme of K14Cre;pNog mice does not deviate until E14.5.
Fig. 1.
Loss of Bmp4 and Msx1 expression coincides with the loss of odontogenic capability of dental mesenchyme in K14Cre;pNog mice. (A-L) In situ hybridization of Bmp4 (A,B,E,F,I,J) and Msx1 (C,D,G,H,K,L) in controls (WT) and in K14Cre;pNog tooth at E13.5 (A-D), E14.5 (E-H) and E15.5 (I-L). Scale bars: 50 µm. (M-P) Tooth formation in tissue recombinants of dental mesenchyme from K14Cre;pNog mice and wild-type dental epithelium at E12.5 (M), E13.5 (N) and E14.5 (O), but not in recombinants of E15.5 dental tissues (P) after 10-day kidney capsule culture. Scale bars: 100 µm.
Overexpression of Nog specifically disrupts BMP non-canonical signaling activities in the dental epithelium
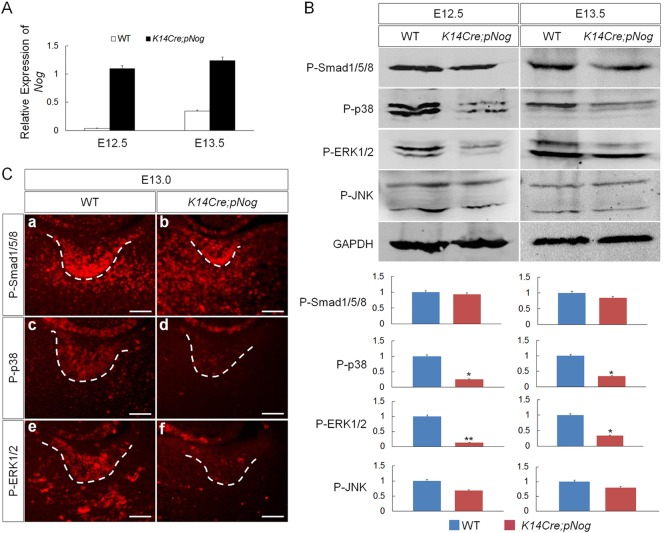
To determine the level of Nog overexpression in the transgenic tooth germs, we performed qRT-PCR on control and K14Cre;pNog tooth germs at E12.5 and E13.5. The results showed that the relative Nog levels in transgenic tooth were rather constant at both stages, but the endogenous Nog level in controls was much higher at E13.5 than E12.5 (Fig. 2A). To determine which BMP-mediated pathway(s) is implicated in the early arrest of tooth development in K14Cre;pNog mice, we examined the activities of BMP canonical and non-canonical signaling pathways, including p38, ERK1/2 and JNK, at E12.5 and E13.5 molar germs by western blot analysis. Similar to previously reported results (Wang et al., 2012), the levels of P-Smad1/5/8 were comparable between transgenic teeth and wild-type controls (Fig. 2B). However, despite an unaltered level of P-JNK, significantly reduced levels of P-p38 and P-ERK1/2 were found in K14Cre;pNog tooth germs at both stages examined, as compared with controls (Fig. 2B). Immunohistochemistry confirmed downregulation of P-p38 and P-ERK1/2, particularly in the dental epithelium (Fig. 2C), suggesting that overexpressed Nog primarily disrupts BMP-mediated non-canonical pathways.
Fig. 2.
Inhibition of non-canonical BMP signaling in K14Cre;pNog tooth. (A) qRT-PCR shows consistently elevated Nog expression levels in K14Cre;pNog tooth germs at E12.5 and E13.5 compared with controls (WT). (B) Representative western blot images and band quantification show significant reduction of P-p38 and P-ERK1/2 but not of P-Smad1/5/8 and P-JNK in K14Cre;pNog tooth germs at E12.5 and E13.5, as compared with controls. *P<0.05, **P<0.01; n=3. (C) Immunofluorescence shows specific inhibition of P-p38 and P-ERK1/2 but not of P-Smad1/5/8 in the epithelium of K14Cre;pNog tooth germs at E13.0. Scale bars: 50 µm.
As either TGFβ or FGF signaling also activate ERK1/2 and p38 pathways, the transgenic Nog might affect FGF and/or TGFβ activities directly or indirectly, attributing to the reduced levels of P-p38 and P-ERK1/2. To exclude this possibility, we examined the expression of ligands (Tgfb1, Tgfb2, Fgf8 and Fgf9) and receptors (Fgfr1 and Fgfr2) of TGFβ or FGF signaling that are known to be expressed in the early developing tooth in E12.5 and E13.5 K14Cre;pNog molar germs (Chai et al., 1994; Pacheco et al., 2008; Liu et al., 2013). The expression levels of Tgfb1 and Tgfb2 (supplementary material Fig. S1A), Fgf8 and Fgf9 (supplementary material Fig. S2A-H), as well as Fgfr1 and Fgfr2 (supplementary material Fig. S2I-L), showed no change in K14Cre;pNog molars compared with controls. We next examined the expression of P-Smad2/3 and the ETS-related factor gene Etv4 that are downstream readouts for TGFβ and FGF signaling, respectively (Xu et al., 2003; Porntaveetus et al., 2011). As expected, the expression of P-Smad2/3 and Etv4 appeared comparable in K14Cre;pNog molar germs and controls (supplementary material Fig. S1B and Fig. S2M,N). All these results demonstrate unaltered activity of TGFβ or FGF signaling in E12.5 and E13.5 K14Cre;pNog molar germs, indicating that inhibition of BMP activity by excess Nog is primarily responsible for the reduced activities of p38 and ERK1/2.
BMP non-canonical pathways regulate cell proliferation in the dental epithelium
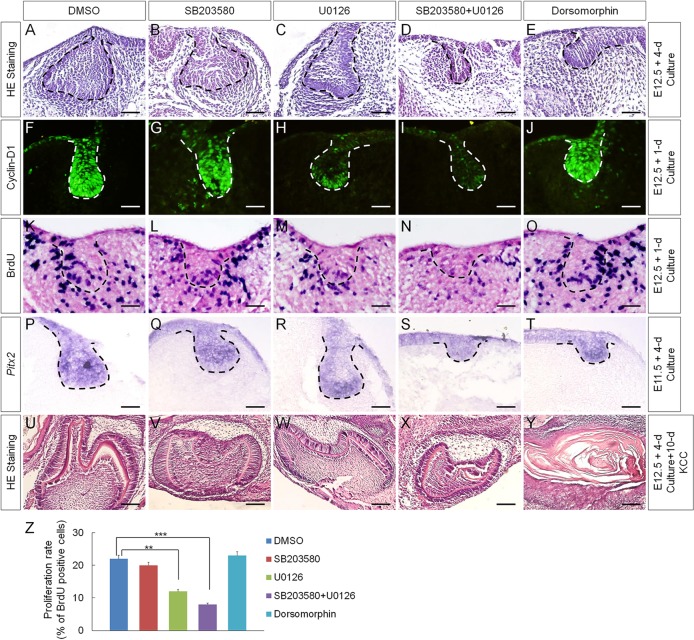
To determine whether the inhibited BMP non-canonical signaling pathways are responsible for the inhibition of cyclin D1 and Pitx2 expression as well as for the reduced cell proliferation rate in the dental epithelium of K14Cre;pNog mice, we cultured E12.5 wild-type molar germs in the presence of small inhibitor molecules. The specificity and efficacy of these inhibitors on P-MAPKAPK-2 (the downstream mediator of P-p38), P-ERK1/2 and P-Smad1/5/8 in organ-cultured tooth germs were optimized and are shown in supplementary material Fig. S3. We found that inhibition of p38 alone by SB203580 affected neither tooth development nor the expression of cyclin D1 and Pitx2 or cell proliferation rate in the dental epithelium, compared with DMSO-treated controls (Fig. 3B,G,L,Q,Z). Whereas inhibition of ERK1/2 pathway by U0126 alone caused dramatic reduction in cyclin D1 expression and statistically significantly reduced rate of cell proliferation in the dental epithelium, Pitx2 expression was not affected, and tooth germs developed to the early cap stage, comparable to DMSO-treated controls (Fig. 3C,H,M,R,Z). However, despite unaltered Pitx2 expression, inhibition of both p38 and ERK1/2 pathways resulted in arrested tooth development at the bud stage, accompanied by almost completely abolished cyclin D1 expression and much reduced rate of cell proliferation, resembling the phenotype observed in K14Cre;pNog mice (Fig. 3D,I,N,S,Z). Interestingly, in the presence of dorsomorphin, a selective type I BMP receptor inhibitor that inhibits BMP-induced phosphorylation of Smad1/5/8 (Yu et al., 2008), tooth development was arrested at the bud stage, but cyclin D1 and Pitx2 expression and cell proliferation in the dental epithelium were not affected (Fig. 3E,J,O,T,Z), suggesting that Smad1/5/8-mediated signaling functions primarily in the dental mesenchyme. These results suggest that inhibition of p38 and ERK1/2 pathways are responsible for the downregulation of cyclin D1 expression and the reduced cell proliferation rate, but not for the inhibition of Pitx2 expression, in the K14Cre;pNog tooth germs.
