Abstract
Objective
The aim of this study was to evaluate the clinical use of a method to assess hemispheric language dominance in pediatric candidates for epilepsy surgery. The method is designed for patients but has previously been evaluated with healthy children.
Methods
Nineteen patients, 8–18 years old, with intractable epilepsy and candidates for epilepsy surgery were assessed. The assessment consisted of two functional MRI protocols (fMRI) intended to target frontal and posterior language networks respectively, and a behavioral dichotic listening task (DL). Regional left/right indices for each fMRI task from the frontal, temporal and parietal lobe were calculated, and left/right indices of the DL task were calculated from responses of consonants and vowels, separately. A quantitative analysis of each patient's data set was done in two steps based on clearly specified criteria. First, fMRI data and DL data were analyzed separately to determine whether the result from each of these assessments were conclusive or not. Thereafter, the results from the individual assessments were combined to reach a final conclusion regarding hemispheric language dominance.
Results
For 14 of the 19 subjects (74%) a conclusion was reached about their hemispheric language dominance. Nine subjects had a left-sided and five subjects had a right-sided hemispheric dominance. In three cases (16%) DL provided critical data to reach a conclusive result.
Conclusions
The success rate of conclusive language lateralization assessments in this study is comparable to reported rates on similar challenged pediatric populations. The results are promising but data from more patients than in the present study will be required to conclude on the clinical applicability of the method.
Keywords: Epilepsy surgery, Children, Language lateralization, fMRI, Dichotic listening, Neurodevelopmental disorders
Highlights
-
•
Language lateralization was assessed in 19 pediatric candidates for epilepsy surgery.
-
•
The assessment involved fMRI and an independent behavioral measure; dichotic listening.
-
•
A two step analysis was employed combining fMRI and dichotic listening data.
-
•
For 74% of the subjects a conclusion was reached about hemispheric language dominance.
-
•
The rate of conclusive assessments in this study is comparable to reported rates on similar challenged pediatric populations.
1. Introduction
Today, mapping of brain networks related to language using fMRI methodology is commonly performed as a part of the planning procedure for the neurosurgical treatment of epilepsy in adults and, increasingly so, in pediatric populations (Medina et al., 2004; Swanson et al., 2007). In a study of language fMRI in 209 healthy children between 5 to 18 years of age, the reported rate of successful assessments was approximately 80%, with age being an important factor (Byars et al., 2002). Other researchers have reported that with thorough preparation and training of children one can expect to obtain reliable and useful data in 95% of typically developing children aged 8 and older and in 80% of typically developing children 4–5 years old (O'Shaughnessy et al., 2008). O'Shaughnessy and colleagues report relatively high success rates with older children and early teenagers with neurodevelopmental disorders as well, which is an important aspect since such disorders are common in children with epilepsy (Davies et al., 2003; O'Shaughnessy et al., 2008; Pellock, 2004).
A comparative analysis of the outcome from the usage of language fMRI and of the intracarotid amobarbital, or Wada test, has shown high concordance (Abou-Khalil, 2007; Adcock et al., 2003; Arora et al., 2009; Woermann et al., 2003) but in patients with atypical language lateralization results tend to have lower concordance (Adcock et al., 2003; Bauer et al., 2014; Gaillard et al., 2002). In a recent review (Spritzer et al., 2012), the authors concluded that the amount of data to support a recommendation of the routine usage of language fMRI in pre-surgical examinations is still insufficient. However, there is a fairly broad consensus that language fMRI in general is superior in reliability and validity (Arora et al., 2009; Binder, 2011; Spanaki et al., 2001), and that WADA usually has disputable added value, unless fMRI is inconclusive. Because of the associated risk factors WADA might be avoided on most patients being evaluated for epilepsy surgery (Sharan et al., 2011).
There are several methodological factors of importance to conduct successful language fMRI examinations of pediatric patients to ensure that the collected data are of high quality. The validity and reliability of language fMRI data profoundly depends on the language task used as well as on the control/baseline condition employed. Ideally, an fMRI paradigm should employ a control condition that contains the same subcomponents as the task condition but exclude the cognitive process to be examined (Swanson et al., 2007). Other basic requirements of language fMRI assessments are that the patient has a good understanding of the tasks in the MR-scanner, sufficient motivation to perform the tasks and a good compliance — since fMRI is sensitive to motion. These requirements can largely be dealt with by careful preparation of the patients prior to the scanning procedure (Byars et al., 2002; O'Shaughnessy et al., 2008). Neurodevelopmental disorders are common in patients with epilepsy, such as Attention Deficit Hyperactivity Disorder (ADHD), Autism Spectrum Disorders (ASD), and different forms of learning disabilities. Therefore the language test material should be designed to take into account such potential difficulties for pediatric patients with epilepsy and it should also be possible to adapt the material in accordance with the patient’s language proficiency.
A potential limitation of analyses of language fMRI data that previously has been mentioned in the literature is that visual inspection of fMRI brain activation patterns has often been used in previous studies (Adcock et al., 2003; Berl et al., 2014; Spritzer et al., 2012). Relying only on visual inspection of the results may weaken the reproducibility of the results and also make it more difficult to compare different studies and instead it is suggested to rely on quantitative analyzes.
In a previous study of language lateralization in 17 healthy 10–11 year old children we evaluated newly developed materials and methods designed to minimize the influence of the aspects mentioned above that potentially could decrease the validity and reliability of language fMRI data (Norrelgen et al., 2012). While planning that study we took into consideration that some uncertainty has been reported regarding the interpretation of language fMRI data for patients with intractable epilepsy (Spritzer et al., 2012; Wellmer et al., 2009). For that reason we decided to add an independent measure of language lateralization in order to get a broader basis of data to build a conclusion of language lateralization on, particularly for those cases when fMRI data are limited or inconclusive. The measure that we considered implementing was Dichotic Listening (DL), which is a behavioral assessment of language lateralization. There are several different versions of DL but they are all based on the principle that contralateral auditory cortical projections are stronger than ipsilateral projections (Rosenweig, 1951). Thus when two competing speech stimuli are presented to each ear simultaneously, many times, the average of responses to the stimuli presented to the contralateral ear of the language dominant hemisphere will show an advantage over stimuli presented to the ipsilateral ear. In individuals with typical left-sided language dominance there is thus a Right Ear Advantage (REA). One version of DL is the Fused Dichotic Words Test (FDWT: Wexler and Halwes, 1983). In two studies the FDWT was compared with fMRI (Fernandes et al., 2006) and with WADA (Fernandes and Smith, 2000) for children with intractable epilepsy, and it was concluded that DL provide valid data of language lateralization in a high proportion of cases and that the concordance with fMRI and WADA is high. A German version of the FDWT (Hattig and Beier, 2000) has been compared with language fMRI in two studies of typical subjects. Hund-Georgiadis et al. (2002) found excellent concordance between fMRI and DL but another study concluded that FDWT was not applicable to determine language laterality and that the concordance with fMRI was poor (Bethmann et al., 2007). No clear explanation to the very different outcomes between these studies was given but Bethmann suggest that different scoring criteria between studies may have caused the discrepancy. However, the version of DL that we decided to implement is based on consonant-vowel stimuli (Hugdahl and Asbjørnsen, 1994). This test is easy to administer also with children and does not involve reading (which the original version of FDWT do). In a number of studies this version of DL has been found to have good concordance with WADA (Hugdahl et al., 1997), PET (Hugdahl et al., 1999), fMRI (e.g. van den Noort et al., 2008) and electro-physiological measures of language (Brancucci et al., 2004, 2005).
