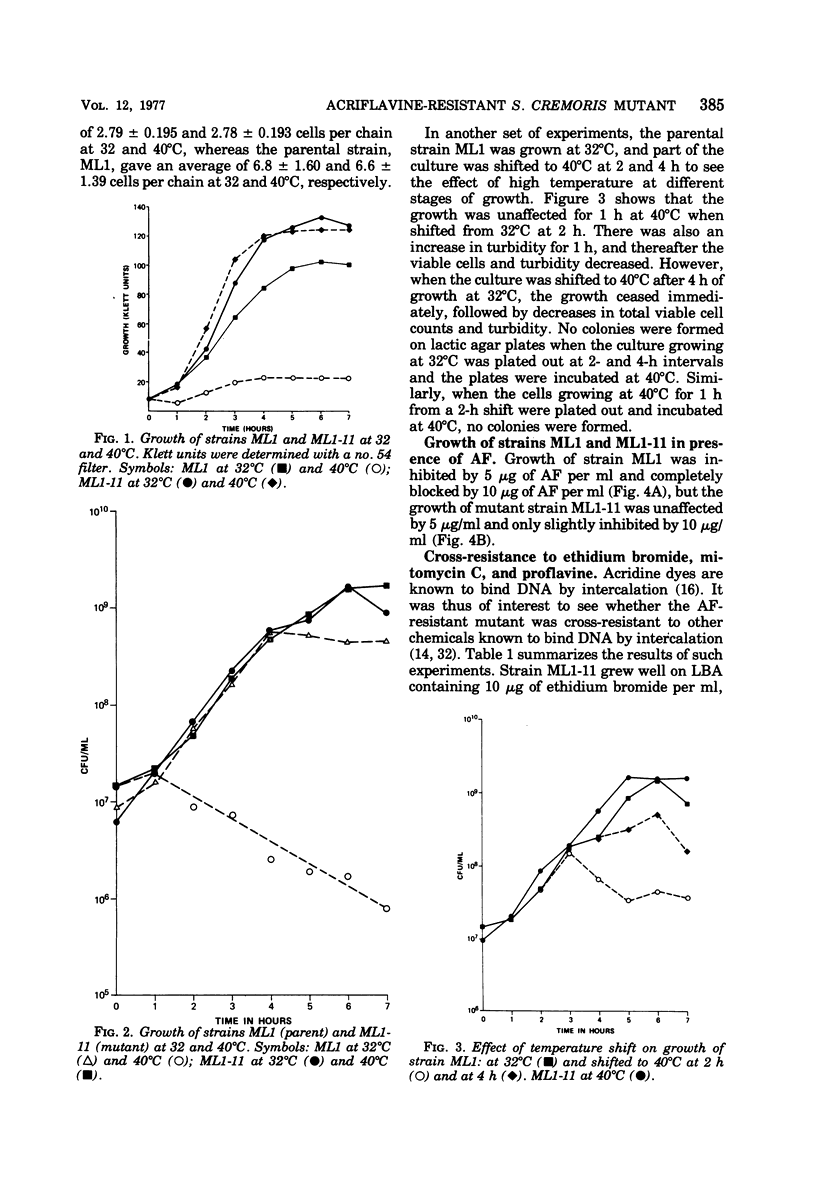
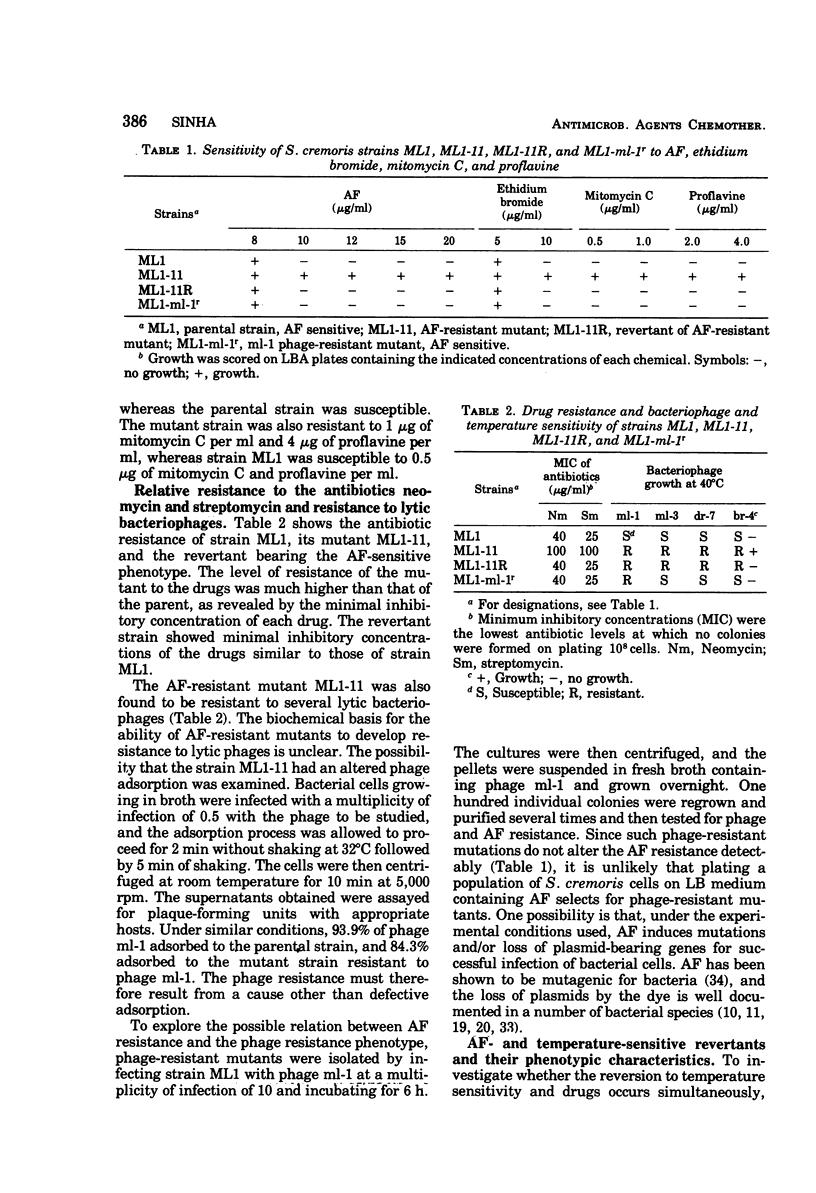
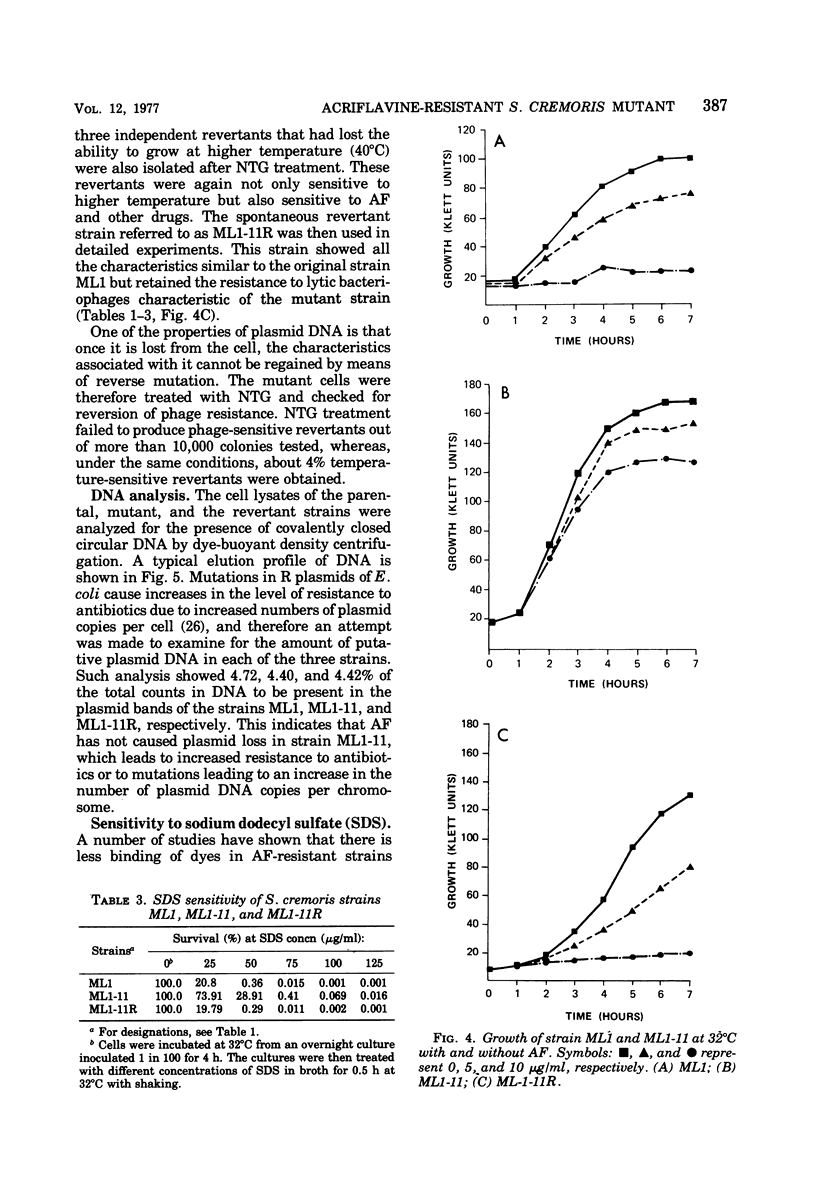
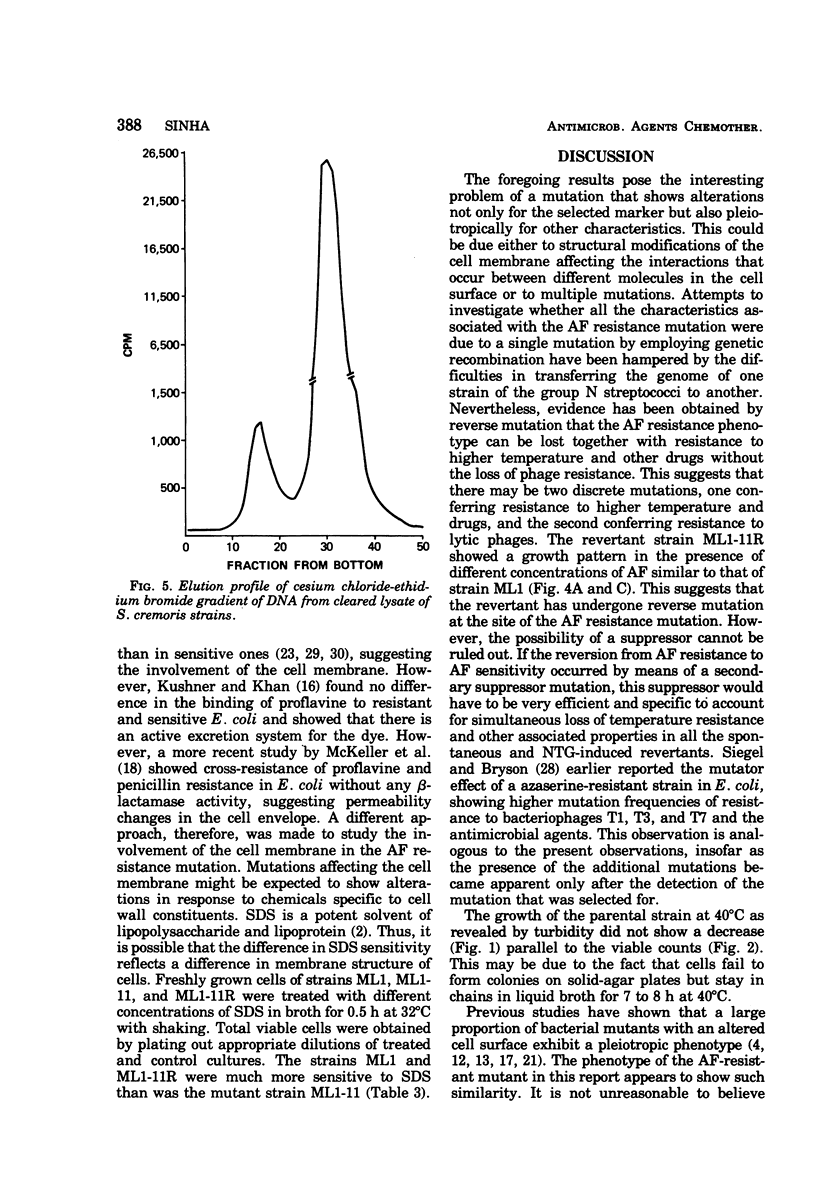
Abstract
Selection for resistance to acriflavine in Streptococcus cremoris resulted in cross-resistance to the drugs neomycin, streptomycin, ethidium bromide, mitomycin C, and proflavine. Furthermore, the mutants showed resistance to lytic bacteriophages to which the parental strain was sensitive, and, unlike the parent, the mutants grew well at higher temperatures (40°C). Revertants selected independently either for temperature sensitivity or for acriflavine sensitivity lost resistance to all the drugs and dyes but retained the bacteriophage resistance phenotype. The acriflavine-resistant mutation resulted in an increase in resistance by the bacterial cells to sodium dodecyl sulfate, a potent solvent of lipopolysaccharide and lipoprotein. It is suggested that the acriflavine resistance mutation determines the synthesis of a membrane substance resistant to higher temperatures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E., Anderson T. F. The surface structure of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1592–1599. doi: 10.1073/pnas.54.6.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Brown L. R. Ethidium bromide-resistant mutant of Bacillus subtilis. J Bacteriol. 1973 Sep;115(3):1077–1083. doi: 10.1128/jb.115.3.1077-1083.