Abstract
Background/Objectives
Fall prevention programs implemented in primary care have had variable success in preventing falls and fall-related injuries. We investigated whether a program that improved the quality of care for falls also reduced the number of episodes of care for serious fall-related injuries.
Design
Non-randomized controlled trial.
Setting
Four community-based primary care practices.
Participants
Patients ≥ 75 years who screened positive for fall risk.
Intervention
A multi component quality improvement program (ACOVE prime) involving face-to-face clinician education about falls and decision support to prompt primary care providers to implement appropriate care, including referral to appropriate community resources, in response to patients screening positive for fall risk.
Measurements
Episodes of care for selected fall-related injuries, based on health care claims.
Results
Of the 1791 patients with data available for analysis, 1187 were in the intervention group and 604 patients were in the control group. Mean age was 83, and over two-thirds of the sample were women. After adjusting for potential confounders there were no statistically significant differences between intervention and control groups in episodes of care for fall-related injuries during the 12 month (incidence rate ratio, 1.27; 95% CI 0.93–1.73) or 24 month (incidence rate ratio, 1.18; 95% CI 0.93–1.49) period subsequent to initiation of the intervention.
Conclusion
Despite improving the care of falls, this quality improvement initiative did not result in a change in the number of episodes of care for serious fall-related injuries. Future work in community-based settings should test higher-intensity interventions to reduce fall-related injuries.
Keywords: quality improvement, practice redesign, ACOVE, falls, fall-related injuries
INTRODUCTION
A large body of evidence suggests that appropriate interventions implemented in research settings can reduce falls and fall-related injuries in community-dwelling older people.1, 2 Single interventions such as exercise appear to be effective, and although results are more heterogeneous for multifactorial interventions, these approaches can be effective as well. Although the efficacy of interventions to reduce falls has been demonstrated in research settings, how broadly these findings apply across typical patients and care settings is unknown. Recent research in fall prevention has been more pragmatic in an attempt to reduce falls across a broader spectrum of care settings and patient populations, with mixed results.3–6 Some investigators have questioned whether single interventions (e.g., exercise) should be preferred to multifactorial interventions given the complexity of implementing a multifactorial program.7 Nonetheless, the American Geriatrics Society/British Geriatrics Society practice guidelines currently recommend a multifactorial approach,8 and the Centers for Disease Control and Prevention has recently created a toolkit to help providers implement a multifactorial fall prevention strategy.9
In a controlled multisite trial, we showed that a primary care practice redesign intervention at five geographically distinct community-based medical groups could improve delivery of recommended care to prevent falls in patients age ≥ 75 at increased risk.10 This intervention is notable in that the research team focused on providing technical assistance to each practice, but the practices carried out the intervention as a quality improvement project using their own staff, with flexibility in implementation. In the current study, we use a pragmatic analysis of health care claims data to determine whether this multifactorial quality improvement intervention was successful in reducing episodes of care for fall-related injuries. Our analysis is pragmatic in including all patients found to be at increased risk for falls, with no exclusions, to determine a realistic estimate of intervention effectiveness among patients being served by the participating practices.
METHODS
This project was approved by the UCLA Institutional Review Board (IRB) and four participating sites either approved the project via their own IRB or deferred to the UCLA IRB. (A fifth site was able to obtain approval only to obtain claims from decedents; data from this site are excluded here.)
Intervention and Participants
The ACOVE prime study was a controlled trial of a practice-based quality improvement intervention to improve care for falls and incontinence in five medical groups, hereafter referred to as sites.10 Each participating site needed to have both an intervention and a control practice (or be able to identify another local practice that could serve as a control); site leaders made their own decision as to which practice would serve as the intervention practice. In both intervention and control practices, the study screened patients age ≥ 75 years to identify individuals at high risk for future falls, with the following questions:11
Have you fallen two or more times in the past 12 months?
Have you fallen and hurt yourself since your last visit to the doctor?
Are you afraid that you might fall because of balance or walking problems?
In both intervention and control practices, screening results were made available to the treating primary care provider. Building on a prior study (ACOVE-2), intervention practices implemented the following components: face-to-face clinician education about falls and incontinence at the start of the intervention period, decision support to prompt primary care providers to take appropriate action in response to a positive screen (either through paper-based structured visit note templates or with computerized electronic health record prompts), and patient education handouts referring patients to appropriate community resources (e.g., exercise programs for fall prevention).11 ACOVE prime also included an audit and feedback component in which providers abstracted their own charts and received feedback where improvement was needed. By design, all sites implemented all components of the intervention, but there was flexibility about how decision support was implemented and how patient education materials were created and used.