In our previous study of healthy children, a conclusion regarding language lateralization of each individual was reached based on a well-defined quantitative analysis of the compiled lateralization indices from the frontal-, temporal- and parietal lobes in the two fMRI paradigms and from the two indices from DL (Norrelgen et al., 2012). A conclusive overall result regarding language dominant hemisphere was obtained for 88% of the subjects. We found no contradictory results between DL and fMRI data and in 12% of the cases DL provided critical information for reaching an overall conclusion about hemispheric language dominance. Our conclusion was that the quantitative analysis method, combining data from fMRI and DL, was useful and that the risk of obtaining incorrect results may have been reduced by this approach.
In the present study our aim was to evaluate the use of the method on a group of pediatric epilepsy patients who were candidates for epilepsy surgery. Specifically we wanted to assess the overall success rate and possible influences on success rate of age and neurodevelopmental problems.
2. Materials and methods
2.1. Ethics statement
All examinations were carried out according to the ethical guidelines and declarations of the Declaration of Helsinki (1975) and the current study was approved by the regional ethics committee at the Stockholm County (2008/1826-31). All participants in the study were potential candidates for epilepsy surgery. The neurologist at the hospital informed the parents and the child/adolescent about the study and asked if they were willing to participate in the study. Oral consent to participate in the study was required for participation.
2.2. Patients
In this study, the criteria for the selection of the pediatric patients in this study were that they had intractable focal epilepsy, were potential candidates for epilepsy surgery, and that the pediatric neurologist deemed it likely that they would be able to collaborate in the presurgical language lateralization assessment. The overall functioning of the patient was taken into account for this selection and an IQ < 70, for example, did not necessarily disqualify a patient for participation. Nineteen patients were contacted about participation in the study by their neurologist at the hospital and all conceded to participate. In a second step a research speech language pathologist met with each of the nineteen patients one to two weeks prior to the planned fMRI assessment in order to further evaluate their ability to participate (for details about the evaluation see “Preparation and pre-training for fMRI session” section). If the patient was deemed likely to be able to participate in the language fMRI assessment, detailed information was given to the patient and the parent about the fMRI procedure. Patient data are displayed in Table 1.
Table 1.
Demographic features of patients, comorbidity, etiology, neuropsychological data and handedness.
| Patient | Gender | Age | Comorbidity | Age at epilepsy onset (years) | Etiology of epilepsy | Full scale IQ | Language comprehension (TROG-II) | Handedness |
|---|---|---|---|---|---|---|---|---|
| 1 | Female | 18.2 | None | 5.0 | Left temporal lobe dysplasia incl hippocampus | 87 | 87 | Right |
| 2 | Male | 16.8 | Hemiplegia sin, mild ID | 10.6 | Large area dysplasia right hemisphere | <70a | No data | Right |
| 3 | Male | 14.1 | None | 4.5 | Dysplasia posterior temporal lobe left | 90 | 92 | Right |
| 4 | Male | 9.4 | None | 5.8 | Dysplasia right temporal lobe | 105 | Right | |
| 5 | Female | 18.6 | None | 13 | Unknown, no MRI lesion | 111 | 97 | Right |
| 6 | Male | 17.5 | PDD-NOS | 1.0 | DNET left posterior hippocampus | Normala | 102 | Right |
| 7 | Male | 10.1 | None | 8.5 | Left temporal lobe multiple cavernomas | 109 | 99 | Left |
| 8 | Male | 12.7 | None | 4.0 | DNET left occipital lobe | 91 | 104 | Right |
| 9 | Male | 15.1 | Hemiplegia dx, mild ID | 0.5 | Prenatal stroke left, mesial temporal sclerosis | <70a | 63 | Left |
| 10 | Female | 16.10 | Asperger syndrome | 9.1 | Rasmussen encephalitis (?) right hemisphere | 86 | 87 | Right |
| 11 | Male | 17.2 | None | 0.5 | Left parieto-occipital dysplasia | Normala | No data | Right |
| 12 | Female | 15.8 | None | 10.0 | No lesion, left temporal epileptogenic zone | 79 | No data | Right |
| 13 | Male | 9.10 | ADHD | 0.1 | Hypothalamic hamartoma | 82 | 106 | Right |
| 14 | Female | 15.6 | None | 3.3 | DNET left frontal lobe | 74 | 92 | Right |
| 15 | Male | 17.9 | ADHD | 3.0 | No lesion | 96 | 92 | Right |
| 16 | Male | 8.4 | None | 5.5 | Left temporal epidermoid | 87 | 96 | Right |
| 17 | Male | 14.8 | None | 12.5 | No lesion | 88 | 87 | Right |
| 18 | Male | 13.3 | Hemiplegia dx, mild ID | 0.1 | Neonatal stroke left a cer. media | <70a | 64 | Left |
| 19 | Female | 8.4 | Speech and language impairment | 4.2 | Uncertain lesion, right insular focus | 79 | 96 | Right |
Ravens Progressive Matrices had been administered in these two cases and the results were percentile 70 and 75 respectively indicating non-verbal cognitive functioning within the normal range.
Full scale IQ scores were not available but the diagnosis was mild intellectual disability (i.e. IQ 55–70).
2.3. Cognitive and language comprehension assessment
The cognitive assessment was carried out by a psychologist according to the WISC-IV (Wechsler, 2005) and language comprehension was assessed by a speech language pathologist with the Test for Reception of Grammar (TROG-II: Bishop, 2003). In two cases the cognitive assessment data were based on Ravens Progressive Matrices (Raven et al., 2003). These two results were not converted to IQ scores, instead percentile scores were used (see Table 1).
2.4. Handedness assessment
Dexterity was assessed either by the Edinburgh Handedness Inventory—revised (Dragovic, 2004) or by an assessment of an occupational therapist at the clinic.
2.5. Verb generation paradigm
In the verb generation task recordings of eighty common Swedish nouns with a likely associated coupling to common Swedish verbs were used (e.g. scissor → cut, pen → write, glass → drink), and in the control condition eighty common Swedish adjectives were used (e.g. green, small, light). From these word lists, we created sets with seven nouns or seven adjectives in each set. These sets were later implemented as epochs in the final verb generation fMRI paradigm; the sets with the nouns in the task condition and the sets with the adjectives in the control condition. To reduce the risk of any confusion about the task a prompt was added before each stimulus (e.g. in test condition: –what can one do with a pen, and in control condition: –repeat the word red).
2.6. Listening paradigm
The language material used in this paradigm consisted of thirty short passages (about 35 s long) that had been adapted for children with three levels of language comprehension corresponding to approximately 7–8, 10–11 years of age or 13 years or older; ten passages for each level (Norrelgen et al., 2012). Copies of each recorded passage with reversed speech (i.e. played backwards) was used in the control condition. Reversed speech has been demonstrated to effectively remove semantic processing in similar fMRI tasks (Ahmad et al., 2003; Roder et al., 2002). The sound files had been compiled into two sets for each of the three age groups with each set containing five passages and five copies with reversed speech. These compilations were then implemented as epoch-related episodes in Presentation (Neurobehavioral Systems, http://www.neurobs.com).