Fig. 3.
Inhibition of BMP-mediated signaling pathways in early tooth germ by small inhibitor molecules. (A-E) Histological examination of E12.5 tooth germs after 4 days in organ culture in the presence of (A) DMSO, (B) SB203580, (C) U0126, (D) SB203580 and U0126 and (E) dorsomorphin. (F-O) Immunofluorescence of cyclin D1 (F-J) and BrdU labeling (K-O) of E12.5 tooth germs after 1 day in organ culture in the presence of inhibitors as indicated. (P-T) In situ hybridization of Pitx2 in E11.5 molar germs after 4 days in organ culture in the presence of inhibitors as indicated. Scale bars in A-T: 50 µm. (U-Y) Tooth-forming capability of E12.5 molar germs that were cultured for 4 days in vitro with each individual inhibitor prior to being subjected to subrenal culture for 10 days. Scale bars: 250 µm. (Z) Statistic comparison of BrdU-labeled cells in the dental epithelium after treatment with individual inhibitors. Error bars show mean±s.d.; **P<0.01, ***P<0.001.
As Pitx2 expression was not affected in SB203580 and U0126 treated tooth germs, we reasoned that inhibition of p38 and/or ERK1/2 pathways would not deviate the odontogenic program. To test this hypothesis, we grafted tooth germs after 4-day treatment in organ culture with either SB203580 or U0126 alone, or with both. Well-differentiated tooth structures were found in all samples after 10-day subrenal culture (Fig. 3U-X; n=5 each). By contrast, dorsomorphin-treated grafts failed to form tooth structures (Fig. 3Y; n=6), consistent with a marked downregulation of Msx1 expression in the mesenchyme of dorsomorphin-treated tooth germs (Yang et al., 2014).
TGFβ signaling is primarily responsible for Smad1/5/8 activation in the dental epithelium
As P-Smad1/5/8 are widely recognized as BMP signaling mediators, the unaffected P-Smad1/5/8 levels in the dental epithelium of K14Cre;pNog tooth germs were puzzling. The fact that TGFβ signaling can activate Smad1/5 in a number of cell types (Moustakas and Heldin, 2009), and that TGFβ signaling molecules and activities are not affected in K14Cre;pNog tooth (supplementary material Fig. S1), prompted us to test whether TGFβ signaling is responsible for Smad1/5/8 activation in the dental epithelium. We cultured E12.5 tooth germs in the presence of SB525334 (a specific inhibitor of TGFβ type I receptor) or Tgfb1 and examined P-Smad1/5/8. We found that P-Smad1/5/8 levels were dramatically reduced in the epithelium of E12.5 molar germ after 24-h culture with SB525334, and significantly increased in the presence of Tgfb1, as compared with controls (supplementary material Fig. S4A,B,G), suggesting that TGFβ signaling accounts largely for Smad1/5/8 activation in the dental epithelium. In line with this notion is the fact that P-Smad1/5/8 levels were neither affected in the dental epithelium of tooth germs treated with dorsomorphin nor in the dental epithelium of Msx1 mutants, in which Bmp4 expression in the dental mesenchyme and Bmp2 expression in the dental epithelium were diminished (supplementary material Fig. S4C-F; Zhang et al., 2000).
Suppression of Wnt/β-catenin signaling activity is responsible for Pitx2 downregulation in the K14Cre;pNog dental epithelium
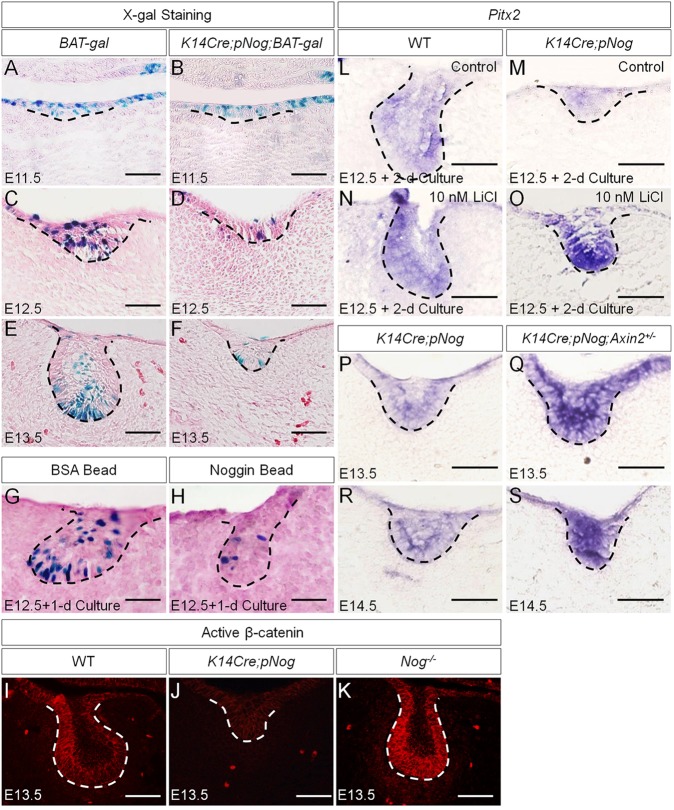
The fact that the arrested tooth development in K14Cre;pNog embryos was associated with a dramatic downregulation of Pitx2, but that the inhibition of p38 and ERK1/2 pathways failed to alter Pitx2 expression, suggested an additional role for Nog. Nog can also bind to Activin (Bayramov et al., 2011), which is essential for early tooth development beyond the bud stage by inducing follistatin expression in the dental epithelium (Ferguson et al., 1998). To test whether overexpressed Nog disrupts Activin signaling, we examined follistatin expression in K14Cre;pNog tooth germs at E12.5 and E13.5, and found comparable follistatin expression in transgenic and control molars (supplementary material Fig. S5), indicating that Activin activity was not affected in K14Cre;pNog mice. Pitx2 is initially induced by FGF8 in the dental epithelium (St. Amand et al., 2000). The observations that Fgf8 and Fgf9 expressions were not affected in the dental epithelium of K14Cre;pNog mice at E12.5, when Pitx2 expression became downregulated (supplementary material Fig. S2; Wang et al., 2012), indicated the existence of an alternative regulatory pathway. As the Wnt/β-catenin pathway also induces Pitx2 expression (Kioussi et al., 2002), we tested whether Pitx2 downregulation is the consequence of suppressed Wnt/β-catenin signaling activity by breeding BAT-gal reporter mice to K14Cre;pNog mice. Although BAT-gal activity was not affected in the epithelium of K14Cre;pNog;BAT-gal tooth germs at E11.5, it became dramatically inhibited at E12.5 and E13.5 compared with controls (Fig. 4A-F). Organ culture experiments further confirmed inhibition of BAT-gal activity by exogenously applied Nog in explanted molar germs (Fig. 4G,H). Consistent with these observations is the almost completely abolished active β-catenin in the dental epithelium of K14Cre;pNog tooth germs (Fig. 4I,J). Interestingly, in Nog mutants (Nog−/−), active β-catenin was dramatically increased in the dental epithelium, indicating that endogenous Nog, which is expressed in the dental epithelium (Hu et al., 2012), indeed modulates Wnt signaling activity in the developing tooth (Fig. 4K). To determine whether the inhibition of Wnt/β-catenin signaling activity is responsible for the downregulation of Pitx2, we examined Pitx2 expression in K14Cre;pNog molar germs after 2-day organ culture in the presence of LiCl and in tooth germs of K14Cre;pNog mice carrying an Axin2 null allele, a negative regulator of Wnt/β-catenin signaling that is expressed in the developing tooth (Lohi et al., 2010). As expected, Pitx2 expression was resumed in both cases, but tooth phenotype was not rescued, remaining at the early bud stage (Fig. 4L-S).
Fig. 4.
Attenuated Wnt/β-catenin signaling leads to downregulation of Pitx2 expression in K14Cre;pNog dental epithelium. (A-F) X-gal staining of BAT-gal (A,C,E) and K14Cre;pNog;BAT-gal (B,D,F) tooth germs at E11.5 (A,B), E12.5 (C,D) and E13.5 (E,F). (G,H) X-gal staining of E12.5 BAT-gal molar germs after 1 day organ culture with BSA beads (G) or Nog beads (H). (I-K) Immunofluorescence of active β-catenin in wild type (I), K14Cre,pNog (J) and Nog mutant (K) tooth germs at E13.5. (L-S) In situ hybridization of Pitx2 in E12.5 wild-type molars (L,N) and E12.5 K14Cre;pNog (M,O) molars cultured in the presence or absence of 10 nM LiCl for 2 days. (P-S) In situ hybridization of Pitx2 in K14Cre;pNog (P,R) and K14Cre;pNog;Axin2+/−(Q,S) molars at E13.5 (P,Q) and E14.5 (R,S). Scale bars: 50 µm.
