Abstract
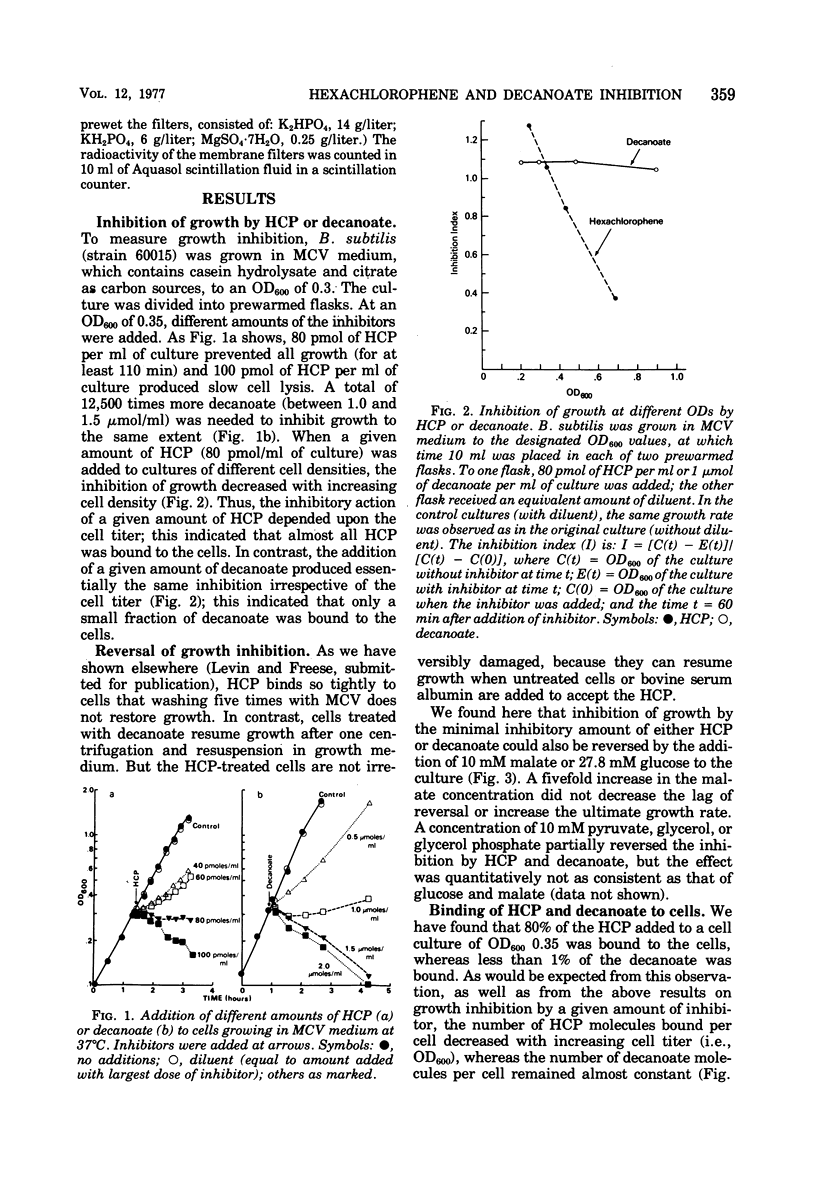
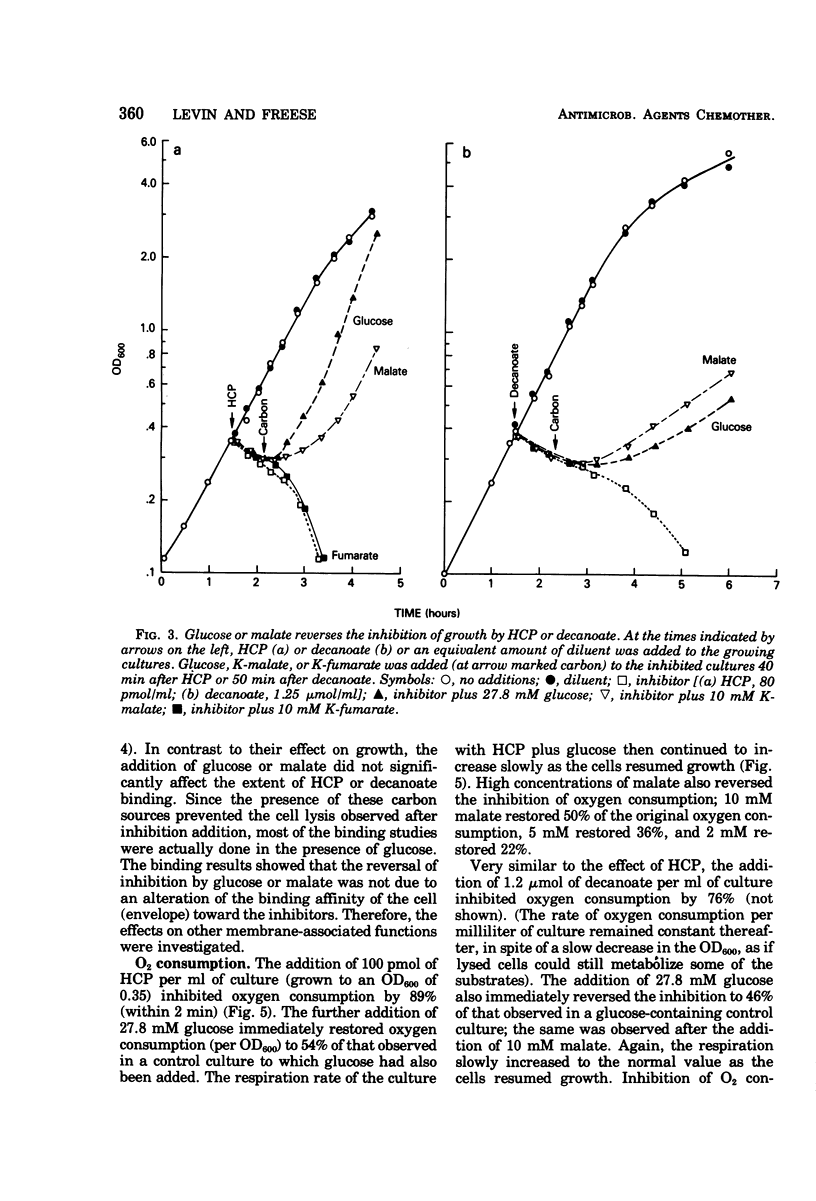
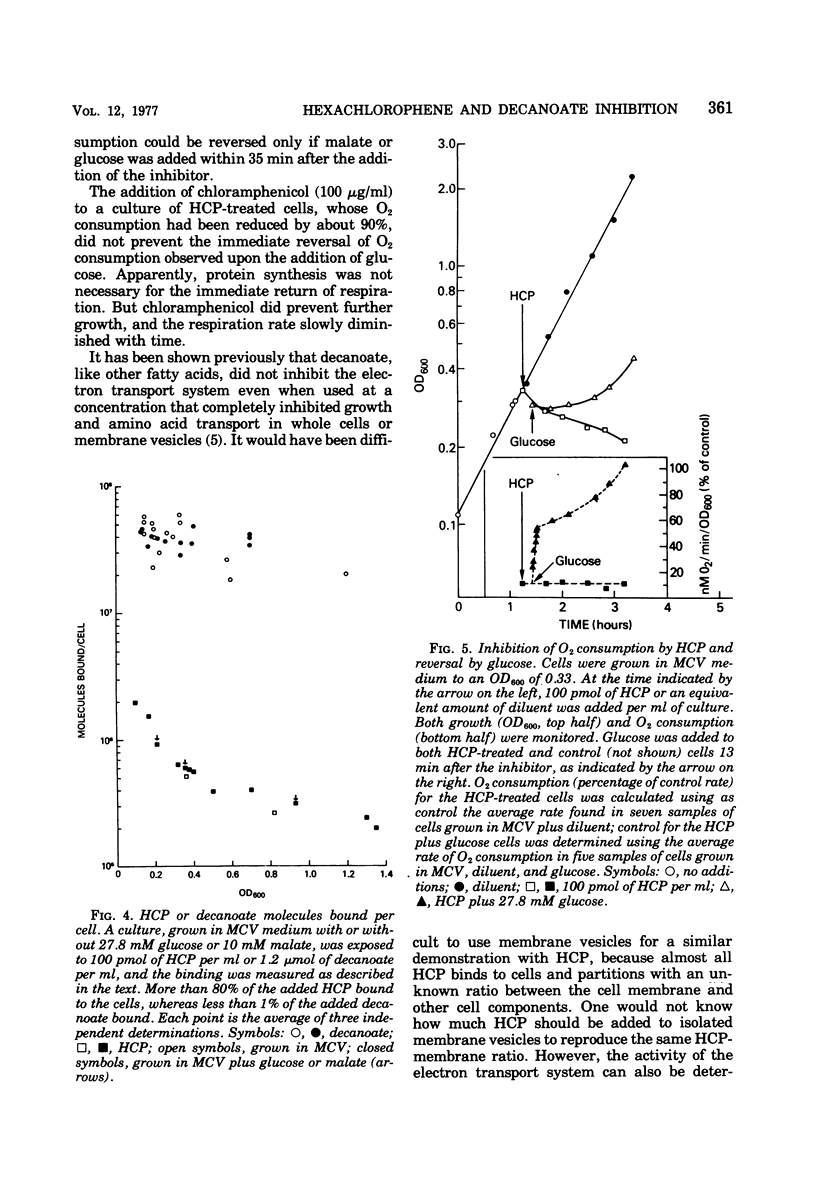
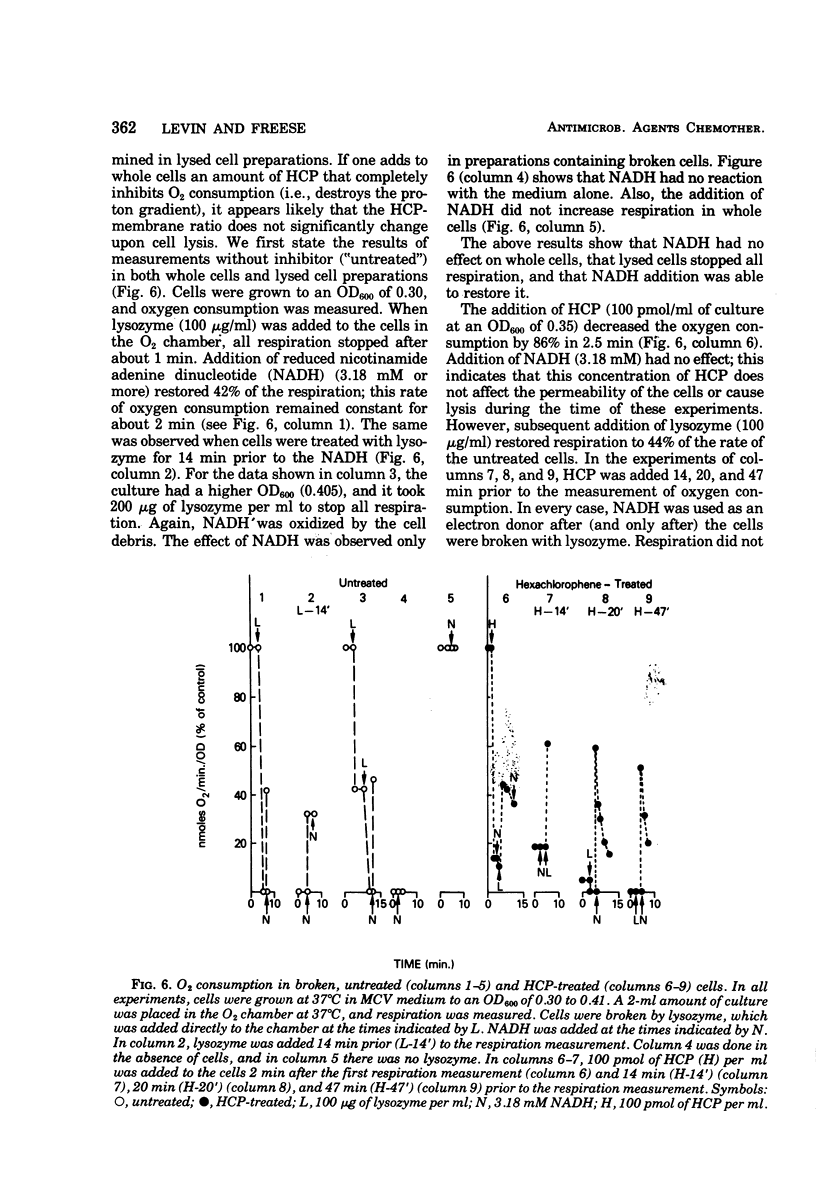
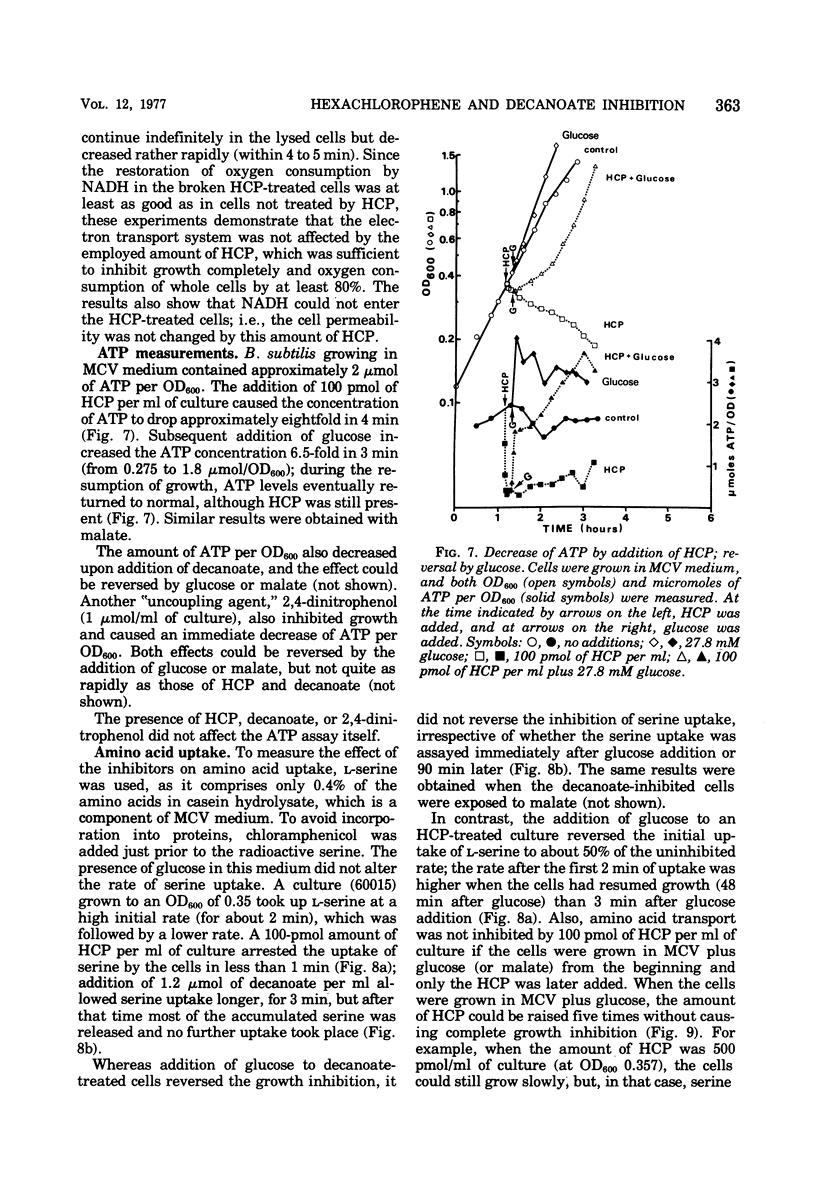
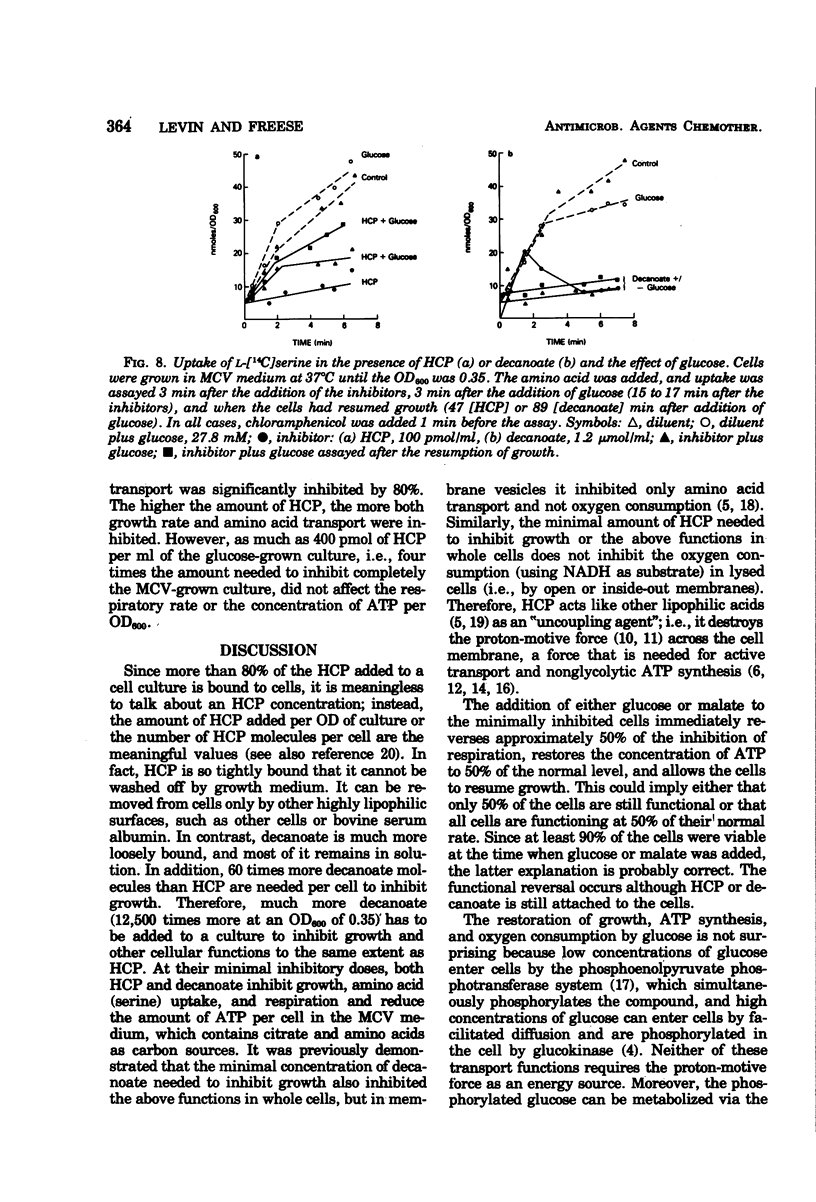
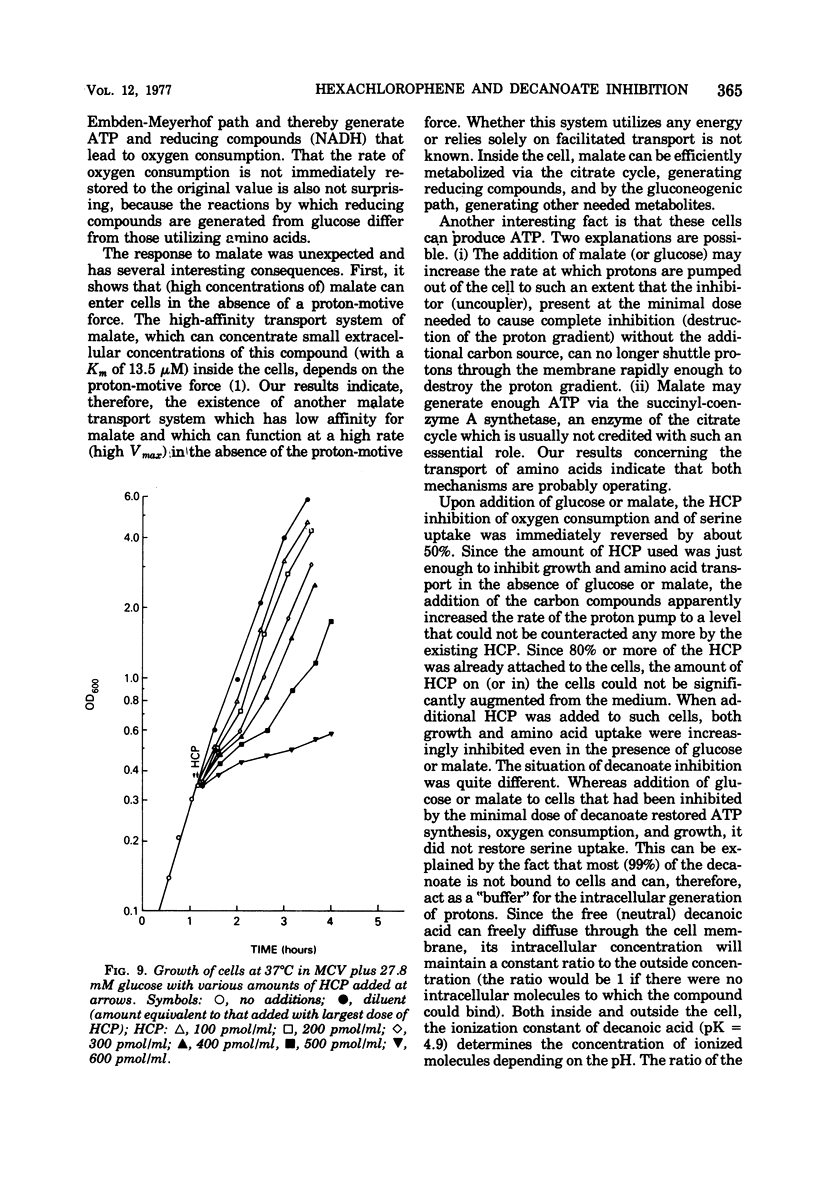
The minimal growth-inhibitory amount of either hexachlorophene (HCP) or decanoate stopped growth, respiration, adenosine 5′-triphosphate synthesis, and amino acid transport of Bacillus subtilis in a culture containing amino acids and citrate as carbon sources. The electron transport system was not affected by this dose. Addition of 27.8 mM glucose or 10 mM malate to an inhibited culture did not reverse the binding of HCP or decanoate to the cells, but it allowed