2.7. Preparation and pre-training for fMRI session
Between one and two weeks before the fMRI scanning session the patients had been informed in detail about the fMRI procedure. At the time of scanning they were introduced to the staff and were then given a detailed preparation of the scanning procedure in the MR-scanner. The preparation included general information about the MR-scanner and a step-by-step instruction of the fMRI scanning tasks. The latter consisted of verb-generation and word-repetition tasks (see below for details), first overtly and then covertly (as in the scanner), followed by performing a short but complete version of the verb generation paradigm (i.e. including verb generation and word repetition). Next, patients were given instructions about the listening task and the corresponding control task. They were instructed to passively listen to the stories in the listening task and to the sounds in the control task (the latter consisted of speech played backwards but was not described as such but as meaningless speech-like sounds). They were also informed that they would be asked questions about the content of the stories after fMRI scanning. Finally, they performed a recorded complete mini-version of the listening paradigm. The language materials used in the pre-training did not include items that were used in the fMRI sessions. The preparation time needed of each subject was 15–20 min. Immediately after preparation the patients were positioned inside the MR-scanner for subsequent fMRI scanning.
2.8. Image acquisition and fMRI paradigm design
A 1.5 Tesla GE (General Electric Healthcare, Milwaukee) HDxt scanner equipped with a quadrature Tr/Tx head coil was used. Anatomical MR imaging included a high-resolution spoiled gradient recalled 3D T1-weighted image sequence (TR/TE = 24/6 ms, flip = 35°, FOV = 220 × 220, matrix size 256 × 192, (0.75% phase FOV)) that provided whole brain coverage with a spatial resolution of 0.9 × 0.9 × 1.5 mm3 in a coronal slice orientation. Functional MRI image volumes of the brain were acquired using a gradient Echo-Planar Image (EPI) sequence (TR/TE = 2500/40 ms, flip = 90°, FOV = 220 × 220 mm, slice thickness = 4.5 mm, slice gap = 0.5 mm, matrix size = 64 × 64). Each EPI BOLD volume consisted of 32 contiguous axial slices with a spatial resolution of 3.44 × 3.44 × 5 mm3.
Both the listening task and the verb generation task were implemented as block-related fMRI designs. The listening task consisted of epochs of approximately 35 s in length interleaved with periods of reversed speech of equal length. The listening task was divided into two separate fMRI session that each entailed four epochs of speech mixed with five epochs of backward speech (i.e. each session starting and ending with a reversed speech epoch). Similarly, the verb generation task consisted of two separate fMRI sessions, for which each session contained four epochs of verb generation and five of word repetition respectively. The total MRI scanning time was approximately 45 min. In each subject, 150 + 134 EPI image volumes were acquired during the listening tasks and 126 + 126 EPI volumes were obtained for the verb generation task.
2.9. Image analysis
All pre-processing and statistical analyses were carried out in SPM5 (Friston et al., 1994). Initially, all EPI volumes were spatially realigned and corrected for movement and subsequently normalized to the MNI (Montreal Neurological Institute) EPI template within SPM and re-sampled to 2 × 2 × 2 mm3 voxel size. Finally, normalized EPI volumes were smoothed using a spatial filter kernel of FWH = 8 mm. BOLD signal increases pertaining to task-evoked responses in brain activity related to passive listening of speech versus backward speech, as well as verb generation versus word repetition, respectively, were modeled using a general linear model (GLM) as implemented in SPM. Six regressors modeling residual movement related variance (translational and rotational movement) were included in the model as covariates of no-interest. At the individual level, statistical parametrical maps showing brain activation related to verb generation and passive listening were thresholded at p < 0.001 uncorrected. It should be noted that the usage of an uncorrected statistical threshold is justified due to the fact that we were foremost interested in brain activation patterns at the individual level. Further, we believe that the statistical thresholding level chosen here provides a good balance between the risk of type-I versus type-II errors in the statistical analysis of fMRI data, in particular when the focus of investigation is to study brain activity at the individual level. This is also in agreement with previous fMRI studies of brain laterality of language function in pediatric populations (Ahmad et al., 2003; Holland et al., 2007; Shurtleff et al., 2010). Language lateralization was quantified using the index as implemented in the Lateralization Index (LI) in the SPM toolbox (Wilke and Lidzba, 2007), values were computed separately in the frontal, temporal and parietal lobe. Positive values indicate a left-sided hemispheric dominance and negative values indicate a right-sided hemispheric dominance. Regional hemispheric dominance was considered to be present for Laterality indices (LI) ≥ ±0.2 (Binder et al., 1996; Gaillard et al., 2001). In accordance with the previous literature (Ahmad et al., 2003; Binder et al., 1996; Gaillard et al., 2001). The LI values were based on the number of significantly activated voxels at the given threshold for each lobe and hemisphere, respectively.
2.10. Dichotic listening test
The dichotic listening test (Hugdahl and Asbjørnsen, 1994) (DL) consists of consonant-vowel syllables presented pair-wise to both ears simultaneously via a headset (consonants: p, t, k, b, d, g, and vowels: i, a, u). During each trial two different consonant-vowel syllables were presented, one to each ear (e.g. da-bi). The subjects were instructed to say out loud both of the two syllables or one of them if they only managed to perceive one. One test session involved 108 stimulus pair presentations. The subject’s responses were manually registered on a computer by the experimenter. Laterality indices for consonants and for vowels were calculated; right ear minus left ear scores of correctly perceived consonants and vowels, respectively, divided by right ear plus left ear scores × 100. Right ear advantage (REA) was defined as a laterality index equal to or greater than 5 and left ear advantage (LEA) when it was equal to or below −5, no ear advantage (NEA) for indices between 5 and −5 (Hugdahl and Hammar, 1997). REA indicate a left-sided hemispheric dominance and LEA indicate a right-sided hemispheric dominance.
Audiometric screening was performed with all subjects (bilaterally; 125–8000 Hz at 20 dbl threshold).
2.11. Procedure for determining hemispheric language dominance
The procedure to reach a conclusion about hemispheric language dominance consisted of two steps. In the first step fMRI data and DL data were analyzed separately to determine whether the result from each test was conclusive or not. In the second step a composite analysis of these two assessments were performed in order to reach a conclusion regarding hemispheric language dominance. The criteria employed in these procedures are specified in detail in Table 2. For a more detailed description of the rationale behind this procedure see Norrelgen et al. (2012).
Table 2.
The criteria applied for the analyses of the fMRI data, the dichotic listening data, and the criteria for the composite analysis based on the results of the fMRI and the dichotic listening analyses (under points 1, 2 and 3 respectively).
Conclusive result:
Inconclusive result:
1d Only one index value.