Nog physically interacts with and inhibits Wnt/β-catenin signaling activity
To reveal the mechanism underlying the inhibition of Wnt/β-catenin signaling activity by Nog, we first examined whether inhibition of BMP activity would attenuate Wnt/β-catenin signaling by culturing E12.5 BAT-gal molar germs in the presence of various inhibitors. Neither inhibition of the Smad-dependent pathway, of p38, or of ERK1/2 alone, nor inhibition of these three pathways simultaneously, decreased Wnt/β-catenin activity in the dental epithelium (supplementary material Fig. S6). This indicated that inhibition of Wnt/β-catenin activity is not the secondary effect of downregulated BMP activity. We next surveyed the expression of Wnt ligands that are known to be expressed in the developing tooth germs, including Wnt4, 5a, 6, 7b, 10a and 10b, but found unchanged expression levels of all Wnt ligands in K14Cre;pNog tooth germs at E12.5 and E13.5 compared with controls (supplementary material Fig. S7).
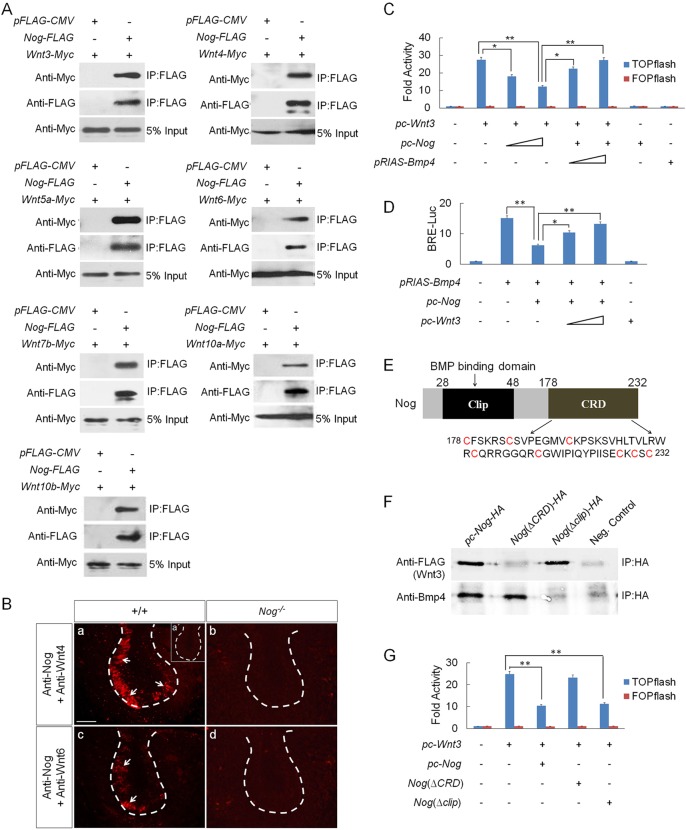
Although Nog is well known for its function as a BMP antagonist, it has been reported that, in Xenopus, Nog was able to physically interact with Wnts at high concentration (Bayramov et al., 2011). To determine whether this is also true in mammals, we conducted co-immunoprecipitation (Co-IP) experiments by co-transfecting into HEK 293T cells the expression vector of FLAG-tagged mouse Nog and each of Myc-tagged Wnt genes that are expressed in the developing tooth. Western blot revealed that Nog formed a complex with each individual Wnt tested (Fig. 5A). To determine whether such physical interaction exists in vivo, we performed an in situ proximity ligation assay (PLA), a technology capable of detecting protein-protein interaction with high specificity and sensitivity in vivo (Soderberg et al., 2008). We chose two representative canonical Wnts, Wnt4 and Wnt6, which are highly expressed in the dental epithelium (Sarkar and Sharpe, 1999). The strong PLA signals in the dental epithelium of E13.5 wild-type molar, compared with the complete absence of signals in Nog−/− tooth germs, indicate a direct interaction of endogenous Nog and Wnts under physiological condition (Fig. 5B).
Fig. 5.
Physical interaction between Nog and Wnts leads to inhibition of Wnt canonical signaling. (A) Co-IP assays show physical interaction between Nog and Wnt3, 4, 5a, 6, 7b, 10a and 10b. (B) In situ PLA shows the bindings of endogenous Nog with Wnt4 (a) and Wnt6 (c) in the dental epithelium of E13.5 wild-type tooth germs, but a lack of signals in Nog−/− tooth germs (b,d). Insert in (a) is a negative control of PLA. White arrows indicate PLA signals. Scale bar: 50 µm. (C) TOPflash reporter assay demonstrates antagonistic effect of Nog on Wnt signaling. TOPflash or FOPflash (0.2 µg), pRL-SV40 (0.02 µg), pc-Wnt3 (0.005 µg) vectors with ascending concentrations of pc-Nog (0.1-0.4 µg) and pRIAS-Bmp4 (0.05-0.1 µg) vectors were co-transfected into HEK 293T and TOPflash activity was measured. (D) BRE-luciferase reporter assay shows attenuation of Nog inhibition on BMP signaling by Wnt3. pGL3-BRE-Luc (0.002 µg), pRL-SV40 (0.02 µg), pTRIS-Bmp4 (0.2 µg) and pc-Nog (0.01 µg) vectors with ascending concentrations of pc-Wnt3 (0.1-0.5 µg) were co-transfected into HEK 293T and luciferase activity was measured. (E) Schematic representation of Nog protein shows the clip domain and CRD domain. The amino acid sequence of CRD is also shown, with cysteine residues highlighted in red. (F) Co-IP assays show physical interactions between truncated Nog and Wnt3 or Bmp4. (G) TOPflash assay shows the effect of truncated Nog on Wnt3 induction of TOPflash activity. TOPflash or FOPflash, pRL-SV40, pc-Wnt3 vectors with pc-Nog, Nog(ΔCRD) or Nog(Δclip) (0.2 µg) were co-transfected into HEK 293T and TOPflash activity was measured. *P<0.05, **P<0.01; n=6.
We next tested whether binding of Nog to Wnts could functionally prevent Wnt signaling, by conducting a TOPflash assay in cell culture with FOPflash as negative control. Our results showed that inclusion of Nog expression vector attenuated Wnt3 induction of TOPflash activity in a dose-dependent manner. The specific inhibitory effect of Nog on Wnt signaling was confirmed by inclusion of Bmp4 expression vector that relieved the inhibitory effect by Nog (Fig. 5C). Conversely, the ability of Wnt3 to attenuate the antagonistic effect of Nog on Bmp4 in a reporter assay, using pGL3-BRE-Luc construct that harbors BMP-response elements (BRE) from the Id1 promoter (Korchynskyi and ten Dijke, 2002), further supported this conclusion (Fig. 5D).
The Nog protein contains two conserved domains, the N-terminal clip domain (residues 28-48) that mediates the binding of Nog to BMPs, and the C-terminal cysteine-rich domain (CRD, residues 178-232), the function of which is currently unknown (Fig. 5E; Groppe et al., 2002). A similar CRD was also found in Wnt receptors, including Frizzled and Lrp5/6, as well as the Wnt antagonists sFRPs to mediate their bindings to Wnts (Nathan and Tzahor, 2009; Ke et al., 2013). We sought to determine whether Nog binding to Wnts is mediated by the CRD and whether it is independent of the clip domain, by generating Nog(ΔCRD) and Nog(Δclip) expression constructs that express truncated noggin proteins lacking the CRD or the clip domain, respectively. Co-IP assays showed that Nog(ΔCRD) bound to Bmp4 but not to Wnt3, and that Nog(Δclip) bound to Wnt3 but not to Bmp4 (Fig. 5F). The importance of the CRD in mediating the inhibitory effect of Nog on Wnt signaling was further revealed by TOPflash assays, evidenced by the ability of Nog(Δclip) but not of Nog(ΔCRD) to attenuate Wnt3-induced TOPflash activity (Fig. 5G).
Genetic activation of Wnt and BMP signaling rescues tooth developmental defect in K14Cre;pNog mice
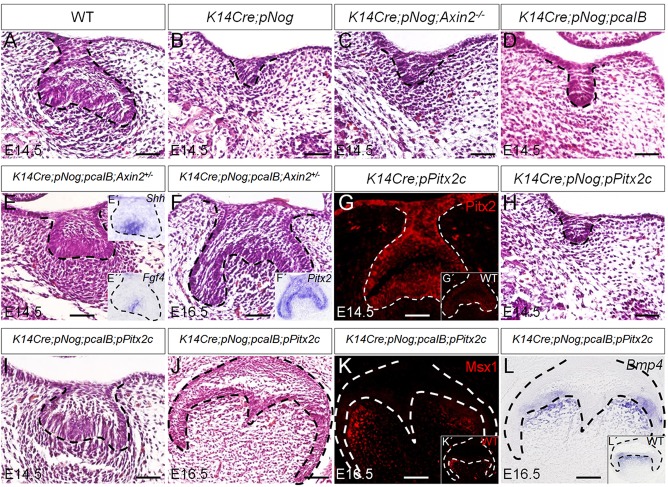
To test whether inhibition of either or both Wnt and BMP signaling pathways is responsible for the tooth phenotype observed in K14Cre;pNog mice, we performed genetic rescue experiments by compounding K14Cre;pNog with either Axin2 null allele or a conditional, constitutively active Bmpr1b transgenic allele (pcaIB) (Li et al., 2011). K14Cre;pNog mice carrying either homozygous Axin2 null gene or the pcaIB allele exhibited identical tooth phenotype as that seen in K14Cre;pNog embryo (Fig. 6B-D). By contrast, mice carrying compounded K14Cre;pNog;pcaIB;Axin2+/− alleles presented rescued tooth development (Fig. 6E,F). These rescued teeth formed an enamel knot at the E14.5 cap stage, assessed by Shh and Fgf4 expression (Fig. 6E′,E″), and developed into a bell-like structure at E16.5 (Fig. 6F). The expression of Pitx2 in the dental epithelium of rescued teeth indicates its retained odontogenic fate (Fig. 6F′). To determine the functional importance of Pitx2 in mediating Wnt/β-catenin signaling pathway in tooth development, we generated a conditional transgenic mouse line (pPitx2c) that expresses Pitx2c (Fig. 6G), one of the three Pitx2 isoforms essential for tooth development (Liu et al., 2003). Whereas K14Cre;pNog mice carrying the pPitx2c allele did not exhibit rescued tooth development (Fig. 6H), compounding the pcaIB allele onto K14Cre;pNog;pPitx2c mice rescued tooth development, evidenced by the formation of the cap-like epithelial structure at E14.5 and the bell-like structure at E16.5 with normal expression levels of Msx1 and Bmp4 (Fig. 6I-L).