The ACOVE prime intervention started at each site in a staggered fashion; the four sites analyzed in this study began the intervention on October 30, 2006 (Site A); January 8, 2007 (Site B); January 1, 2007 (Site C); and March 26, 2007 (Site D). Patient screening that was tracked by the research team ended 12 months after initiation of the intervention at the site, or on December 31, 2007, whichever came first. In a sample of 1037 patients whose medical records were reviewed, intervention patients received 60% of recommended care for falls during the intervention period, compared to 37.6% for controls (p<0.001).10
Data
We obtained Medicare enrollment and claims data on participants at four of the five sites for calendar years 2005–2009, for all individuals screening positive for at least one of the fall-related screening items. These data create a follow-up period of at least 33 months from intervention onset, and a minimum 22-month pre-intervention period, for all individuals with claims. Medicare eligibility was determined based on the Master Beneficiary Summary File, and fee-for-service Medicare claims data were obtained from MedPAR, outpatient, hospice, home health agency, durable medical equipment, and carrier files. In addition, for participants enrolled in a Medicare Advantage plan at the time of the intervention, we attempted to obtain both institutional and professional encounter data from the six participating health plans that had at least 50 members in the study, and were successful in obtaining data from five plans. Of the 2,022 patients potentially eligible from the four sites, 77 were either not enrolled in Medicare or sites did not have a valid Medicare ID for the patient. In addition, for 154 patients, we could not obtain managed care data. We analyzed data on the remaining 1,791 patients.
Outcome
Since we could not find any claims-based algorithms for fall-related injuries that were validated against medical records, we adapted an approach used for osteoporotic fractures.12 We extracted information on five types of serious injuries: hip fractures, other non-vertebral fractures, inpatient head trauma, joint dislocations, and health care claims where an external cause of injury code (“E code”) for falls was used. Vertebral fractures were excluded because only one quarter are caused by falls from standing height or less.13 Similar to other studies using claims, the definition of the outcome of fall-related injury was designed to provide a pragmatic balance of sensitivity and specificity, based on clinical judgment and prior work.4, 14, 15 Episodes of care where an E-code indicated that a fall had occurred were automatically included, regardless of the underlying injury. Conversely, if an E-code indicated a cause that was not a fall, we excluded these cases, even if the underlying injury is commonly fall-related. We made this decision because E-codes describing the cause of injury are likely to be specific (E-codes are not required for payment, therefore there is little incentive to use an E-code to indicate a cause of injury unless that cause actually occurred). We used combinations of ICD-9-CM, Healthcare Common Procedure Coding System (HCPCS), and E-codes to identify incident cases, using a coding hierarchy that built on previous work.12 Our coding hierarchy is available upon request; Table 2 shows detailed types of injuries with associated ICD-9-CM and E-codes.
Table 2.
Number and type of fall-related injuries since intervention inception*
| Total | Intervention | Control | ||
|---|---|---|---|---|
| Total number of person-years** | 4579.4 | 3068.0 | 1511.4 | |
| Total number of episodes (incidence rate per 1000 person years)*** | 679 (148) | 462 (151) | 217 (144) | |
| Injury Type: | Diagnosis codes | |||
| Hip fracture episodes (incidence rate per 1000 person years) | 122 (27) | 79 (26) | 43 (28) | |
| Hip | 820.xx | 122 | 79 | 43 |
| Other fracture episodes (incidence rate per 1000 person years) | 260 (57) | 172 (56) | 88 (58) | |
| Pelvis | 808.xx | 31 | 18 | 13 |
| Rib(s) | 807.0x – 807.1x | 33 | 26 | 7 |