Conclusive result:
Inconclusive result:
3 Composite results of fMRI and dichotic listening Conclusive result:
Inconclusive result: 3d Inconclusive fMRI (1c, 1f or 1g) regardless of DL result (2a, 2b or 2c). 3e fMRI result as in 1a contradicted by two indices in DL or, 1b contradicted by one or two indices in DL. |
3. Results
3.1. fMRI data: whole brain analyses
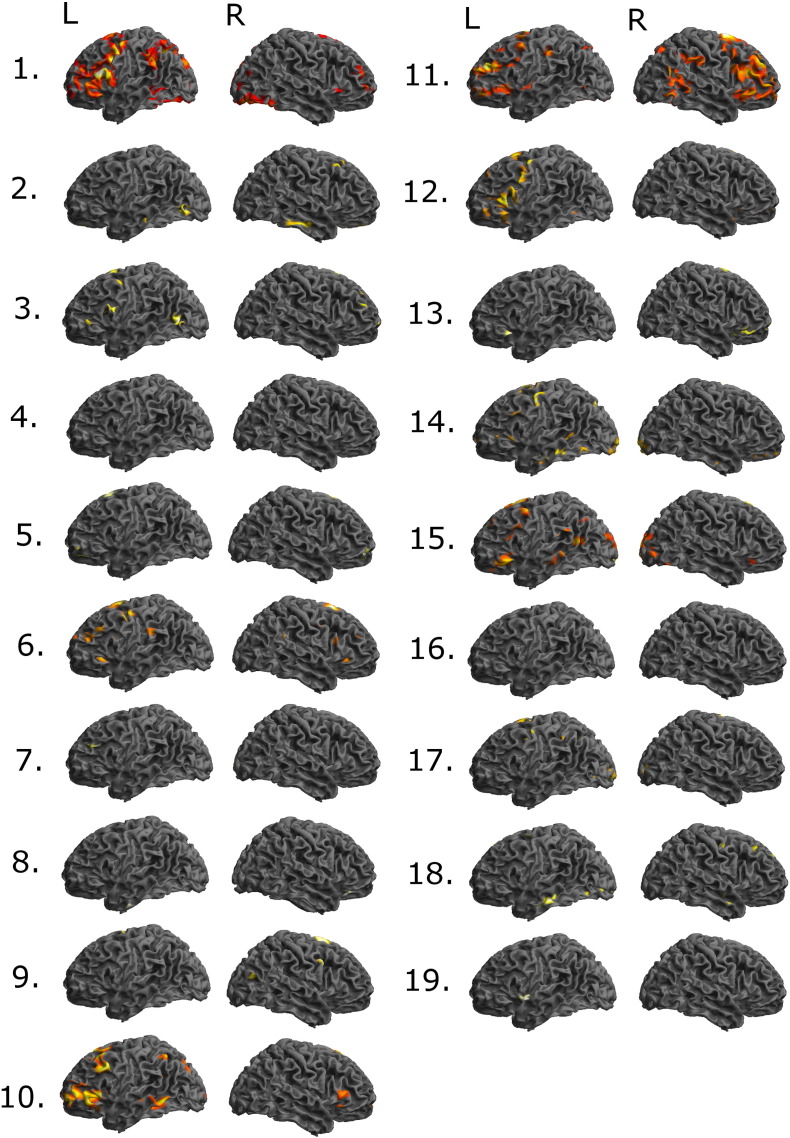
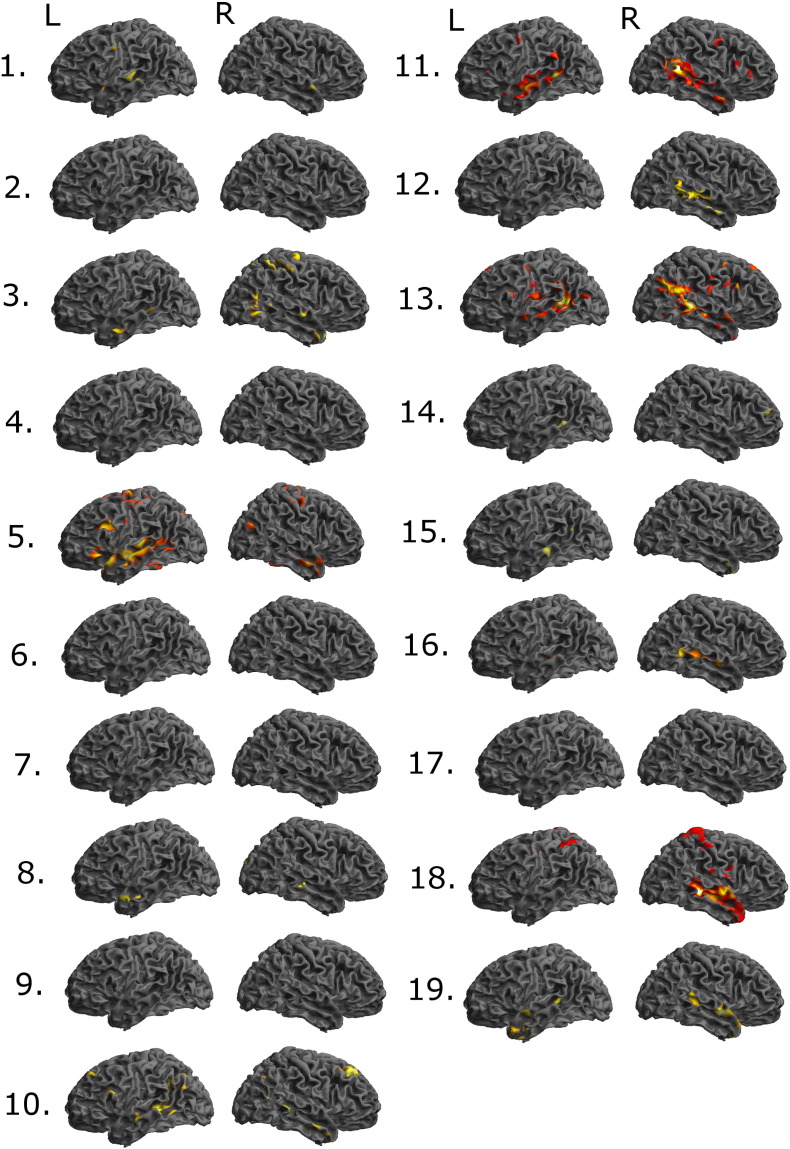
The whole brain analyses of the verb generation task and of the listening task were based on significant BOLD signal changes in response to the task condition contrasted by the control condition (thresholded at p < 0.001, uncorrected at peak level). The results of subjects 1–19 are displayed in 3D surface renderings (Figs. 1 and 2) and in Maximum Intensity Maps (Figs. S1 and S2). In one case activations were deemed to be caused by movement artifacts (patient no 7; Figs. 1 and S1).
Fig. 1.
3D surface renderings of fMRI data of significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the verb-generation paradigm at p < 0.001 uncorrected. Orientation of all projections is shown on the top row (L = left, R = right).
Fig. 2.
3D surface renderings of fMRI data of significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the listening paradigm at p < 0.001 uncorrected. Orientation of all projections is shown on the top row (L = left, R = right).