Fig. 6.
Genetic rescue of tooth developmental defect in K14Cre;pNog mice by simultaneous activation of Wnt and BMP signaling pathways. (A-F,H-J) H&E staining of molar germs from (A) E14.5 wild type, (B) E14.5 K14Cre;pNog, (C) E14.5 K14Cre;pNog;Axin2−/−, (D) E14.5 K14Cre;pNog;pcaIB, (E) E14.5 K14Cre;pNog;pcaIB;Axin2+/−, (F) E16.5 K14Cre;pNog;pcaIB;Axin2+/−, (H) E14.5 K14Cre;pNog;pPitx2c, (I) E14.5 K14Cre;pNog; pcaIB;pPitx2c and (J) E16.5 K14Cre;pNog;pcaIB;pPitx2c mice. (E′,E″,F′) In situ hybridization of Shh (E′) and Fgf4 (E″) in E14.5 K14Cre;pNog;pcaIB;Axin2+/− molar germs, and (F′) Pitx2 in E16.5 K14Cre;pNog;pcaIB;Axin2+/− molar germ. (G) Immunofluorescence of Pitx2 in E14.5 K14Cre;pPitx2c tooth germ and in control (G′). (K) Immunofluorescence of Msx1 in E16.5 K14Cre;pNog;pcaIB;pPitx2c and in control (K′). (L) In situ hybridization of Bmp4 in E16.5 K14Cre;pNog;pcaIB;pPitx2c and in control (L′). Scale bars: 50 µm.
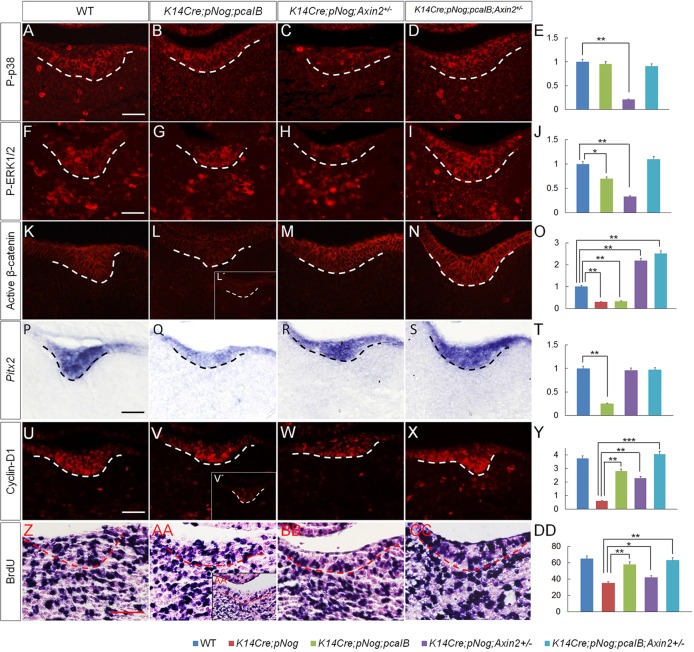
To further dissect the exact function of BMP and Wnt signaling pathways in dental epithelium development, we examined the expression of P-p38, P-ERK1/2, active β-catenin, Pitx2 and cyclin D1, as well as cell proliferation rate in the dental epithelium of K14Cre;pNog mice carrying various transgenic alleles at E12.5. The level of P-p38 was fully restored by the pCaIB allele (Fig. 7A-E). P-ERK1/2 expression was mostly restored by pCaIB, but fully restored by pCaIB and Axin2+/− alleles (Fig. 7F-J). β-catenin was highly activated by Axin2+/− allele, but not by pCaIB allele (Fig. 7K-O). Meanwhile, Pitx2 expression was resumed in K14Cre;pNog;Axin2+/− and K14Cre;pNog;pcaIB;Axin2+/− mice but not in K14Cre;pNog;pCaIB mice (Fig. 7P-T). The level of cyclin D1 was partially restored (Fig. 7V,W) in both K14Cre;pNog;pCaIB and K14Cre;pNog;Axin2+/− mice compared with controls and K14Cre;pNog teeth (Fig. 7U,V′,Y). Accordingly, cell proliferation rate was also partially resumed in mice with each genotype, with K14Cre;pNog;pCaIB mice showing a much significantly resumed rate (Fig. 7Z-DD). In K14Cre;pNog;pcaIB;Axin2+/− mice, however, Pitx2 and cyclin D1 expression and cell proliferation rate were fully resumed to levels comparable to controls (Fig. 7S,T,X,Y,CC,DD). These observations indicate that BMP non-canonical signaling plays a major role in regulating cell proliferation, whereas Wnt/β-catenin signaling functions primarily to control the odontogenic fate in the dental epithelium during early odontogenesis.
Fig. 7.
Rescue of cell fate and proliferation defects in K14Cre;pNog mice by activation of BMP and Wnt signaling pathways. (A-D,F-I,K-N,U-X) Immunofluorescence of P-p38 (A-D), P-ERK1/2 (F-I), active β-catenin (K-N) and cyclin D1 (U-X) in E12.5 tooth germs of control and transgenic mice as indicated. (P-S, Z-CC) In situ hybridization of Pitx2 expression (P-S) and BrdU labeling (Z-CC) in E12.5 tooth germs of control and transgenic mice. (L′,V′,AA′) E12.5 K14Cre;pNog tooth germ for comparison. Scale bars: 50 µm. (E,J,O,T,Y) Qualification and statistical comparison for expressions of P-p38 (E), P-ERK1/2 (J), active β-catenin (O), Pitx2 (T) and cyclin D1 (Y). (DD) Statistical comparison of cell proliferation rate (percentage of BrdU-positive cells) in E12.5 molars of control and transgenic mice. *P<0.05, **P<0.01, ***P<0.001; n=3.
DISCUSSION
Non-canonical BMP signaling functions in early dental epithelium to regulate cell proliferation
Although the key mediators of BMP canonical and non-canonical signaling pathways, including Smad1/5/8, Smad4, p38, ERK1/2 and JNK, are widely activated in the developing tooth (Xu et al., 2003; Cho et al., 2008; Moriguchi et al., 2011; and this study), the biological function of each pathway during odontogenesis remains elusive. Here, we show that Nog overexpression leads to an arrested tooth development at the early bud stage, associated with significantly suppressed cell proliferation and inhibition of p38 and ERK1/2-mediated BMP non-canonical signaling pathways specifically in the dental epithelium. However, the levels of P-Smad1/5/8 in both the dental epithelium and mesenchyme were not affected. In addition, the expression of the dental mesenchymal markers Msx1 and Bmp4, both downstream targets of BMP signaling (Vainio et al., 1993; Chen et al., 1996), appeared unaltered until E14.5, indicating the sustained BMP activity and odontogenic fate in the dental mesenchyme of K14Cre;pNog mice. This could be explained by a potentially limited diffusion of the transgenically expressed Nog into the dental mesenchyme. It has been reported that Nog could be retained at the cell surface by direct binding to cell surface heparin sulfate proteoglycans (HSPGs) without affecting its function as a BMP antagonist (Paine-Saunders et al., 2002). As dental epithelial cells express multiple HSPGs (Bai et al., 1994), these molecules could confine Nog function within the dental epithelium of K14Cre;pNog mice. The ultimate disappearance of the molecular markers and the odontogenic capability in the dental mesenchyme of K14Cre;pNog mice apparently is the consequence of an altered odontogenic program in the epithelium.