| Clavicle | 810.xx | 10 | 5 | 5 |
| Humerus | 812.xx | 42 | 25 | 17 |
| Radius & ulna | 813.xx | 61 | 39 | 22 |
| Carpal - navicular, etc. | **** | 20 | 11 | 9 |
| Hand - metacarpal | 815.xx | 14 | 10 | 4 |
| Hand - phalanges | 816.xx | 13 | 10 | 3 |
| Hand - multiple | 817.xx | 0 | 0 | 0 |
| Femur, tibia, fibula | 821.xx, 823.xx | 67 | 44 | 23 |
| Patella | 822.xx | 5 | 5 | 0 |
| Ankle | 824.xx | 23 | 16 | 7 |
| Head Injury episodes (incidence rate per 1000 person years) | 55 (12) | 28 (9) | 27 (18) | |
| Head fracture - vault | 800.xx | 0 | 0 | 0 |
| Head fracture - base | 801.xx | 1 | 0 | 1 |
| Head fracture - face | 802.xx | 18 | 7 | 11 |
| Head fracture - other | 803.xx | 0 | 0 | 0 |
| Head fracture - multiple | 804.xx | 0 | 0 | 0 |
| Head trauma | 850.xx–854.xx | 41 | 22 | 19 |
| Joint dislocation episodes (incidence rate per 1000 person years) | 20 (4) | 15 (5) | 5 (3) | |
| Shoulder | 831.xx | 7 | 5 | 2 |
| Elbow | 832.xx | 0 | 0 | 0 |
| Wrist | 833.xx | 1 | 0 | 1 |
| Knee | 836.xx | 12 | 10 | 2 |
| Fall requiring medical care (incidence rate per 1000 person years) | E Codes | 492 (107) | 338 (110) | 154 (102) |
| Stairs or steps | E880.x | 10 | 6 | 4 |
| Ladders or scaffolding | E881.x | 1 | 1 | 0 |
| Building or structure | E882 | 0 | 0 | 0 |
| Hole | E883.x | 0 | 0 | 0 |
| One level to another | E884.x | 57 | 47 | 10 |
| Same level - tripping | E885.x | 124 | 92 | 32 |
| Same level - pushed | E886.x | 1 | 0 | 1 |
| Other and unspecified | E888.x | 397 | 270 | 127 |
This table provides counts of fall-related injuries, by injury type, as well as counts of different types of external cause of injury codes.
The table also provides the counts and incidence rate for all injuries combined, as well as for the major injury categories (hip fracture, other fractures, head injury, joint dislocation, and falls requiring medical care).
Based on primary and secondary ICD 9 diagnosis codes, as well as external cause of injury (“E”) codes.
Number of person-years of observation from the inception of the intervention at each site to the end date for availability of claims data.
The number of episodes of fall-related injuries from the inception of the intervention at each site to the end date for availability of claims data. Since a given episode may involve multiple injuries, listed injuries are not mutually exclusive; therefore, the numbers for each specific type of injury do not add up to the total number in the larger categories of injury, and the number of episodes in the larger categories do not add up to the total number of episodes.
ICD-9 codes 814, 814.0, 814.00, 814.01, 814.09, 814.1, 814.10, 814.11, 814.19
The coding hierarchy classifies episodes of care as either inpatient or outpatient, based on the highest resource intensity of care received during the episode. For fee-for-service Medicare claims, inpatient care included any claim in the MedPAR file, which covers short-term acute care hospital, long-stay hospital, and post-acute skilled nursing facility claims paid by Medicare. Outpatient care reflects claims from any of the other fee-for-service claims files (predominantly the carrier file and outpatient facility file), and includes Emergency Department care. For data received from Medicare Advantage plans, we created definitions of inpatient and outpatient care paralleling the approach to fee-for-service claims.
We took certain precautions to minimize the potential for misclassification of events based on claims data. First, unless an E-code for falls was provided, we restricted eligible claims to serious injuries that were likely to be fall-related in people age ≥ 75.14 Second, to avoid double-counting fall-related injuries due to multiple claims for a single injury, a fall-related injury claim could only count towards defining a unique injury episode if there were no prior claims for fall-related injury, or the claim occurred at least 30 days after a previous claim that counted toward the definition of an earlier fall-related injury episode. We also conducted sensitivity analyses analyzing only each participant’s first fall-related injury episode.