resumption of growth, respiration, and adenosine 5′-triphosphate synthesis, as the glucose or malate then supplied the needed carbon. The addition of glucose or malate did not reverse amino acid transport inhibition caused by decanoate, but it did reverse that due to HCP. However, if the dose of HCP was raised in the presence of glucose or malate, only growth and amino acid transport were affected; this indicates that both HCP and decanoate act at their minimal growth inhibitory doses by inhibiting substrate transport. As active transport of amino acids and ketoacids depends on the proton gradient and the membrane potential of the cells, we conclude that the primary effect of these lipophilic acids is the destruction of the proton-motive force.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisschop A., Doddema H., Konings W. N. Dicarboxylic acid transport in membrane vesicles from Bacillus subtilis. J Bacteriol. 1975 Nov;124(2):613–622. doi: 10.1128/jb.124.2.613-622.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corner T. R., Joswick H. L., Silvernale J. N., Gerhardt P. Antimicrobial actions of hexachlorophene: lysis and fixation of bacterial protoplasts. J Bacteriol. 1971 Oct;108(1):501–507. doi: 10.1128/jb.108.1.501-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick J. J., Corner T. R., Gerhardt P. Antimicrobial actions of hexachlorophene: inhibition of respiration in Bacillus megaterium. Antimicrob Agents Chemother. 1974 Dec;6(6):712–721. doi: 10.1128/aac.6.6.