Our in vitro organ culture experiments using inhibitors confirm the function of p38 and ERK1/2, but not of P-Smad1/5/8-mediated BMP signaling in the regulation of dental epithelial cell proliferation by controlling cyclin D1 expression (Fig. 3), which is further supported by the findings of the in vivo rescue studies (Fig. 7). Strikingly, despite the presence of abundant P-Smad1/5/8 and Smad4 in the developing tooth germs, the canonical BMP signaling activity, mediated by Smad1/5/8-Smad4 complexes, was not detectable using transgenic alleles harboring the BMP-response element (Monteiro et al., 2008; Yang et al., 2014). In line with this notion is the fact that epithelial inactivation of Smad4 does not affect early tooth development (Xu et al., 2008). The dramatic reduction or increase in P-Smad1/5/8 levels in the dental epithelium of organ-cultured tooth germs in the presence of TGFβ type I receptor inhibitor or Tgfb1, respectively, indicates that TGFβ signaling is responsible for Smad1/5/8 activation in the dental epithelium, consistent with a previous report that TGFβ is able to activate Smad1/5 (Moustakas and Heldin, 2009). TGFβ has been reported to be able to activate Smad1/5 in several cell types, especially in Alk1 (Acvrl1 – Mouse Genome Informatics)-expressing endothelial cells (Goumans et al., 2002). In Alk1 highly expressing cells, Alk1 can be recruited into the TGFβ receptor complex with TGFβ induction and activates Smad1/5 (Goumans et al., 2003). In fact, our preliminary studies identified Alk1 expression in the dental epithelium (G. Yang, G. Yuan and Y.C., unpublished data). Whereas the biological function of the TGFβ-induced activation of Smad1/5/8 in dental epithelium warrants further investigation, this observation explains the unchanged P-Smad1/5/8 level in the dental epithelium of K14Cre;pNog mice. Additionally, TGFβ signaling also inhibits BMP canonical signaling in the dental mesenchyme by limiting Smad4 availability, leading to activation of Msx1 expression by an atypical canonical BMP signaling (Smad4-independent and Smad1/5/8-dependent) pathway, but not by p38- and ERK1/2-mediated non-canonical BMP signaling (Yang et al., 2014).
A novel function of Nog as Wnt signaling antagonist
In K14Cre;pNog tooth germs, the inhibition of cyclin D1 expression and cell proliferation is accompanied by the loss of odontogenic fate in the dental epithelium, as assessed by the loss of Pitx2 expression. This is in contrast to the inhibition of both p38 and ERK1/2 pathways in organ-cultured tooth germs, which did not affect Pitx2 expression and the capability of the tooth germ to form tooth organ after removal of the inhibitors, thus suggesting an additional role for Nog. Pitx2 is a known downstream target of the Wnt/β-catenin signaling pathway (Kioussi et al., 2002). Accordingly, we found that Wnt/β-catenin signaling activity is attenuated significantly in K14Cre;pNog tooth germs as well as in Nog-treated tooth germs in vitro.
BMPs have dual roles in regulating Wnt signaling activity. For example, BMPs can either antagonize Wnt signaling or induce the expression of Wnt ligands and receptors, and activate the Wnt canonical pathway, depending on the type of tissues and cells (Soshnikova et al., 2003; Chesnutt et al., 2004; Liu et al., 2006; Chen et al., 2007; Ille et al., 2007; Lee et al., 2009). In the developing tooth, however, inhibition of Smad-dependent and -independent BMP pathways did not alter BAT-gal activity and Pitx2 expression, suggesting that the suppression of Wnt/β-catenin signaling in K14Cre;pNog tooth is not a secondary effect of BMP inhibition by Nog. This notion is supported by the fact that Nog can bind directly to Wnts and functionally inhibits Wnt signaling activity in TOPflash assays. The in situ PLA experiments further revealed a physical interaction between Nog and Wnts in tooth germs under physiological conditions, and the elevated level of active β-catenin in Nog mutant tooth germ supports a physiological function of Nog in modulating Wnt signaling activity in vivo. Although targeted inactivation of Nog causes defects in incisors but not in molars (Hu et al., 2012), it is possible that the enhanced Wnt signaling activities in Nog−/− molars do not reach a level that could alter the molar developmental program, similar to that seen in Axin2 heterozygotes (Lohi et al., 2010). Nevertheless, our results establish a novel function for Nog as a Wnt antagonist in vivo. Several other secreted proteins, such as Cerberus, Coco and Ectodin, could also modulate both Wnt and BMP signals but act through different mechanisms. Similar to Nog, Cerberus and Coco modulate BMP and Wnt signaling pathways by directly binding to BMP and Wnt ligands (Bell et al., 2003; Piccolo et al., 1999). Ectodin antagonizes BMP signaling by binding to BMP ligands, whereas its Wnt modulation function is through binding to the extracellular domain of the Wnt co-receptor, including LRP5/6 and LRP4, thus blocking the binding of Wnts to the receptors (Lintern et al., 2009).
Wnt/β-catenin signaling controls the odontogenic fate of the dental epithelium by sustaining Pitx2 expression
As the earliest molecular marker for the dental epithelium fate, Pitx2 is induced by FGF8 (St. Amand et al., 2000), and is essential for tooth development beyond the bud stage (Lin et al., 1999). However, the mechanism that sustains Pitx2 expression in the dental epithelium remains unknown. In K14Cre;pNog tooth, Wnt/β-catenin activity was not altered in the dental epithelium at E11.5, similar to Pitx2 expression (Wang et al., 2012). However, at E12.5, Pitx2 expression became downregulated in accordance with the reduced Wnt/β-catenin signaling activity, suggesting that Wnt/β-catenin signaling is responsible for sustaining Pitx2 expression. This notion is supported by the observations that Pitx2 expression was resumed in K14Cre;pNog tooth germs treated with LiCl or lacking an Axin2 allele. The functional importance of Pitx2 in mediating Wnt signaling in early tooth development is further strengthened by the observation that the transgenic Pitx2 expression in the dental epithelium could substitute for reactivation of Wnt signaling (by deletion of an Axin2 allele) to rescue tooth development defect in K14Cre;pNog;pCaIb mice.
Orchestration of non-canonical BMP and Wnt/β-catenin signaling in regulating early tooth development
Whereas reactivation of BMP signaling by expression of pcaIB or reactivation of Wnt signaling by deletion of Axin2 in the dental epithelium of K14Cre;pNog tooth germs partially resumed the expression of cyclin D1 and cell proliferation rate (Fig. 7), the tooth phenotype was not rescued in either case (Fig. 6). However, the early tooth defect was rescued, as associated with fully resumed levels of cyclin D1 and Pitx2 expression as well as cell proliferation rate in the K14Cre;pNog dental epithelium, by simultaneous activation of both BMP and Wnt/β-catenin signaling pathways (Figs 6 and 7). This indicates an orchestrated function of BMP and Wnt signaling in regulating early tooth development. The partially rescued cell proliferation rate associated with partially resumed cyclin D1 level, in addition to resumed Pitx2 expression, in K14Cre;pNog;Axin2+/− tooth germs, is consistent with the fact that Ccnd1 is a direct transcriptional target of Wnt signaling (Shtutman et al., 1999; Tetsu and McCormick, 1999) and its mRNA is stabilized by Wnt/β-catenin-induced Pitx2 (Briata et al., 2003). These results indicate that Wnt/β-catenin signaling also controls cell proliferation in a synergistic manner together with BMP signaling in the dental epithelium.
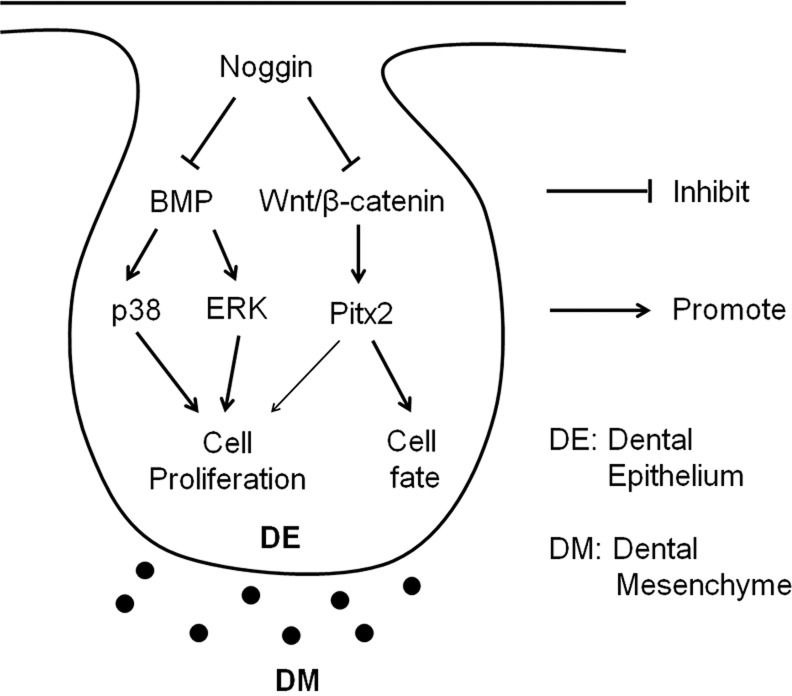
In summary, using a transgenic animal model combined with in vitro organ culture and genetic rescue approaches, we have dissected the distinct and orchestrated function of non-canonical BMP and Wnt/β-catenin signaling pathways during early tooth development (Fig. 8). Here, non-canonical BMP signaling plays a major role in regulating dental epithelial cell proliferation, whereas Wnt/β-catenin signaling functions primarily to control the odontogenic fate and partly to regulate cell proliferation in the dental epithelium during early odontogenesis. We have also established a novel function for Nog as an antagonist of Wnts, which raises an alert for future studies using Nog as a BMP antagonist, and calls for a possible re-evaluation of previous studies involving Nog.