Statistical analysis
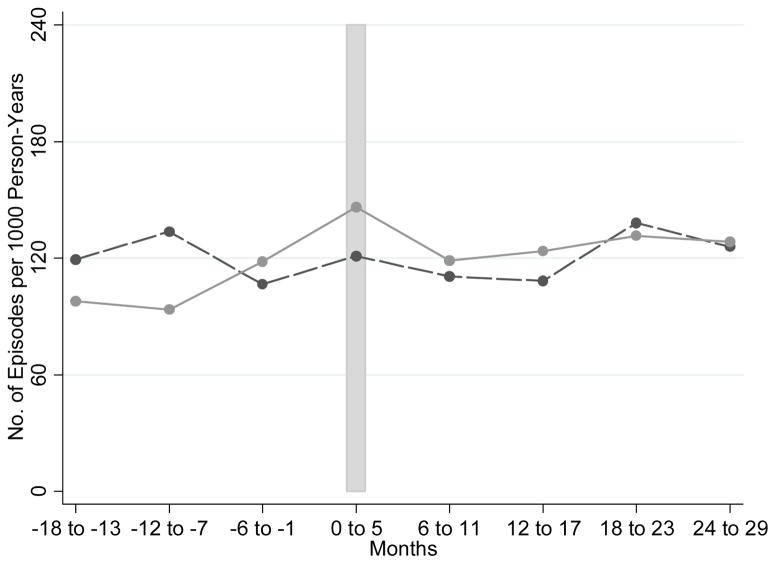
Our analysis began with descriptive statistics on available data, including age, gender, Elixhauser comorbidity count,16 the presence/absence of at least one fall (using our claims-based episode definition) during the 22-month period prior to the start date of the intervention at each site, and the responses to the falls screening questions. All of these variables have a potential relationship with treatment assignment (since assignment was at the level of the practice and practices often vary in their patient demographics) and with the outcome of interest, and were therefore candidates for multivariable modeling. We did not include race/ethnicity in our model because, although race/ethnicity may influence osteoporosis risk, and therefore fracture risk, only 1.7% of our sample was non-white. We examined unadjusted outcomes using a graph showing fall-related injury episodes per 1000 person-years of follow-up(Figure 1).
Figure 1.
Episodes of care for fall-related injuries per 1000 person years, reported semi-annually (solid line = intervention group; dashed line = control group).
The vertical bar represents the intervention start period for each site. Each dot represents the total number of episodes of care for fall-related injuries per 1000 person-years for a six month time interval. Month zero represents the intervention start month for each site.
Because participants were not randomized, primary analyses were based on a multivariable regression framework to adjust for potential observed confounders. We hypothesized that the strongest intervention effect would be seen in the first 12 months (the time period during which the intervention was formally ongoing). Accordingly, our primary analysis focused on the 12 months beginning with the intervention start date at each site. We also calculated results using 24 months of data, beginning with the intervention start date.
We selected negative binomial regression as the primary modeling approach to allow all fall-related injury episodes, including recurrent episodes in a given participant, to be counted. Negative binomial regression is often used in utilization count analysis because of its interpretation as a mixture of Poisson distributions with rates that reflect individuals’ unobserved propensities to fall.17, 18 We included the independent variables described above and dummy variables for study sites in an initial model, and all variables were retained, except for the variable indicating a positive screen for urinary incontinence. Although urinary incontinence is a risk factor for falls,19 the variable as measured represents only the subset of individuals with urinary incontinence seeking additional information about treatment; we found no association between this variable and the outcome in preliminary analyses. In the model, we also included a dichotomous variable for the small proportion (4.6%) of cases where there was evidence of missing claims data in the 22-month period prior to intervention start, since such cases might have a falsely low rate of claims-detected injuries or comorbidities in the baseline period.
Once we finalized the negative binomial regression model, we also ran Cox proportional hazards regression models with time to first fall-related injury as the outcome, as a sensitivity analysis. Since both approaches resulted in similar findings, we present only the negative binomial models here. All statistical analyses were performed using STATA/SE 11.2 (College Station, TX).
Prior to obtaining data, we performed power calculations. We assumed capture of data from all ACOVE prime participants. We calculated the percent reduction in injuries needed for 80% power in a 1-sided test of proportions at the 5% significance level. Given a 14.3% injury rate we were powered to detect a 24.5% reduction in injuries.
RESULTS
Of the 1,791 patients with data available for analysis, 1,187 were in the intervention group and 604 were in the control group. Table 1 shows participant characteristics. Intervention patients were younger than controls (mean age 82.7 years versus 83.3 years, p=0.03) and had fewer comorbidities (3.3 versus 3.9, p<0.0001). The percent of women in the intervention and control groups was 71% and 74%, respectively (p=0.09). A large majority of the sample reported being afraid of falling due to balance or walking problems; over one-third reported two or more falls in the past year, and about one quarter reported having had a fall with injury since their last visit to the doctor. There was no statistically significant difference in the percent of intervention and control patients responding positively to each screening question. The percent of intervention and control participants from each site varied (p<0.0001); site A had 66% of the total intervention participants but only 35% of controls.