3.2. fMRI data: laterality index analyses
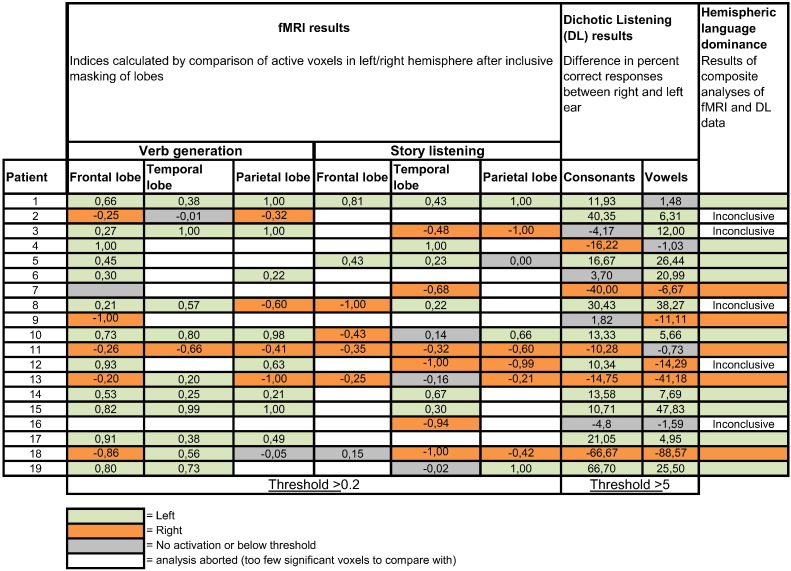
We analyzed the results from the verb generation paradigm and from the listening paradigm in accordance with the criteria (Table 2) to reach a conclusion about the overall result of the fMRI assessment (see Fig. 3). The analysis showed that in thirteen of the nineteen patients (68%) a conclusive result was reached and in six cases (32%) the result was inconclusive (patient nos. 3, 7–9, 12 and 16).
Fig. 3.
Results of the analyses for subject nos. 1 to 19 of the two language fMRI tasks, dichotic listening (DL) and of the composite analyses of fMRI and DL. The hemispheric language dominance results are based on the composite analyses of the fMRI and the DL data according to the criteria specified in Table 2.
3.3. Dichotic listening data analyses
The analyses of the DL data revealed that the results for 17/19 of the patients (89%) were conclusive (see Fig. 3). The results on consonant- and vowel discrimination were contradictory in one case (no 12) and below the threshold in another case (no 16). For two patients (nos. 2 and 4) DL results were contradictory to the fMRI results (in case no. 4 this was of no consequence for the conclusive analysis of hemispheric dominance based on criterion 3b, Table 2).
All subjects passed the audiometric screening.
3.4. Composite analyses of hemispheric language dominance
The analyses of fMRI and DL data combined, based on the criteria under point 3 (see Table 2), revealed that a conclusive overall result regarding hemispheric language dominance was reached for fourteen patients (74 %). In eleven cases criterion 3a was fulfilled (nos. 1, 5, 6, 10, 11, 13–15 and 17–19), one case fulfilled criterion 3b (no. 4) and in two cases criterion 3c was fulfilled (nos. 7 and 9). The results of five patients were inconclusive; one case fulfilled criterion 3e (no. 2) and the four other cases fulfilled criteria 3d (nos. 3, 8 12 and 16) and c (nos. 2, 7 and 9). Nine of the fourteen patients (64 %) with a conclusive result, displayed a left sided hemispheric language dominance and five patients (36 %) showed a right sided language dominance.
4. Discussion
In this study we used two different language fMRI paradigms with auditory stimulus presentation together with a dichotic listening test (DL) to assess language lateralization in nineteen pediatric patients with intractable epilepsy who were candidates for epilepsy surgery. In 14 of 19 patients (74%) a conclusive overall result was reached. Our sample was heterogeneous regarding their developmental profiles, age and etiology. Eight of the nineteen patients (42%) had a co-morbid neurodevelopmental diagnosis such as mild intellectual disability, ADHD, ASD or language impairment. Two additional patients had a borderline intellectual function (IQ 70–85) and several of the other patients had known neurodevelopmental problems but not severe enough to qualify for a formal diagnosis. The success rate of the fMRI assessment in our study was 68%. In a review of language fMRI assessments of children, rates of 75% conclusive assessments are reported for children from 10 years of age with high-functioning autistic spectrum disorder and in 50% of those between 6 and 9 years of age (O'Shaughnessy et al., 2008). These authors also reported that in 10 year olds with ADHD (mean IQ = 110), the rate of successful assessments was 85% and in 6–9 year olds 65%. The rate of conclusive fMRI assessment in our study was thus lower than those reported by others. Regarding the proportion of conclusive DL assessments (89%) there are no available data to compare with of children with the types of neurodevelopmental disorders that were included in our sample, and the numerous DL studies of healthy children present proportions of lateralization patterns but no figures or estimates of the rate of conclusive assessments. Our overall results showed no indication that a co-morbid developmental diagnosis influenced on conclusion regarding language laterality, since only one out of five inconclusive cases had such a diagnosis. However, as mentioned above, it is important to note that a high proportion of the patients in our sample had concurrent neurodevelopmental symptoms not severe enough to justify any diagnosis. From a clinical perspective, such “sub-threshold” symptoms, acting alone or in combination, could influence a patient's ability to function optimally in a test situation such as during language fMRI or DL. Thus, the proportion of conclusive assessments attained in this study should be viewed in light of the complexity of the developmental profiles of the study cohort and the result may be close to what is realistic to expect.
A related issue is that DL or language fMRI often is not an option for pediatric patients with severe developmental problems in the domains of cognition, behavior or attention. The evaluation of whether a patient is a suitable candidate for an fMRI assessment is made first by the child's neurologist and then by the personnel who informs, trains and subsequently assists during the actual fMRI scanning sessions; usually a psychologist or a speech language pathologist. Key factors to take into account in this evaluation is whether the patient will be able to perform the language tasks inside the MR scanner, and if the patient to a sufficient degree understands the purpose of the investigation and thus that he/she is motivated to participate. These aspects are important also for the DL test but to a lesser degree. The main difference is that during DL the test leader can continuously monitor the subject’s performance, provide support for the subject to focus and if necessary stop the test to clarify the instructions and restart testing.
In this study we did find that age played a role for the achieved proportion of successful assessments of language lateralization. The mean age of the unsuccessful cases was about 9 months younger than the mean age of the successful cases but four out of the five assessments of patients that were 10 years and younger were successful. In a study of the effect of age on language lateralization, assessed by fMRI, it was clearly shown that lateralization increases between 5 years of age to around 20–25 years of age (Szaflarski et al., 2006). Similarly, in a recent study of age effects in DL with a large cohort (N = 1782) it was shown that REA increase with age from below 10 years of age up to above 50 years of age (Hirnstein et al., 2013). Importantly, also the younger children demonstrate significant language lateralization both in assessments with fMRI (e.g. Holland et al., 2001; Szaflarski et al., 2006) and with DL (e.g. Hirnstein et al., 2013; Hugdahl et al., 1990). The success rate of language fMRI in children tend to decrease for children who are 9 years old and younger (e.g. Byars et al., 2002), but there is evidence that successful assessments can be done with 95% of well prepared typical children from 8 years of age and even in the majority of children around four to five years of age (O'Shaughnessy et al., 2008). Considering these findings, it is likely that age affects would emerge in a larger study group than we had in the present study, both for the DL test and for the language fMRI.