Fig. 8.
Model diagram for orchestration of non-canonical BMP and Wnt/β-catenin signaling in regulating early tooth development.
MATERIALS AND METHODS
Animals and embryo collection
The use of animals in this study was approved by the Institutional Animal Care and Use Committee of Tulane University. The generation of Axin2lacZ (Axin2+/−), Nog−/−, BAT-gal, K14-Cre, pMes-Nog (pNog) and pMes-caBmpr1b (pcaIB) transgenic mice has been described previously (McMahon et al., 1998; Lustig et al., 2002; Maretto et al., 2003; Andl et al., 2004; Xiong et al., 2009; He et al., 2010; Wang et al., 2013). Production of pMes-Pitx2c (pPitx2c) transgenic mice is described in the methods in the supplementary material. Embryos were collected from timed-pregnant females, and tail samples from each embryo were subjected to PCR-based genotyping.
Histology, in situ hybridization, immunofluorescence and X-gal staining
Standard hematoxylin and eosin (H&E) staining was used for histological analysis, and section in situ hybridization, using non-radioactive probes, was conducted as described previously (Wang et al., 2012). For immunofluorescence and X-gal staining, samples were fixed in 4% PFA and subsequently dehydrated with 30% sucrose/PBS, embedded in O.C.T. and cryo-sectioned. Immunofluorescence and X-gal staining were conducted as described previously (Ito et al., 2003; He et al., 2010). The following antibodies for immunoflurescence were used: anti-P-Smad1/5/8 (Cell Signaling, #13820, 1:50), anti-P-p38 (R&D Systems, AF869, 1:50), anti-P-ERK1/2 (R&D Systems, AF1018, 1:50), anti-cyclin D1 (Abcam, ab16663, 1:400), anti-active β-catenin (EMD Millipore, 05-665, 1:200), anti-Pitx2 (Capra Science, PA1021-100, 1:100), anti-Msx1 (R&D Systems, AF5045, 1:200), anti-P-Smad2/3 (Santa Cruz Biotechnology, sc-11769, 1:50), anti-Fgfr1 (Abcam, ab823, 1:200) and anti-Fgfr2 (Abcam, ab58201, 1:200).
Organ culture and bead implantation
Mandibular molar germs were carefully isolated from staged embryos and placed in semisolid culture medium as described previously (Hu et al., 2006). For bead implantation, Affi-Gel Blue agarose beads (Bio-Rad) were washed with PBS prior to being incubated with 100 ng/µl Nog protein or 10 ng/µl Tgfb1 (both from R&D Systems) at 37°C for 30 min. Control beads were soaked with 100 or 10 ng/µl BSA. Protein-soaked beads were placed on the top of each tooth germ. For small molecule inhibition experiments, SB203580 (Cell Signaling), U0126 (Cell Signaling) or dorsomorphin (Sigma) at a final concentration of 20 µM, or SB525334 (Sigma) at a final concentration of 10 µM, were added to the culture medium. LiCl was added in culture medium at a final concentration of 10 nM. DMSO was used in control groups.
Tissue recombination and subrenal culture
Tissue recombination and subrenal culture were carried out as described previously (Yuan et al., 2008). Briefly, mandibular molar germs were isolated from staged K14Cre;pNog and wild-type embryos, respectively. Dental mesenchyme from K14Cre;pNog embryo was recombined with dental epithelium isolated from littermate wild-type embryos. Tissue recombinants were cultured in semisolid culture medium for 1 day prior to being subjected to subrenal culture in adult male CD-1 mice. Samples were harvested after 10 days in subrenal culture and processed for histological analysis.
BrdU labeling
Cell proliferation rate was assessed by a 5-Bromo-2'-deoxyuridine (BrdU) incorporation assay using a BrdU labeling and detection kit II (Roche), as described previously (Xiong et al., 2009). Cell proliferation rates were measured by counting BrdU-positive cells and total cells within defined arbitrary areas, and are presented as percentage of labeled cells against total cells in the area. Three samples were used for BrdU labeling in each group. Data were collected from three continuous sections from each sample.
In situ proximity ligation assay (PLA)
The binding of endogenous Nog with Wnts in wild-type and Nog−/− molar germs was conducted using an in situ PLA kit (Duolink kit, Sigma-Aldrich). Briefly, tissue sections were blocked with Duolink blocking reagent, and then incubated with anti-Nog (rabbit polyclonal, Abcam, ab16054, 1:100) and anti-Wnt4 (goat polyclonal, R&D Systems, AF475, 1:100) or anti-Wnt6 (goat polyclonal, Santa Cruz Biotechnology, sc-241734, 1:100) antibodies overnight at 4°C. Samples were washed and incubated with secondary antibodies conjugated with oligonucleotides (anti-goat PLA probe Plus or anti-rabbit PLA probe Minus) in a humidity chamber for 1 h at 37°C. Ligation of the oligonucleotide probes was followed by amplification at 37°C for 100 min. PLA signals were detected using a fluorescence microscope. Negative controls were included by replacing anti-Wnt antibodies with goat-negative IgG on wild-type samples.
Co-IP and western blot
The constructions of Myc-tagged mouse Wnt expression vectors, Nog-FLAG, FLAG-tagged pc-Wnt3 and HA-tagged pc-Nog are described in the methods in the supplementary material. Expression vectors were transfected into HEK 293T cells by Lipofectamine 2000 (Invitrogen), and Co-IP and western blot were conducted as described previously (Zhu et al., 2013). To examine the level of BMP signaling mediators in tooth germ by western blot, proteins were extracted from molar germs using 2× protein loading buffer (LI-COR Biosciences). Immunoreactive bands were visualized with Odyssey imaging system (LI-COR Biosciences) using IRDye 800 secondary antibody (LI-COR Biosciences). Western blots were performed at least three times independently. The following antibodies were used: anti-P-Smad1/5/8 (1:2000), anti-P-p38 (1:2000), anti-P-ERK1/2 (1:2000), anti-P-JNK (R&D Systems, AF1205, 1:2000), anti-GAPDH (GeneTex, GTX10118, 1:4000), anti-Myc and anti-FLAG (Thermo Scientific, MA1-91878, 1:2000), anti-Bmp4 (Abcam, ab39973, 1:2000), and anti-P-MAPKAPK-2 (Thermo Scientific, PA5-12619, 1:2000).
Reporter assays
HEK 293T cells in 48-well plates were co-transfected by TOPflash or FOPflash (Upstate Biotechnology) or pGL3-BRE-Luc (Addgene), with pc-Wnt3, pc-Nog or Nog mutant expression vectors, and/or pRIAS-Bmp4 (Addgene). pRL-SV40 (Renilla luciferase, Promega) was included as an internal control. Cells were harvested and lysed 48 h after transfection, and luciferase activities were measured with a dual luciferase reporter assay system (Promega) and determined using the GloMax luminometer (Promega). Firefly luciferase expression was normalized against Renilla luciferase expression to determine relative luciferase activity. Duplicated wells were assayed for each transfection and three independent transfection assays were performed.
Mutagenesis, RT-PCR and quantitative real-time RT-PCR (qRT-PCR)
The methods for the generation of truncated Nog expression vectors Nog(ΔCRD) and Nog(Δclip), and primer information for RT-PCR or qRT-PCR are provided in the supplementary material.
Data quantification and statistical analysis
All experiments were repeated at least three times and at least four samples of each phenotype were collected for each genetic rescue experiment. For the western blot, densitometry of immunoblot bands was performed and relative quantification was processed with the ImageJ software (NIH). For histological sections, fluorescence intensity was collected from three continuous sections of each sample with ImageJ software. Quantification data are presented as the mean±s.d. of at least three independent experiments. Statistical analysis was performed using Student's t-test or ANOVA, followed by post hoc Bonferroni test, with SPSS (IBM). Significance was defined as P<0.05.
Supplementary Material
Footnotes
Competing interests
The authors declare no competing financial interests.
Author contributions
G.Yuan and G.Yang performed most of the experiments, collected and analyzed data, prepared figures and wrote the manuscript. Y.Z. participated in the initial phase of this study. X.Z. and Z.Z. performed Co-IP assays on binding of Nog with Wnts. Z.C. provided advice on the project and revised the manuscript. Y.C. conceived the project and made a final revision and edit of the manuscript.