Table 1.
Participant Characteristics
| Intervention (N=1187) Mean (SD) or percent |
Control (N=604) Mean (SD) or percent |
P-value*** | |
|---|---|---|---|
|
| |||
| Age at start of intervention (yrs) | 82.7 (5.4) | 83.3 (5.2) | 0.03 |
|
| |||
| Women (%) | 71 | 74 | 0.09 |
|
| |||
| Elixhauser comorbidity count | 3.3 (2.5) | 3.9 (2.8) | <0.0001 |
|
| |||
| Claim for fall-related injury in pre-intervention period (%) | 15 | 17 | 0.04 |
|
| |||
| Responses to screening questions (%)* | |||
| ≥ 2 falls in the past year | 38 | 35 | 0.18 |
| Fall with injury since last visit to MD | 23 | 25 | 0.39 |
| Fear of falling due to balance or walking problems | 83 | 86 | 0.14 |
| Bothersome urinary incontinence, desiring treatment | 37 | 34 | 0.34 |
|
| |||
| Site (%)** | <0.0001 | ||
| Site A | 66 | 35 | |
| Site B | 5 | 32 | |
| Site C | 11 | 14 | |
| Site D | 18 | 19 | |
Participants could choose more than one response to the fall screening items.
Expressed as percent of intervention and control participants from each site. Column total equals 100%.
P value measured by t-test for continuous variables and Pearson chi square test for categorical variables.
In the 22-month period prior to intervention start, there were 103 episodes of fall-related injuries per 1000 person-years for the intervention group and 115 episodes per 1000 person-years for the control group, across 2233.6 and 1174.8 person-years of observation, respectively. Subsequent to the start of the intervention, there were 151 episodes of fall-related injuries per 1000 person-years for the intervention group and 144 episodes per 1000 person-years for the control group, across 3,068.0 and 1,511.4 person-years of observation, respectively. Figure 1 shows the incidence of episodes of care for fall-related injuries by intervention and control group status, over time.
Table 2 shows the types of injuries patients experienced. Hip fractures were the most common single type of injury, but collectively, other non-vertebral fractures were more frequent. Head injuries and joint dislocations were less common. E-codes were generally non-specific about the mechanism of the fall, with the “other and unspecified” category most commonly used. Incidence rates for different types of fall-related injuries did not show unusual imbalances by intervention and control group status.
Negative binomial regression analyses (Table 3) showed no statistically significant difference in the number of fall-related injury episodes between intervention and control groups during the 12 month period (incidence rate ratio, 1.27; 95% CI 0.93–1.73) or 24 month period (incidence rate ratio, 1.18; 95% CI 0.93–1.49) subsequent to the initiation of the intervention, after adjusting for potential confounders. Positive predictors of fall-related injuries included older age, higher Elixhauser comorbidity score, a claims-detected fall-related injury prior to intervention start, a positive screen for at least two prior falls in the past year, and a positive screen for fall with injury since the last visit to the physician. Sites varied in their fall-related injury rates, with site D having lower rates of fall-related injuries compared to site A at 12 and 24 months, and site C having lower rates of fall-related injuries than site A at 24 months.
Table 3.
Adjusted Results Associated with Fall-related Injuries*
| Incidence Rate Ratio (IRR) and 95% Confidence Interval | ||
|---|---|---|
|
| ||
| 12 months | 24 months | |
|
| ||
| Intervention group | 1.27 (0.93–1.73) | 1.18 (0.93–1.49) |
|
| ||
| Age (per additional decade) | 1.27 (1.00–1.62) | 1.41 (1.17–1.70) |
|
| ||
| Female | 1.20 (0.88–1.64) | 1.16 (0.92–1.47) |
|
| ||
| Claims-based Elixhauser comorbidity score (per additional comorbidity) | 1.09 (1.04–1.15) | 1.11 (1.07–1.15) |
|
| ||
| Claim for fall-related injury in pre-intervention period | 1.44 (1.05–1.97) | 1.38 (1.08–1.77) |
|
| ||
| Responses to screening questions | ||
| ≥2 falls in the past year | 1.59 (1.20–2.11) | 1.61 (1.30–2.00) |
| Fall with injury since last visit to MD | 1.84 (1.37–2.46) | 1.42 (1.12–1.79) |
| Fear of falling due to balance or walking problems | 0.98 (0.70–1.37) | 1.09 (0.82–1.43) |
|
| ||
| Site** | ||
| Site B | 0.88 (0.59–1.33) | 0.97 (0.72–1.32) |
| Site C | 0.69 (0.43–1.10) | 0.61 (0.42–0.89) |
| Site D | 0.66 (0.44–0.99) | 0.62 (0.45–0.84) |
Time zero represents the start date of the intervention at each site.