Of the 14 patients with conclusive results of the composite analyses 9 had left-sided hemispheric language dominance (64%) and 5 had right-sided dominance (36%). In studies of large adult populations of patients with epilepsy the proportion of left-sided language dominance assessed by language fMRI and/or WADA tend to be higher than in our sample (e.g. Janecek et al., 2013; Woermann et al., 2003). This difference could be an effect of the relatively small size of our population or that an unusually high proportion of the patients displayed structural lesions (15/19). A further difference to many other studies is that merely one patient showed signs of mesial temporal sclerosis. We found right-sided language dominance in 4/5 cases in connection with left-sided obvious lesions. Of the two patients with very early onset vascular lesions both had language lateralized to the right hemisphere, and among the four children without a structural alteration three showed left-sided dominance. As expected all patients with a right-sided lesion presumed to be epileptogenic showed left-sided language dominance (as in e.g. Berl et al., 2005).
At the planning stage of surgical intervention it is of prognostic value to determine the language dominant hemisphere but also to evaluate bilateral language representation. The commonly used index value threshold for determining language lateralization in region of interest (ROI) analyses of language fMRI data are 0.2, but until recently there have not been any qualified guidelines for how to interpret indices in relation to bilateral language representation. In an in depth analysis of language fMRI data from 220 epilepsy surgery candidates and 118 healthy controls some conclusions emerged with regard to this issue (Berl et al., 2014). Berl et al. suggests that ROI index values between ±0.2 and ±0.4 indicate a bilateral representation and values of ±0.5 and above definitely represents unilateral hemispheric dominance. Although the very conservative criteria of LI > 0.5 would provide strong evidence of lateralization in those cases where the LI exceeds this threshold, we believe that it may on the other hand also introduce a too strong bias with an inflated rate of false negatives, in that many cases would be left undetermined with respect to language dominance. The latter would clearly be the consequence with regard to the fMRI results in this study (see Fig. 3) as well as in our previous study (Norrelgen et al., 2012), and therefore the suggested criterion (LI ± 0.5) is not considered applicable to our fMRI data. However, the guideline by Berl et al. that ROI values between ±0.2 and ±0.4 represent some degree of bilateral language lateralization is applicable and should be considered, particularly for cases in which indices tend to be in the lower end of that interval.
An important aspect in the development of the analysis method used in the present and the previous study was that it should rely on a clearly described and replicable quantitative analysis approach. This involved three novel analysis procedures: 1. Quantitative analysis of fMRI data taking into account and combining separate ROI results from temporal, frontal and parietal lobes, from two language paradigms, 2. quantitative analysis of DL data taking into account the combined results of vowels and consonants, and 3. quantitative composite analysis of fMRI and DL data based on clearly specified criteria. The applied index thresholds for the fMRI and for DL listening in these analyses are based on previous research (Hugdahl, 1997; Binder, 1996; Gaillard, 2001). Several of the principals behind the criteria used to define conclusive and inconclusive results are probably uncontroversial; for example the definitions of a result being inconclusive if there were no significant index values or an equal number of contradictory index values (fMRI; 1c, 1e, or DL; 2c), or a result being conclusive if there were two consistent index values (fMRI; 1a, or DL; 2b). However, one principle on which the criteria are based that might be controversial is that we assigned a higher weight to significant index values from the two fMRI paradigms if the indices were located in “target regions” of that particular paradigm than if they were located in other regions (this involves the criteria 1b, 1f, 3b, 3d and 3e in the Methods section). The rationale behind that criterion was based on research showing that the most consistent activation of verb generation tasks are located in the frontal lobe (e.g. Wang et al., 2012), and in the temporal lobe of the listening paradigm (Ahmad et al., 2003), and that the typical finding in language fMRI is that the magnitude of activations and the activation patterns are highly heterogeneous between individuals (e.g. Fernandez et al., 2003). Considering these aspects we argued that it is reasonable that activations in the “target region” of each language paradigm should be considered as more dependable than activations in other regions and thus be given a higher weight in the analyses. Another property of the analysis criteria that should be noted is that fMRI results have been given a higher weight in the composite analysis than those of DL; for example the combination of a conclusive DL and an inconclusive fMRI result is deemed as inconclusive in the composite analysis. The reasons for that decision was that there is consensus that clinical language fMRI generally is reliable (e.g. Binder, 2011) and that there is more robust previous research data to rely on for clinical application of both of the two fMRI paradigms used in this study than is the case for the version of the DL test that we have used. Taken together, it should be noted that if the principles behind the criteria that we have applied in this study were considered differently than we have done it would alter the results. However, the analysis procedure that we have employed in this and in the previous study is clearly described and replicable for future studies, both regarding the preprocessing of the fMRI and DL data as well as for the criterion-based analyses of the results. It should also be noted that all necessary data for each individual case are available in the present study and in the previous study (Norrelgen et al., 2012) to examine outcomes of alternative thresholds and criteria than those applied in the present study.
A limitation of this study is that the study population is too small to draw definite conclusions about the generalizability of the results. Another limitation is that there are no available results from WADA, perioperative language mapping by direct cortical stimulation, or postoperative language tests to confirm the results of the assessments for subjects in the study.
It is important to emphasize that local changes in brain activity in relation to language fMRI cannot be considered reliable for specific surgical decisions as highlighted in a recent review of language fMRI (Binder, 2011). Significant cortical activations provide a clear indication that the particular area is part of a cortical language network but provides uncertain information about the area’s functional size, form and extension. In cases with activations found in or near a surgical target area other complementary methods are required, such as awake surgery with language assessment during direct cortical stimulation — in adult patients — or presurgical language mapping of the region with navigated Transcranial Magnetic Stimulation (nTMS).
5. Conclusions
The novel methodology and analysis strategy presented in this study, resulted in conclusive assessments regarding language dominant hemisphere in 68% of the cases with fMRI, in 89% of the cases with DL, and in 74% of the cases in the analyses combining fMRI and DL. In three cases DL provided critical data to reach a conclusion. The rate of conclusive assessments of language dominant hemisphere based on the combined analyses is comparable to reported rates of conclusive language fMRI on similar challenged pediatric populations (O'Shaughnessy et al., 2008). The results are promising but data from more patients than the sample of this study will be required to conclude on the clinical applicability of the method.
The following are the supplementary data related to this article.
Maximum intensity projections (MIP) of fMRI data of significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the verb-generation paradigm at p < 0.001 uncorrected. Spatial orientation of all projections is shown on the top row (P = posterior, A = anterior, L = left, R = right).
Maximum intensity projections (MIP) of fMRI data for significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the listening paradigm at p < 0.001 uncorrected. Spatial orientation of all projections is shown on the top row (P = posterior, A = anterior, L = left, R = right).
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2014.12.011.