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- Freese E., Sheu C. W., Galliers E. Function of lipophilic acids as antimicrobial food additives. Nature. 1973 Feb 2;241(5388):321–325. doi: 10.1038/241321a0. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joswick H. L., Corner T. R., Silvernale J. N., Gerhardt P. Antimicrobial actions of hexachlorophene: release of cytoplasmic materials. J Bacteriol. 1971 Oct;108(1):492–500. doi: 10.1128/jb.108.1.492-500.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrough R. D. Review of recent evidence of toxic effects of hexachlorophene. Pediatrics. 1973 Feb;51(2):391–394. [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4(3):399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- Mullick F. G. Hexachlorophene toxicity. Human experience at the Armed Forces Institute of Pathology. Pediatrics. 1973 Feb;51(2):395–399. [PubMed] [Google Scholar]
- Niven D. F., Hamilton W. A. Mechanisms of energy coupling to the transport of amino acids by Staphylococcus aureus. Eur J Biochem. 1974 May 15;44(2):517–522. doi: 10.1111/j.1432-1033.1974.tb03510.x. [DOI] [PubMed] [Google Scholar]
- Powell H., Swarner O., Gluck L., Lampert P. Hexachlorophene myelinopathy in premature infants. J Pediatr. 1973 Jun;82(6):976–981. doi: 10.1016/s0022-3476(73)80428-7. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The electrochemical gradient of protons and its relationship to active transport in Escherichia coli membrane vesicles. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1892–1896. doi: 10.1073/pnas.73.6.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Konings W. N., Freese E. Effects of acetate and other short-chain fatty acids on sugar and amino acid uptake of Bacillus subtilis. J Bacteriol. 1972 Aug;111(2):525–530. doi: 10.1128/jb.111.2.525-530.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvernale J. N., Joswick H. L., Corner T. R., Gerhardt P. Antimicrobial actions of hexachlorophene: cytological manifestations. J Bacteriol. 1971 Oct;108(1):482–491. doi: 10.1128/jb.108.1.482-491.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towfighi J., Gonatas N. K., McCree L. Hexachlorophene-induced changes in central and peripheral myelinated axons of developing and adult rats. Lab Invest. 1974 Dec;31(6):712–721. [PubMed] [Google Scholar]