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRICK F. H., BARNETT L., BRENNER S., WATTS-TOBIN R. J. General nature of the genetic code for proteins. Nature. 1961 Dec 30;192:1227–1232. doi: 10.1038/1921227a0. [DOI] [PubMed] [Google Scholar]
- HIROTA Y., LIJIMA T. Acriflavine as an effective agent for eliminating F-factor in Escherichia coli K-12. Nature. 1957 Sep 28;180(4587):655–656. doi: 10.1038/180655a0. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Wakil S., Shapiro B., Ryter A., Hurwitz J., Jacob F. Sur un mutant thermosensible d'Escherichia coli présentant des anomalies de la membrane. C R Acad Sci Hebd Seances Acad Sci D. 1969 Oct;269(14):1346–1348. [PubMed] [Google Scholar]
- Holland I. B., Threlfall E. J., Holland E. M., Darby V., Samson A. C. Mutants of Escherichia coli with altered surface properties which are refractory to colicin E2, sensitive to ultraviolet light and which can also show recombination deficiency, abortive growth of bacteriophage lambda and filament formation. J Gen Microbiol. 1970 Aug;62(3):371–382. doi: 10.1099/00221287-62-3-371. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner D. J., Khan S. R. Proflavine Uptake and Release in Sensitive and Resistant Escherichia coli. J Bacteriol. 1968 Oct;96(4):1103–1114. doi: 10.1128/jb.96.4.1103-1114.