Also adjusted with dichotomous variable for presence of missing claims data in the pre-intervention period.
Site A is reference group
DISCUSSION
In this study, we did not find a discernible effect of a fall prevention quality improvement program on episodes of care for serious fall-related injuries. There are many possible explanations that either independently or in combination may account for our findings. One possibility is that the non-randomized design prevented our controlling sufficiently for unmeasured differences, such as differences in functional status or mobility, between intervention and control groups; these unmeasured differences may obscure a small intervention effect. Another possibility is that some key care processes that prevent fall-related injuries were already being performed at a high level in the control group, higher than might have been the case among control groups of the traditional clinical trials whose evidence base we relied upon for our power calculations. For example, exercise programs, which are powerful interventions to reduce falls and fall-related injuries in their own right,1, 2 were offered to 68% of control participants and 89% of intervention participants who were found to have deficits in gait, balance, strength and endurance.10 Since two-thirds of eligible control participants were offered a key intervention to prevent falls, the ability to detect differences in outcomes between intervention and control participants may be limited.
A third explanation relates to the lack of exclusion criteria in this quality improvement program. The entire population at risk was studied in this analysis, potentially including individuals often excluded from clinical trials (e.g., individuals with cognitive impairment and patients with limited life expectancy). This may have diluted the ability to detect an effect, although it enhanced the study’s generalizability. A fourth and related possibility is that individuals in the study sample were too frail to benefit from the intervention. Although outcome definitions differed, the incidence rate of fall-related injuries in our overall sample subsequent to the start of the intervention was 148 per 1,000 person years, about double the rate of fall-related use of medical services reported in the control population for a successful intervention to reduce falls in Connecticut.4
Finally, the program may have had insufficient intensity to reduce serious fall-related injuries, even though it increased performance of care processes that have been associated with reduced fear of falling20 and are part of successful multifactorial interventions to prevent falls.10, 21 Although the quality indicators for falls used in this study cover both an appropriate assessment for falls as well as evidence-based interventions,21 the intensity of the intervention required to pass the quality indicator was likely lower than in the research studies that demonstrated a reduction in falls. For example, appropriate care offered by the provider and documented in the medical record may not have been accepted by the patient; or care may have been accepted by the patient but logistical factors (e.g., transportation or finances) may have interfered with receiving the intervention (e.g., exercise, home modification). In either scenario, the quality indicator would have been satisfied but there would be no reason to expect a reduction in fall risk.
Interventions to prevent falls in non-research settings have met with varying degrees of success. Using a quasi-experimental population-based design, the evaluation of the Connecticut Collaboration for Fall Prevention demonstrated a 9% statistically significant relative reduction in fall-related injuries in an intervention service area, relative to a control area, among people age ≥ 70.4 The study was notable for its long duration (3 years) and high intensity, with outreach to multiple groups of providers in different settings (primary care, home care, rehabilitation, Emergency Department), senior center personnel, and older adults; it also included a patient activation component.22 Two other programs, one in New Zealand and one in the Netherlands, were pragmatic randomized trials. Both were based in the health care system, focused mainly on healthcare providers, and used the existing delivery system to varying degrees to provide necessary interventions. Both studies were lower intensity and shorter duration than the Connecticut study, and both found statistically non-significant reductions in falls among patients participating in the intervention (4% relative reduction in New Zealand; 14% relative reduction in the Netherlands).5, 6 These findings cannot be compared directly with the Connecticut study, because they are not population-based and they did not include fall-related injury rates. In comparison, our study used a quality improvement approach that combined time-limited technical assistance with full reliance on the existing delivery system to provide care, and would be categorized as low cost but very low intensity; our outcome assessment focused on patients screening positive for high fall risk, but no further exclusions were applied.