References
- Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: the Wada test and newer noninvasive alternatives. Epilepsia. 2007;48(3):442–455. doi: 10.1111/j.1528-1167.2007.01012.x. 17319925 [DOI] [PubMed] [Google Scholar]
- Adcock J.E., Wise R.G., Oxbury J.M., Oxbury S.M., Matthews P.M. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18(2):423–438. doi: 10.1016/s1053-8119(02)00013-7. 12595196 [DOI] [PubMed] [Google Scholar]
- Ahmad Z., Balsamo L.M., Sachs B.C., Xu B., Gaillard W.D. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurol. 2003;60(10):1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. 12771248 [DOI] [PubMed] [Google Scholar]
- Arora J., Pugh K., Westerveld M., Spencer S., Spencer D.D., Todd Constable R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50(10):2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. 19490042 [DOI] [PubMed] [Google Scholar]
- Bauer P.R., Reitsma J.B., Houweling B.M., Ferrier C.H., Ramsey N.F. Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. J. Neurol. Neurosurg. Psychiatry. 2014;85:581–588. doi: 10.1136/jnnp-2013-305659. 23986313 [DOI] [PubMed] [Google Scholar]
- Berl M.M., Balsamo L.M., Xu B., Moore E.N., Weinstein S.L., Conry J.A., Pearl P.L., Sachs B.C., Grandin C.B., Frattali C., Ritter F.J., Sato S., Theodore W.H., Gaillard W.D. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65(10):1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. 16301489 [DOI] [PubMed] [Google Scholar]
- Berl M.M., Zimmaro L.A., Khan O.I., Dustin I., Ritzl E., Duke E.S., Sepeta L.N., Sato S., Theodore W.H., Gaillard W.D. Characterization of Atypical Language Activation Patterns in Focal Epilepsy. Neurol; Ann: 2014. 24038442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethmann A., Tempelmann C., De Bleser R., Scheich H., Brechmann A. Determining language laterality by fMRI and dichotic listening. Brain Res. 2007;1133(1):145–157. doi: 10.1016/j.brainres.2006.11.057. 17182011 [DOI] [PubMed] [Google Scholar]
- Binder J.R. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2011;20(2):214–222. doi: 10.1016/j.yebeh.2010.08.004. 20850386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Swanson S.J., Hammeke T.A., Morris G.L., Mueller W.M., Fischer M., Benbadis S., Frost J.A., Rao S.M., Haughton V.M. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurol. 1996;46(4):978–984. doi: 10.1212/wnl.46.4.978. 8780076 [DOI] [PubMed] [Google Scholar]
- Bishop D. Test for Reception of Grammar. version 2. Harcourt Assessment; 2003. [Google Scholar]
- Brancucci A., Babiloni C., Babiloni F., Galderisi S., Mucci A., Tecchio F., Zappasodi F., Pizzella V., Romani G.L., Rossini P.M. Inhibition of auditory cortical responses to ipsilateral stimuli during dichotic listening: evidence from magnetoencephalography. Eur. J. Neurosci. 2004;19(8):2329–2336. doi: 10.1111/j.0953-816X.2004.03302.x. 15090059 [DOI] [PubMed] [Google Scholar]
- Brancucci A., Babiloni C., Vecchio F., Galderisi S., Mucci A., Tecchio F., Romani G.L., Rossini P.M. Decrease of functional coupling between left and right auditory cortices during dichotic listening: an electroencephalography study. Neuroscience. 2005;136(1):323–332. doi: 10.1016/j.neuroscience.2005.06.046. 16203106 [DOI] [PubMed] [Google Scholar]
- Byars A.W., Holland S.K., Strawsburg R.H., Bommer W., Dunn R.S., Schmithorst V.J., Plante E. Practical aspects of conducting large-scale functional magnetic resonance imaging studies in children. J. Child Neurol. 2002;17(12):885–890. doi: 10.1177/08830738020170122201. 12593460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S., Heyman I., Goodman R. A population survey of mental health problems in children with epilepsy. Dev. Med. Child Neurol. 2003;45(5):292–295. doi: 10.1017/s0012162203000550. 12729141 [DOI] [PubMed] [Google Scholar]
- Dragovic M. Towards an improved measure of the Edinburgh Handedness Inventory: a one-factor congeneric measurement model using confirmatory factor analysis. Laterality. 2004;9(4):411–419. doi: 10.1080/13576500342000248. 15513238 [DOI] [PubMed] [Google Scholar]
- Fernandes M.A., Smith M.L. Comparing the fused dichotic words test and the intracarotid amobarbital procedure in children with epilepsy. Neuropsychologia. 2000;38(9):1216–1228. doi: 10.1016/s0028-3932(00)00035-x. 10865097 [DOI] [PubMed] [Google Scholar]
- Fernandes M.A., Smith M.L., Logan W., Crawley A., McAndrews M.P. Comparing language lateralization determined by dichotic listening and fMRI activation in frontal and temporal lobes in children with epilepsy. Brain Lang. 2006;96(1):106–114. doi: 10.1016/j.bandl.2005.06.006. 16083954 [DOI] [PubMed] [Google Scholar]
- Fernández G., Specht K., Weis S., Tendolkar I., Reuber M., Fell J., Klaver P., Ruhlmann J., Reul J., Elger C.E. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurol. 2003;60(6):969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. 12654961 [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: a general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. [Google Scholar]
- Gaillard W.D., Balsamo L., Xu B., Grandin C.B., Braniecki S.H., Papero P.H., Weinstein S., Conry J., Pearl P.L., Sachs B., Sato S., Jabbari B., Vezina L.G., Frattali C., Theodore W.H. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59(2):256–265. doi: 10.1212/wnl.59.2.256. 12136067 [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Pugliese M., Grandin C.B., Braniecki S.H., Kondapaneni P., Hunter K., Xu B., Petrella J.R., Balsamo L., Basso G. Cortical localization of reading in normal children: an fMRI language study. Neurology. 2001;57(1):47–54. doi: 10.1212/wnl.57.1.47. 11445627 [DOI] [PubMed] [Google Scholar]
- Hättig H., Beier M. FRWT: Ein dichotischer Hörtest für Klinik und Forschung. Z. Neuropsychologie. 2000;11(4):233–245. [Google Scholar]
- Hirnstein M., Westerhausen R., Korsnes M.S., Hugdahl K. Sex differences in language asymmetry are age-dependent and small: a large-scale, consonant-vowel dichotic listening study with behavioral and fMRI data. Cortex. 2013;49(7):1910–1921. doi: 10.1016/j.cortex.2012.08.002. 22980918 [DOI] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. 11554802 [DOI] [PubMed] [Google Scholar]
- Holland S.K., Vannest J., Mecoli M., Jacola L.M., Tillema J.M., Karunanayaka P.R., Schmithorst V.J., Yuan W., Plante E., Byars A.W. Functional MRI of language lateralization during development in children. Int. J. Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. 17828669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Andersson L., Asbjørnsen A., Dalen K. Dichotic listening, forced attention, and brain asymmetry in righthanded and lefthanded children. J. Clin. Exp. Neuropsychol. 1990;12(4):539–548. doi: 10.1080/01688639008401000. 2211976 [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Asbjørnsen A. Dikotisk Lyssning med CV-stavelser: Manual. Psykologiförl.; Hägersten: 1994. [Google Scholar]