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Gottfried S., Rothfield L. Leakage of periplasmic enzymes by mutants of Escherichia coli and Salmonella typhimurium: isolation of "periplasmic leaky" mutants. J Bacteriol. 1972 Feb;109(2):520–525. doi: 10.1128/jb.109.2.520-525.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITSUHASHI S., HARADA K., KAMEDA M. Elimination of transmissible drug-resistance by treatment with acriflavin. Nature. 1961 Mar 18;189:947–947. doi: 10.1038/189947a0. [DOI] [PubMed] [Google Scholar]
- MITSUHASHI S., MORIMURA M., KONO K., OSHIMA H. ELIMINATION OF DRUG RESISTANCE OF STAPHYLOCOCCUS AUREUS BY TREATMENT WITH ACRIFLAVINE. J Bacteriol. 1963 Jul;86:162–164. doi: 10.1128/jb.86.1.162-164.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar R. C., McKenzie C. N., Kushner K. J. Correlation of resistance to proflavine and penicillin in Escherichia coli. Antimicrob Agents Chemother. 1976 Oct;10(4):765–767. doi: 10.1128/aac.10.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Acriflavine-binding capacity of Escherichia coli in relation to acriflavine sensitivity and metabolic activity. J Bacteriol. 1966 Nov;92(5):1447–1452. doi: 10.1128/jb.92.5.1447-1452.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Gene-Controlled Resistance to Acriflavine and Other Basic Dyes in Escherichia coli. J Bacteriol. 1965 Jul;90(1):8–14. doi: 10.1128/jb.90.1.8-14.