These findings should be interpreted in the context of the study’s limitations. First, we had limited statistical power to detect small reductions in fall-related injuries. Although injury rates were consistent with our pre-specified power calculations, the actual number of patients on whom we were able to obtain data was below what we expected due to inability to obtain claims data from one site and some Medicare Advantage patients. Second, the claims data algorithm we developed has not been validated against medical record review. Previous work involving actual medical record review shows that the vast majority of injuries we included are expected to be fall-related,14 and claims for fractures have shown high positive predictive value for fractures in the medical record,23 but injuries could still be incorrectly coded in claims. Third, because this work was based on a quality improvement study, only claims data and patient screening information were available to adjust for baseline differences between the intervention and control groups; thus, the observed findings may be biased by residual unmeasured differences between intervention and control groups. Fourth, our claims-based approach could not capture fall-related injuries that did not come to medical attention, thus limiting the injuries we captured to more serious events. Fifth, we likely underestimated non-fracture-related injuries since most non-fracture events (other than selected joint dislocations and head injuries) would only be included if an E-code were also present.
Strengths of this work include a pragmatic approach to gathering data, selection of an outcome that is both meaningful to patients and to the healthcare system, and an intervention that included a generalizable study population and allowed for flexible implementation.
In summary, we conclude that a program that improved the quality of care for falls in a primary care setting had insufficient intensity to reduce episodes of care for fall-related injuries. Future research should involve medical record validation of claims-based approaches to gathering data on fall-related injuries, as well as testing of higher-intensity interventions to reduce falls and fall-related injuries in a general population of older people who seek care in community settings.
Acknowledgments
We thank Beate Danielsen, Health Information Solutions; James Byrkit, MD and Mark Fischl, MD, Salem Clinic; Therese M. Pedace and Thomas R. Loepfe, MD, Mayo Clinic Health System-Franciscan Healthcare; Alison Condon, RN and Carl Sahler, MD, Canandaigua Medical Group; Patricia Gallacher, Patricia Kane and Edward Dzielak, DO, Physicians Health Alliance; and Ethan Corona, Excellus Blue Cross Blue Shield; for help in obtaining data. We thank Robin Beckman for programming assistance.
Support: This project was supported by a grant from the National Institute on Aging (R01AG036776). Dr. Jennings was supported by the UCLA Claude Pepper Older Americans Independence Center funded by the National Institute on Aging (5P30AG028748)33 and the NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR000124.
Sponsor’s Role: None - The sponsor had no role in the design, methods, subject recruitment, data collection, analysis or preparation of paper
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
- David A. Ganz, MD, PhD: conception and design, analysis and interpretation of data; drafting the article; revising the article critically for important intellectual content; final approval.
- Sung-Bou Kim: analysis and interpretation of data; revising the article critically for important intellectual content; final approval.
- David S. Zingmond, MD, PhD: conception and design; acquisition of data, analysis and interpretation of data; revising the article critically for important intellectual content; final approval
- Karina D. Ramirez, BA: acquisition of data, analysis and interpretation of data; revising the article critically for important intellectual content; final approval.
- Carol P. Roth, RN, MPH: analysis and interpretation of data; revising the article critically for important intellectual content; final approval
- Lee Jennings, MD, MSHS: analysis and interpretation of data; revising the article critically for important intellectual content; final approval
- Takahiro Mori, MD, MSHS: analysis and interpretation of data; revising the article critically for important intellectual content; final approval
- Emmett B. Keeler, PhD: conception and design; analysis and interpretation of data; revising the article critically for important intellectual content; final approval
- Neil S. Wenger, MD, MPH: conception and design, acquisition of data, analysis and interpretation of data; revising the article critically for important intellectual content; final approval.
- David B. Reuben, MD: conception and design, acquisition of data, analysis and interpretation of data; revising the article critically for important intellectual content; final approval.