Funding
This work was supported by grants from the National Institutes of Health (NIH) [R01 DE014044 and DE024152 to Y.C.] and by the National Natural Science Foundation of China [81470708 to G.Yu.; 81371105 to G.Ya.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.117887/-/DC1
References
- Andl T., Ahn K., Kairo A., Chu E. Y., Wine-Lee L., Reddy S. T., Croft N. J., Cebra-Thomas J. A., Metzger D., Chambon P. et al. (2004). Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131, 2257-2268 10.1242/dev.01125 [DOI] [PubMed] [Google Scholar]
- Bai X. M., Van der Schueren B., Cassiman J. J., Van den Berghe H. and David G. (1994). Differential expression of multiple cell-surface heparan sulfate proteoglycans during embryonic tooth development. J. Histochem. Cytochem. 42, 1043-1054 10.1177/42.8.8027524 [DOI] [PubMed] [Google Scholar]
- Bayramov A. V., Eroshkin F. M., Martynova N. Y., Ermakova G. V., Solovieva E. A. and Zaraisky A. G. (2011). Novel functions of Nog proteins: inhibition of Activin/Nodal and Wnt signaling. Development 138, 5345-5356 10.1242/dev.068908 [DOI] [PubMed] [Google Scholar]
- Bell E., Muñoz-Sanjuán I., Altmann C. R., Vonica A. and Brivanlou A. H. (2003). Cell fate specification and competence by Coco, a maternal BMP, TGFbeta and Wnt inhibitor. Development 130, 1381-1389 10.1242/dev.00344 [DOI] [PubMed] [Google Scholar]
- Briata P., Ilengo C., Corte G., Moroni C., Rosenfeld M. G., Chen C.-Y. and Gherzi R. (2003). The Wnt/β-catenin→Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol. Cell 12, 1201-1211 10.1016/S1097-2765(03)00407-6 [DOI] [PubMed] [Google Scholar]
- Chai Y., Mah A., Crohin C., Groff S., Bringas P. Jr, Le T., Santo V. and Slavkin H. C. (1994). Specific transforming growth factor-beta subtypes regulate embryonic mouse Meckel's cartilage and tooth development. Dev. Biol. 162, 85-103 10.1006/dbio.1994.1069 [DOI] [PubMed] [Google Scholar]
- Chen Y., Bei M., Woo I., Satokata I. and Maas R. (1996). Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122, 3035-3044. [DOI] [PubMed] [Google Scholar]
- Chen Y., Whetstone H. C., Youn A., Nadesan P., Chow E. C. Y., Lin A. C. and Alman B. A. (2007). Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 282, 526-533 10.1074/jbc.M602700200 [DOI] [PubMed] [Google Scholar]
- Chen J., Lan Y., Baek J.-A., Gao Y. and Jiang R. (2009). Wnt/beta-catenin signaling plays an essential role in activation of odontogenic mesenchyme during early tooth development. Dev. Biol. 334, 174-185 10.1016/j.ydbio.2009.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnutt C., Burrus L. W., Brown A. M. C. and Niswander L. (2004). Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev. Biol. 274, 334-347 10.1016/j.ydbio.2004.07.019 [DOI] [PubMed] [Google Scholar]
- Cho K.-W., Cho S.-W., Lee J.-M., Lee M.-J., Gang H.-S. and Jung H.-S. (2008). Expression of phosphorylated forms of ERK, MEK, PTEN and PI3K in mouse oral development. Gene Expr. Patterns 8, 284-290 10.1016/j.gep.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Ferguson C. A., Tucker A. S., Christensen L., Lau A. L., Matzuk M. M. and Sharpe P. T. (1998). Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 12, 2636-2649 10.1101/gad.12.16.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans M.-J., Valdimarsdottir G., Itoh S., Rosendahl A., Sideras P. and ten Dijke P. (2002). Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 21, 1743-1753 10.1093/emboj/21.7.1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goumans M.-J., Valdimarsdottir G., Itoh S., Lebrin F., Larsson J., Mummery C., Karlsson S. and ten Dijke P. (2003). Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGFbeta/ALK5 signaling. Mol. Cell 12, 817-828 10.1016/S1097-2765(03)00386-1 [DOI] [PubMed] [Google Scholar]
- Groppe J., Greenwald J., Wiater E., Rodriguez-Leon J., Economides A. N., Kwiatkowski W., Affolter M., Vale W. W., Izpisua Belmonte J. C. and Choe S. (2002). Structural basis of BMP signalling inhibition by the cystine knot protein Nog. Nature 420, 636-642 10.1038/nature01245 [DOI] [PubMed] [Google Scholar]
- He F., Xiong W., Wang Y., Matsui M., Yu X., Chai Y., Klingensmith J. and Chen Y. (2010). Modulation of BMP signaling by Nog is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev. Biol. 347, 109-121 10.1016/j.ydbio.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Nadiri A., Kuchler-Bopp S., Perrin-Schmitt F., Peters H. and Lesot H. (2006). Tissue engineering of tooth crown, root, and periodontium. Tissue Eng. 12, 2069-2075 10.1089/ten.2006.12.2069 [DOI] [PubMed] [Google Scholar]
- Hu X., Wang Y., He F., Li L., Zheng Y., Zhang Y. and Chen Y. P. (2012). Nog is required for early development of murine upper incisors. J. Dent. Res. 91, 394-400 10.1177/0022034511435939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille F., Atanasoski S., Falk S., Ittner L. M., Märki D., Büchmann-Møller S., Wurdak H., Suter U., Taketo M. M. and Sommer L. (2007). Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev. Biol. 304, 394-408 10.1016/j.ydbio.2006.12.045 [DOI] [PubMed] [Google Scholar]
- Ito Y., Yeo J. Y., Chytil A., Han J., Bringas P. Jr, Nakajima A., Shuler C. F., Moses H. L. and Chai Y. (2003). Conditional inactivation of Tgfbr2 in cranial neural crest causes cleft palate and calvaria defects. Development 21, 5280-5296. [DOI] [PubMed] [Google Scholar]
- Järvinen E., Salazar-Ciudad I., Birchmeier W., Taketo M. M., Jernvall J. and Thesleff I. (2006). Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta-catenin signaling. Proc. Natl. Acad. Sci. USA 103, 18627-18632 10.1073/pnas.0607289103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Zhou J., Gao Y., Baek J.-A., Martin J. F., Lan Y. and Jiang R. (2013). Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development 140, 423-432 10.1242/dev.081927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke J., Harikumar K. G., Erice C., Chen C., Gu X., Wang L., Parker N., Cheng Z., Xu W., Williams B. O. et al. (2013). Structure and function of Norrin in assembly and activation of a frizzled 4-Lrp5/6 complex. Genes Dev. 27, 2305-2319 10.1101/gad.228544.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C., Briata P., Baek S. H., Rose D. W., Hamblet N. S., Herman T., Ohgi K. A., Lin C., Gleiberman A., Wang J. et al. (2002). Identification of a Wnt/Dvl/beta-catenin→Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111, 673-685 10.1016/S0092-8674(02)01084-X [DOI] [PubMed] [Google Scholar]
- Korchynskyi O. and ten Dijke P. (2002). Identification and functional characterization of distinct critically important bone morphogenetic protein-specific response elements in the Id1 promoter. J. Biol. Chem. 277, 4883-4891 10.1074/jbc.M111023200 [DOI] [PubMed] [Google Scholar]
- Lee M. Y., Lim H. W., Lee S. H. and Han H. J. (2009). Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells 27, 1858-1868 10.1002/stem.124 [DOI] [PubMed] [Google Scholar]
- Li L., Lin M., Wang Y., Cserjesi P., Chen Z. and Chen Y. (2011). BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev. Biol. 349, 451-461 10.1016/j.ydbio.2010.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. R., Kioussi C., O'Connel S., Briata P., Szeto D., Liu F., Izpisúa-Belmonte J. C. and Rosenfeld M. G. (1999). Pitx2 regulates lung asymmetry, cardiac positioning and pituitary and tooth morphogenesis. Nature 401, 279-282 10.1038/45803 [DOI] [PubMed] [Google Scholar]
- Lintern K. B., Guidato S., Rowe A., Saldanha J. W. and Itasaki N. (2009). Characterization of wise protein and its molecular mechanism to interact with both Wnt and BMP signals. J. Biol. Chem. 284, 23159-23168 10.1074/jbc.M109.025478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F. and Millar S. E. (2010). Wnt/β-catenin signaling in oral tissue development and disease. J. Dent. Res. 89, 318-330 10.1177/0022034510363373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Selever J., Lu M.-F. and Martin J. F. (2003). Genetic dissection of Pitx2 in craniofacial development uncovers new functions in branchial arch morphogenesis, late aspects of tooth morphogenesis and cell migration. Development 130, 6375-6385 10.1242/dev.00849 [DOI] [PubMed] [Google Scholar]
- Liu W., Sun X., Braut A., Mishina Y., Behringer R. R., Mina M. and Martin J. F. (2005). Distinct functions for Bmp signaling in lip and palate fusion in mice. Development 132, 1453-1461 10.1242/dev.01676 [DOI] [PubMed] [Google Scholar]
- Liu Z., Tang Y., Qiu T., Cao X. and Clemens T. L. (2006). A dishevelled-1/Smad1 interaction couples WNT and bone morphogenetic protein signaling pathways in uncommitted bone marrow stromal cells. J. Biol. Chem. 281, 17156-17163 10.1074/jbc.M513812200 [DOI] [PubMed] [Google Scholar]