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H. Genetic determination of resistance to acriflavine, phenethyl alcohol, and sodium dodecyl sulfate in Escherichia coli. J Bacteriol. 1968 Oct;96(4):987–996. doi: 10.1128/jb.96.4.987-996.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Suganuma A. Membrane mutation associated with sensitivity to acriflavine in Escherichia coli. J Bacteriol. 1972 Apr;110(1):329–335. doi: 10.1128/jb.110.1.329-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORGEL A., BRENNER S. Mutagenesis of bacteriophage T4 by acridines. J Mol Biol. 1961 Dec;3:762–768. doi: 10.1016/s0022-2836(61)80081-8. [DOI] [PubMed] [Google Scholar]
- SILVER S. ACRIFLAVINE RESISTANCE: A BACTERIOPHAGE MUTATION AFFECTING THE UPTAKE OF DYE BY THE INFECTED BACTERIAL CELLS. Proc Natl Acad Sci U S A. 1965 Jan;53:24–30. doi: 10.1073/pnas.53.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Levine E., Spielman P. M. Acridine binding by Escherichia coli: pH dependency and strain differences. J Bacteriol. 1968 Feb;95(2):333–339. doi: 10.1128/jb.95.2.333-339.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. II. Elimination of resistance factors with acridine dyes. J Bacteriol. 1961 May;81:679–683. doi: 10.1128/jb.81.5.679-683.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J. Structural requirements for the binding of ethidium to nucleic acids. Biochim Biophys Acta. 1966 Feb 21;114(2):234–244. doi: 10.1016/0005-2787(66)90305-4. [DOI] [PubMed] [Google Scholar]