References
- 1.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Khoury F, Cassou B, Charles MA, et al. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f6234. doi: 10.1136/bmj.f6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faes MC, Reelick MF, Melis RJ, et al. Multifactorial fall prevention for pairs of frail community-dwelling older fallers and their informal caregivers: A dead end for complex interventions in the frailest fallers. J Am Med Dir Assoc. 2011;12:451–458. doi: 10.1016/j.jamda.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Tinetti ME, Baker DI, King M, et al. Effect of dissemination of evidence in reducing injuries from falls. N Engl J Med. 2008;359:252–261. doi: 10.1056/NEJMoa0801748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elley CR, Robertson MC, Garrett S, et al. Effectiveness of a falls-and-fracture nurse coordinator to reduce falls: A randomized, controlled trial of at-risk older adults. J Am Geriatr Soc. 2008;56:1383–1389. doi: 10.1111/j.1532-5415.2008.01802.x. [DOI] [PubMed] [Google Scholar]
- 6.Hendriks MR, Bleijlevens MH, van Haastregt JC, et al. Lack of effectiveness of a multidisciplinary fall-prevention program in elderly people at risk: A randomized, controlled trial. J Am Geriatr Soc. 2008;56:1390–1397. doi: 10.1111/j.1532-5415.2008.01803.x. [DOI] [PubMed] [Google Scholar]
- 7.Campbell AJ, Robertson MC. Fall prevention: single or multiple interventions? Single interventions for fall prevention. J Am Geriatr Soc. 2013;61:281–284. doi: 10.1111/jgs.12095_2. discussion 286–287. [DOI] [PubMed] [Google Scholar]
- 8.Summary of the Updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 9.Stevens JA, Phelan EA. Development of STEADI: A fall prevention resource for health care providers. Health Promot Pract. 2013;14:706–714. doi: 10.1177/1524839912463576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wenger NS, Roth CP, Hall WJ, et al. Practice redesign to improve care for falls and urinary incontinence: Primary care intervention for older patients. Arch Intern Med. 2010;170:1765–1772. doi: 10.1001/archinternmed.2010.387. [DOI] [PubMed] [Google Scholar]
- 11.Reuben DB, Roth C, Kamberg C, et al. Restructuring primary care practices to manage geriatric syndromes: The ACOVE-2 intervention. J Am Geriatr Soc. 2003;51:1787–1793. doi: 10.1046/j.1532-5415.2003.51565.x. [DOI] [PubMed] [Google Scholar]
- 12.Taylor AJ, Gary LC, Arora T, et al. Clinical and demographic factors associated with fractures among older Americans. Osteoporosis Int. 2011;22:1263–1274. doi: 10.1007/s00198-010-1300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper C, Atkinson EJ, O’Fallon WM, et al. Incidence of clinically diagnosed vertebral fractures: A population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 14.Tamblyn R, Reid T, Mayo N, et al. Using medical services claims to assess injuries in the elderly: Sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–194. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 15.Tinetti ME, Han L, Lee DS, et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588–595. doi: 10.1001/jamainternmed.2013.14764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Robertson MC, Campbell AJ, Herbison P. Statistical analysis of efficacy in falls prevention trials. J Gerontol A Biol Sci Med Sci. 2005;60:530–534. doi: 10.1093/gerona/60.4.530. [DOI] [PubMed] [Google Scholar]
- 18.Ullah S, Finch CF, Day L. Statistical modelling for falls count data. Accid Anal Prev. 2010;42:384–392. doi: 10.1016/j.aap.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Deandrea S, Lucenteforte E, Bravi F, et al. Risk factors for falls in community-dwelling older people: A systematic review and meta-analysis. Epidemiology. 2010;21:658–668. doi: 10.1097/EDE.0b013e3181e89905. [DOI] [PubMed] [Google Scholar]
- 20.Min LC, Reuben DB, Adams J, et al. Does better quality of care for falls and urinary incontinence result in better participant-reported outcomes? J Am Geriatr Soc. 2011;59:1435–1443. doi: 10.1111/j.1532-5415.2011.03517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang JT, Ganz DA. Quality indicators for falls and mobility problems in vulnerable elders. J Am Geriatr Soc. 2007;55:S327–S334. doi: 10.1111/j.1532-5415.2007.01339.x. [DOI] [PubMed] [Google Scholar]
- 22.Baker DI, King MB, Fortinsky RH, et al. Dissemination of an evidence-based multicomponent fall risk-assessment and -management strategy throughout a geographic area. J Am Geriatr Soc. 2005;53:675–680. doi: 10.1111/j.1532-5415.2005.53218.x. [DOI] [PubMed] [Google Scholar]
- 23.Ray WA, Griffin MR, Fought RL, Adams ML. Identification of fractures from computerized Medicare files. J Clin Epidemiol. 1992;45:703–714. doi: 10.1016/0895-4356(92)90047-q. [DOI] [PubMed] [Google Scholar]