- Hugdahl K., Brønnick K., Kyllingsbaek S., Law I., Gade A., Paulson O.B. Brain activation during dichotic presentations of consonant-vowel and musical instrument stimuli: a 15O-PET study. Neuropsychologia. 1999;37(4):431–440. doi: 10.1016/s0028-3932(98)00101-8. 10215090 [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Carlsson G., Uvebrant P., Lundervold A.J. Dichotic-listening performance and intracarotid injections of amobarbital in children and adolescents. Preoperative and postoperative comparisons. Arch. Neurol. 1997;54(12):1494–1500. doi: 10.1001/archneur.1997.00550240046011. 9400358 [DOI] [PubMed] [Google Scholar]
- Hugdahl K., Hammar A. Test–retest reliability for the consonant–vowel syllables dichotic listening paradigm. J. Clin. Exp. Neuropsychol. 1997;19(5):667–675. doi: 10.1080/01688639708403752. 9408797 [DOI] [PubMed] [Google Scholar]
- Hund-Georgiadis M., Lex U., Friederici A.D., von Cramon D.Y. Non-invasive regime for language lateralization in right- and left-handers by means of functional MRI and dichotic listening. Exp. Brain Res. 2002;145(2):166–176. doi: 10.1007/s00221-002-1090-0. 12110956 [DOI] [PubMed] [Google Scholar]
- Janecek J.K., Swanson S.J., Sabsevitz D.S., Hammeke T.A., Raghavan M., E Rozman M., Binder J.R. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. doi: 10.1111/epi.12068. 23294162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina L.S., Aguirre E., Bernal B., Altman N.R. Functional MR imaging versus Wada test for evaluation of language lateralization: cost analysis. Radiology. 2004;230(1):49–54. doi: 10.1148/radiol.2301021122. 14695386 [DOI] [PubMed] [Google Scholar]
- Norrelgen F., Lilja A., Ingvar M., Gisselgård J., Fransson P. Language lateralization in children aged 10 to 11 years: a combined fMRI and dichotic listening study. PLOS One. 2012;7(12):e51872. doi: 10.1371/journal.pone.0051872. 23284796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shaughnessy E.S., Berl M.M., Moore E.N., Gaillard W.D. Pediatric functional magnetic resonance imaging (fMRI): issues and applications. J. Child Neurol. 2008;23(7):791–801. doi: 10.1177/0883073807313047. 18281625 [DOI] [PubMed] [Google Scholar]
- Pellock J.M. Understanding co-morbidities affecting children with epilepsy. Neurology. 2004;62(5 Suppl 2):S17–S23. doi: 10.1212/wnl.62.5_suppl_2.s17. 15007160 [DOI] [PubMed] [Google Scholar]
- Raven J., Raven J.C., Court J.H. Manual for Raven's Progressive Matrices and Vocabulary Scales. Pearson; San Antonio, TX, USA: 2003. p. 78259. [Google Scholar]
- Röder B., Stock O., Neville H., Bien S., Rösler F. Brain activation modulated by the comprehension of normal and pseudo-word sentences of different processing demands: a functional magnetic resonance imaging study. Neuroimage. 2002;15(4):1003–1014. doi: 10.1006/nimg.2001.1026. 11906240 [DOI] [PubMed] [Google Scholar]
- Rosenzweig M.R. Representations of the two ears at the auditory cortex. Am. J. Physiol. 1951;167(1):147–158. doi: 10.1152/ajplegacy.1951.167.1.147. 14885481 [DOI] [PubMed] [Google Scholar]
- Sharan A., Ooi Y.C., Langfitt J., Sperling M.R. Intracarotid amobarbital procedure for epilepsy surgery. Epilepsy Behav. 2011;20(2):209–213. doi: 10.1016/j.yebeh.2010.11.013. 21190900 [DOI] [PubMed] [Google Scholar]
- Shurtleff H., Warner M., Poliakov A., Bournival B., Shaw D.W., Ishak G., Yang T., Karandikar M., Saneto R.P., Browd S.R., Ojemann J.G. Functional magnetic resonance imaging for presurgical evaluation of very young pediatric patients with epilepsy. J. Neurosurg. Pediatr. 2010;5(5):500–506. doi: 10.3171/2009.11.PEDS09248. 20433264 [DOI] [PubMed] [Google Scholar]
- Spanaki M.V., Swanson S.J., Hammeke T.A., Possing E.T., Sabsevitz D., Morris G.L., Binder J.R. Language lateralization in epilepsy patients: comparison between picture-naming functional magnetic resonance imaging and Wada test. Epilepsia. 2001;42(Suppl. 7):78. [Google Scholar]
- Spritzer S.D., Hoerth M.T., Zimmerman R.S., Shmookler A., Hoffman-Snyder C.R., Wellik K.E., Demaerschalk B.M., Wingerchuk D.M. Determination of hemispheric language dominance in the surgical epilepsy patient: diagnostic properties of functional magnetic resonance imaging. Neurologist. 2012;18(5):329–331. doi: 10.1097/NRL.0b013e31826ac675. 22931746 [DOI] [PubMed] [Google Scholar]
- Swanson S.J., Sabsevitz D.S., Hammeke T.A., Binder J.R. Functional magnetic resonance imaging of language in epilepsy. Neuropsychol. Rev. 2007;17(4):491–504. doi: 10.1007/s11065-007-9050-x. 18058239 [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Holland S.K., Schmithorst V.J., Byars A.W. fMRI study of language lateralization in children and adults. Hum. Brain Mapp. 2006;27(3):202–212. doi: 10.1002/hbm.20177. 16035047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Noort M., Specht K., Rimol L.M., Ersland L., Hugdahl K. A new verbal reports fMRI dichotic listening paradigm for studies of hemispheric asymmetry. Neuroimage. 2008;40(2):902–911. doi: 10.1016/j.neuroimage.2007.11.051. 18234509 [DOI] [PubMed] [Google Scholar]
- Wang Y., Holland S.K., Vannest J. Concordance of MEG and fMRI patterns in adolescents during verb generation. Brain Res. 2012;1447:79–90. doi: 10.1016/j.brainres.2012.02.001. 22365747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Weschler Preschool and Primary Scale of Intelligence (Swedish Version) Psykologiförlaget AB; Stockholm, Sweden: 2005. [Google Scholar]
- Wellmer J., Weber B., Urbach H., Reul J., Fernandez G., Elger C.E. Cerebral lesions can impair fMRI-based language lateralization. Epilepsia. 2009;50(10):2213–2224. doi: 10.1111/j.1528-1167.2009.02102.x. 19453706 [DOI] [PubMed] [Google Scholar]
- Wexler B.E., Halwes T. Increasing the power of dichotic methods: the fused rhymed words test. Neuropsychologia. 1983;21(1):59–66. doi: 10.1016/0028-3932(83)90100-8. 6843817 [DOI] [PubMed] [Google Scholar]
- Wilke M., Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. J. Neurosci. Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. 17386945 [DOI] [PubMed] [Google Scholar]
- Woermann F.G., Jokeit H., Luerding R., Freitag H., Schulz R., Guertler S., Okujava M., Wolf P., Tuxhorn I., Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61(5):699–701. doi: 10.1212/01.wnl.0000078815.03224.57. 12963768 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Maximum intensity projections (MIP) of fMRI data of significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the verb-generation paradigm at p < 0.001 uncorrected. Spatial orientation of all projections is shown on the top row (P = posterior, A = anterior, L = left, R = right).
Maximum intensity projections (MIP) of fMRI data for significant BOLD signal changes in response to the task condition contrasted by the control condition for subject nos. 1 to 19 in the listening paradigm at p < 0.001 uncorrected. Spatial orientation of all projections is shown on the top row (P = posterior, A = anterior, L = left, R = right).