- Liu F., Chu E. Y., Watt B., Zhang Y., Gallant N. M., Andl T., Yang S. H., Lu M.-M., Piccolo S., Schmidt-Ullrich R. et al. (2008). Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 313, 210-224 10.1016/j.ydbio.2007.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Gu S., Sun C., Ye W., Song Z., Zhang Y. and Chen Y. (2013). FGF signaling sustains the odontogenic fate of dental mesenchyme by suppressing β-catenin signaling. Development 140, 4375-4385 10.1242/dev.097733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi M., Tucker A. S. and Sharpe P. T. (2010). Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev. Dyn. 239, 160-167. [DOI] [PubMed] [Google Scholar]
- Lustig B., Jerchow B., Sachs M., Weiler S., Pietsch T., Karsten U., van de Wetering M., Clevers H., Schlag P. M., Birchmeier W. et al. (2002). Negative feedback loop of Wnt signaling through upregulation of conductin/Axin2 in colorectal and liver tumors. Mol. Cell. Biol. 22, 1184-1193 10.1128/MCB.22.4.1184-1193.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M. and Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304 10.1073/pnas.0434590100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon J. A., Takada S., Zimmerman L. B., Fan C.-M., Harland R. M. and McMahon A. P. (1998). Nog-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 12, 1438-1452 10.1101/gad.12.10.1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R. M., de Sousa Lopes S. M. C., Bialecka M., de Boer S., Zwijsen A. and Mummery C. L. (2008). Real time monitoring of BMP Smads transcriptional activity during mouse development. Genesis 46, 335-346 10.1002/dvg.20402 [DOI] [PubMed] [Google Scholar]
- Moriguchi M., Yamada M., Miake Y. and Yanagisawa T. (2011). Immunolocalization of TAK1, TAB1, and p38 in the developing rat molar. Anat. Sci. Int. 86, 69-77 10.1007/s12565-010-0089-z [DOI] [PubMed] [Google Scholar]
- Moustakas A. and Heldin C.-H. (2009). The regulation of TGFbeta signal transduction. Development 136, 3699-3714 10.1242/dev.030338 [DOI] [PubMed] [Google Scholar]
- Mucchielli M.-L., Mitsiadis T. A., Raffo S., Brunet J.-F., Proust J.-P. and Goridis C. (1997). Mouse Otlx2/RIEG expression in the odontogenic epithelium precedes tooth initiation and requires mesenchyme-derived signals for its maintenance. Dev. Biol. 189, 275-284 10.1006/dbio.1997.8672 [DOI] [PubMed] [Google Scholar]
- Nathan E. and Tzahor E. (2009). sFRPs: a declaration of (Wnt) independence. Nat. Cell Biol. 11, 13 10.1038/ncb0109-13 [DOI] [PubMed] [Google Scholar]
- Pacheco M. S., Reis A. H., Aguiar D. P., Lyons K. M. and Abreu J. G. (2008). Dynamic analysis of the expression of the TGFbeta/SMAD2 pathway and CCN2/CTGF during early steps of tooth development. Cells Tissues Organs 187, 199-210 10.1159/000112640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine-Saunders S., Viviano B. L., Economides A. N. and Saunders S. (2002). Heparan sulfate proteoglycans retain Nog at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J. Biol. Chem. 277, 2089-2096 10.1074/jbc.M109151200 [DOI] [PubMed] [Google Scholar]
- Piccolo S., Agius E., Leyns L., Bhattacharyya S., Grunz H., Bouwmeester T. and De Robertis E. M. (1999). The head inducer Cerberus is a multifunctional antagonist of Nodal, BMP and Wnt signals. Nature 397, 707-710 10.1038/17820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porntaveetus T., Otsuka-Tanaka Y., Basson M. A., Moon A. M., Sharpe P. T. and Ohazama A. (2011). Expression of fibroblast growth factors (Fgfs) in murine tooth development. J. Anat. 218, 534-543 10.1111/j.1469-7580.2011.01352.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar L. and Sharpe P. T. (1999). Expression of Wnt signalling pathway genes during tooth development. Mech. Dev. 85, 197-200 10.1016/S0925-4773(99)00095-7 [DOI] [PubMed] [Google Scholar]
- Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R. and Ben-Ze'ev A. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96, 5522-5527 10.1073/pnas.96.10.5522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber C., Kopf J., Hiepen C. and Knaus P. (2009). Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 20, 343-355 10.1016/j.cytogfr.2009.10.007 [DOI] [PubMed] [Google Scholar]
- Söderberg O., Leuchowius K.-J., Gullberg M., Jarvius M., Weibrecht I., Larsson L.-G. and Landegren U. (2008). Characterizing proteins and their interactions in cells and tissues using the in situ proximity ligation assay. Methods 45, 227-232 10.1016/j.ymeth.2008.06.014 [DOI] [PubMed] [Google Scholar]
- Soshnikova N., Zechner D., Huelsken J., Mishina Y., Behringer R. R., Taketo M. M., Crenshaw E. B. III and Birchmeier W. (2003). Genetic interaction between Wnt/beta-catenin and BMP receptor signaling during formation of the AER and the dorsal-ventral axis in the limb. Genes Dev. 17, 1963-1968 10.1101/gad.263003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Amand T. R., Zhang Y., Semina E. V., Zhao X., Hu Y., Nguyen L., Murray J. C. and Chen Y. (2000). Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev. Biol. 217, 323-332 10.1006/dbio.1999.9547 [DOI] [PubMed] [Google Scholar]
- Tetsu O. and McCormick F. (1999). Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422-426 10.1038/18884 [DOI] [PubMed] [Google Scholar]
- Tummers M. and Thesleff I. (2009). The importance of signal pathway modulation in all aspects of tooth development. J. Exp. Zool. B Mol. Dev. Evol. 312B, 309-319 10.1002/jez.b.21280 [DOI] [PubMed] [Google Scholar]
- Vainio S., Karavanova I., Jowett A. and Thesleff I. (1993). Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell 75, 45-58 10.1016/0092-8674(93)90678-J [DOI] [PubMed] [Google Scholar]
- Wang X.-P., O'Connell D. J., Lund J. J., Saadi I., Kuraguchi M., Turbe-Doan A., Cavallesco R., Kim H., Park P. J., Harada H. et al. (2009). Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 136, 1939-1949 10.1242/dev.033803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Li L., Zheng Y., Yuan G., Yang G., He F. and Chen Y. (2012). BMP activity is required for tooth development from the lamina to bud stage. J. Dent. Res. 91, 690-695 10.1177/0022034512448660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zheng Y., Chen D. and Chen Y. (2013). Enhanced BMP signaling prevents degeneration and leads to endochondral ossification of Meckel's cartilage in mice. Dev. Biol. 381, 301-311 10.1016/j.ydbio.2013.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W., He F., Morikawa Y., Yu X., Zhang Z., Lan Y., Jiang R., Cserjesi P. and Chen Y. (2009). Hand2 is required in the epithelium for palatogenesis in mice. Dev. Biol. 330, 131-141 10.1016/j.ydbio.2009.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Jeong L., Han J., Ito Y., Bringas P. Jr and Chai Y. (2003). Developmental expression of Smad1-7 suggests critical function of TGF-beta/BMP signaling in regulating epithelial-mesenchymal interaction during tooth morphogenesis. Int. J. Dev. Biol. 47, 31-39 10.1387/14 [DOI] [PubMed] [Google Scholar]
- Xu X., Han J., Ito Y., Bringas P. Jr, Deng C. and Chai Y. (2008). Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-β/BMP signaling during tooth and palate development. Dev. Cell 15, 322-329 10.1016/j.devcel.2008.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G., Yuan G., Ye W., Cho K. W. Y. and Chen Y. (2014). An atypical canonical BMP signaling pathway regulates msh homeobox 1 (Msx1) expression during odontogenesis. J. Biol. Chem. Published October 1, 2014 (Epub ahead of print) 10.1074/jbc.M114.600064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S. E., Daluiski A., Pederson R., Rosen V. and Lyons K. M. (2000). The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development 127, 621-630. [DOI] [PubMed] [Google Scholar]
- Yu P. B., Hong C. C., Sachidanandan C., Babitt J. L., Deng D. Y., Hoyng S. A., Lin H. Y., Bloch K. D. and Peterson R. T. (2008). Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat. Chem. Biol. 4, 33-41 10.1038/nchembio.2007.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G. H., Zhang L., Zhang Y. D., Fan M. W., Bian Z. and Chen Z. (2008). Mesenchyme is responsible for tooth suppression in the mouse lower diastema. J. Dent. Res. 87, 386-390 10.1177/154405910808700412 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang Z., Zhao X., Yu X., Hu Y., Geronimo B., Fromm S. H. and Chen Y. P. (2000). A new function of BMP4: dual role of BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development 127, 1431-1443. [DOI] [PubMed] [Google Scholar]
- Zhu X., Zhao P., Liu Y., Zhang X., Fu J., Ivy Yu H.-M., Qiu M., Chen Y., Hsu W. and Zhang Z. (2013). Intra-epithelial requirement of canonical Wnt signaling for tooth development. J. Biol. Chem. 288, 12080-12089 10.1074/jbc.M113.462473 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.