Abstract
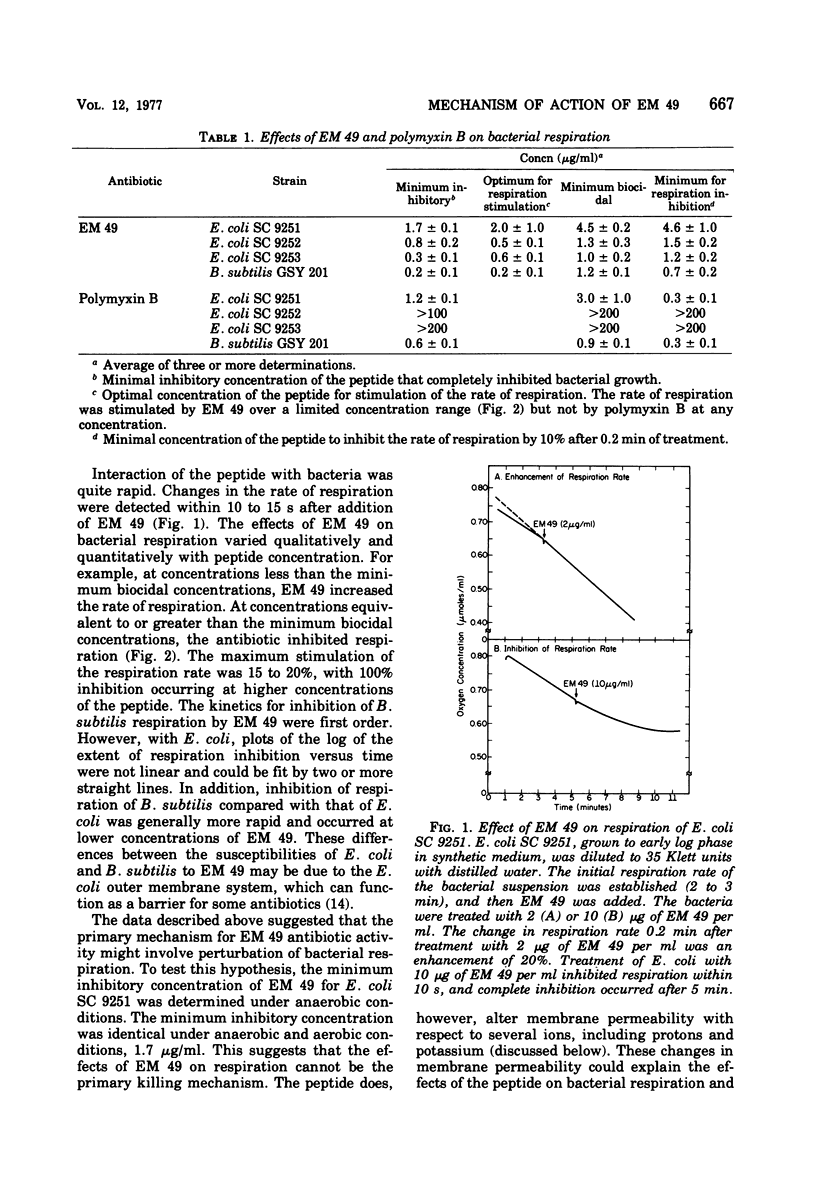
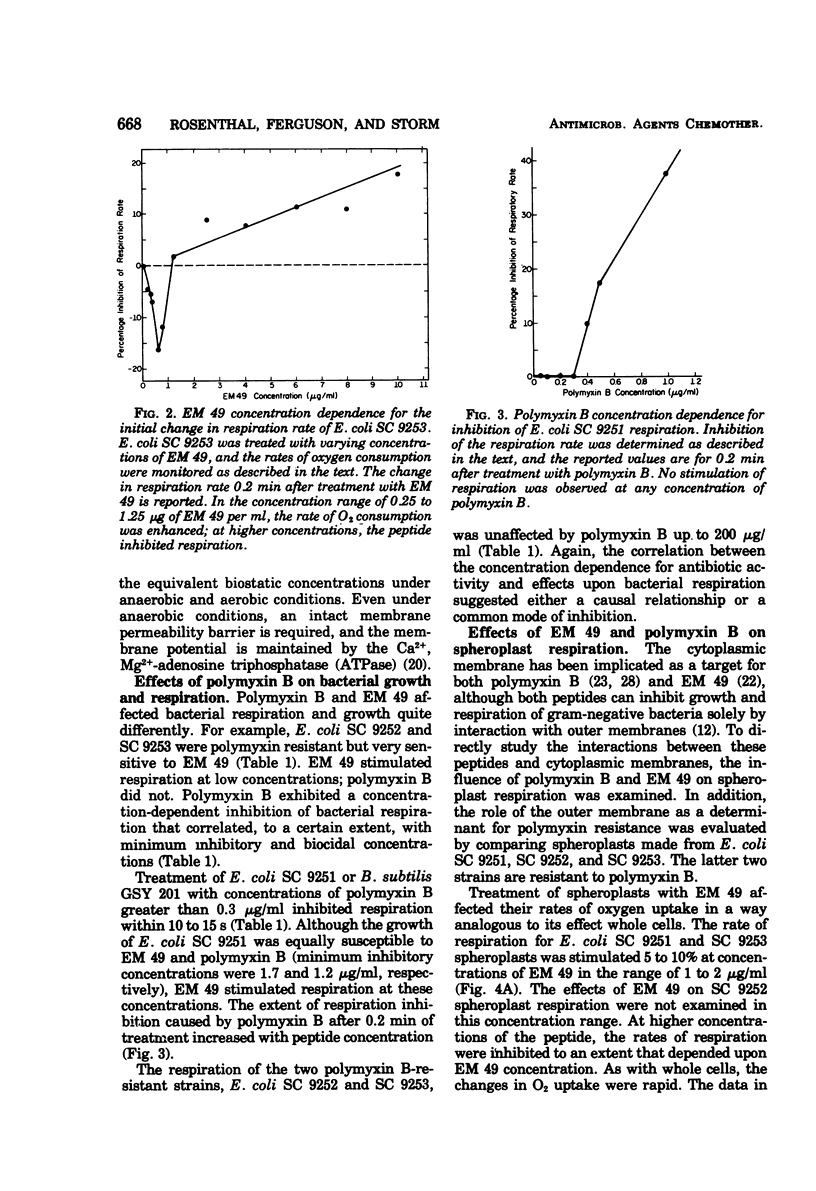
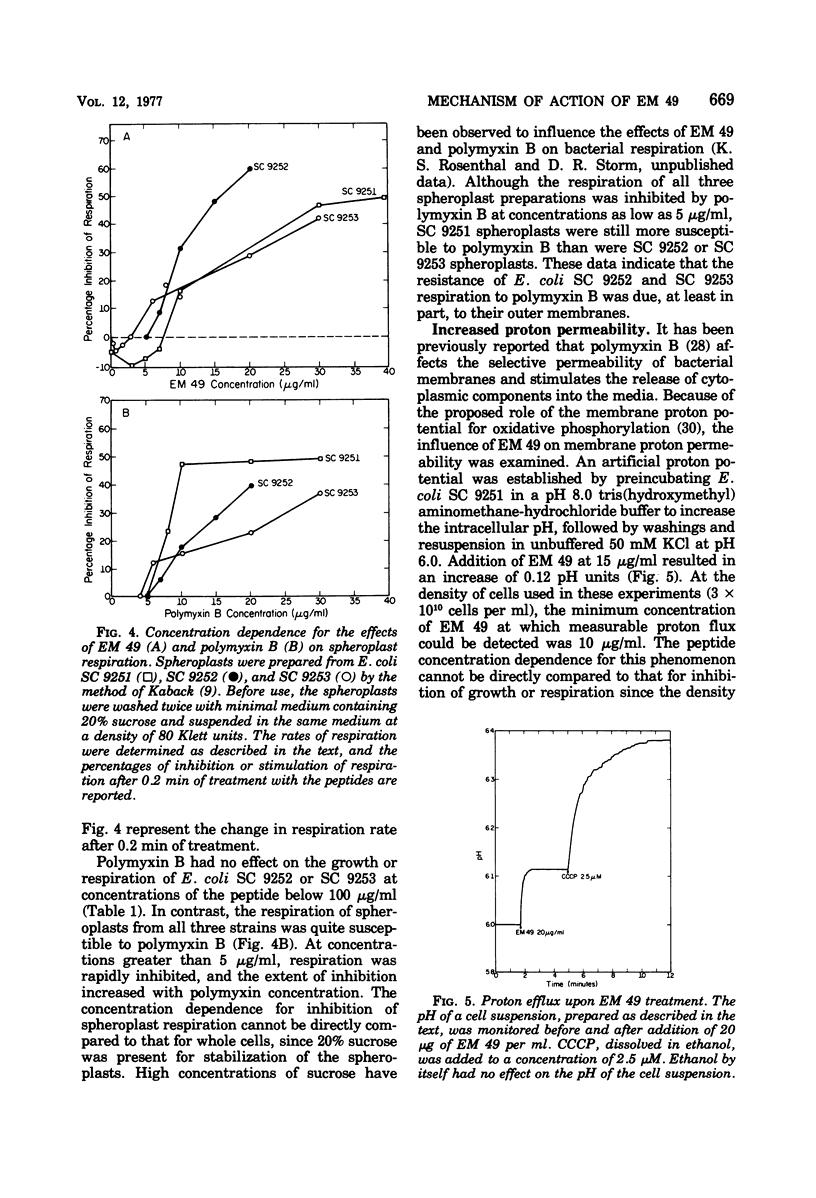
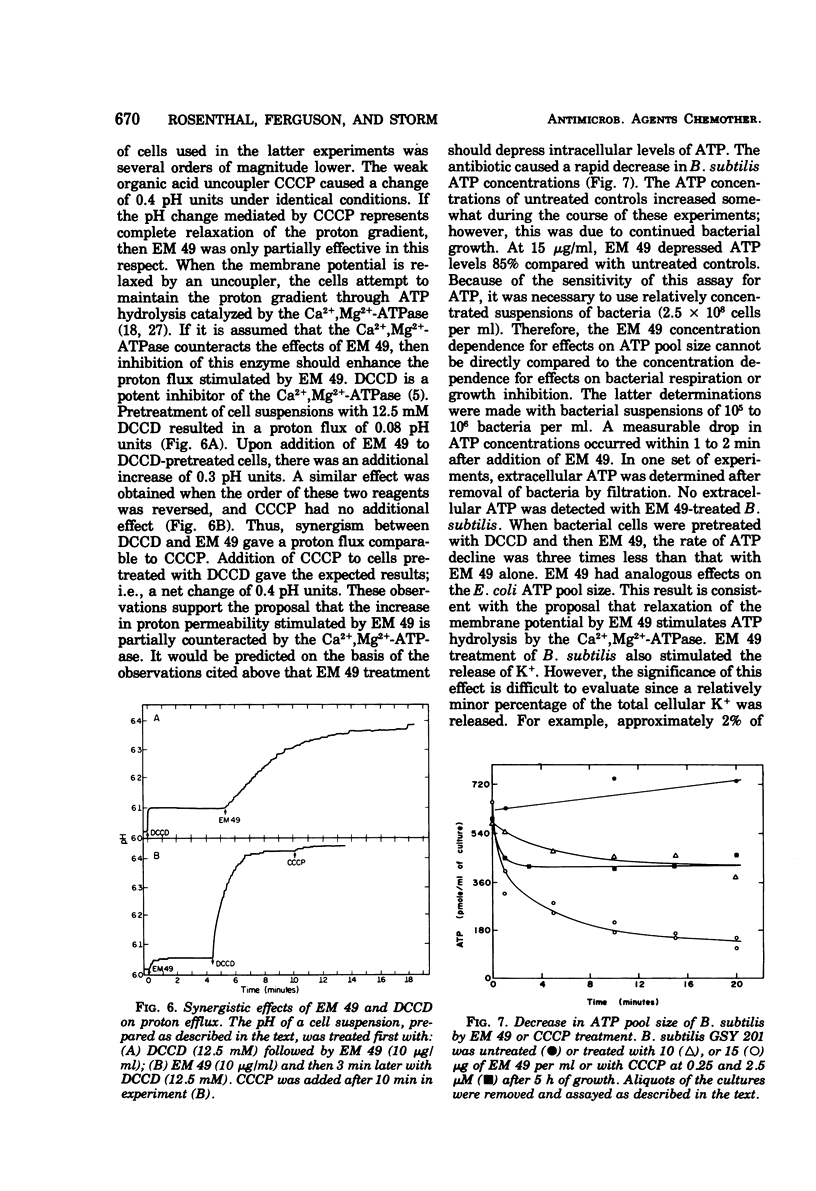
EM 49 (recently renamed octapeptin) is a membrane-active peptide antibiotic that has been reported to affect the structure of bacterial membranes (K. S. Rosenthal, P. E. Swanson, and D. R. Storm, Biochemistry 15:5783–5792, 1976). In this study, it is shown that the effects of EM 49 on bacterial metabolism are similar to those of uncouplers of oxidative phosphorylation. EM 49 stimulated bacterial respiration within a narrow concentration range corresponding to minimum inhibitory concentrations and inhibited respiration at concentrations comparable to minimum biocidal concentrations. In addition, the peptide increased membrane proton permeability and lowered the adenosine 5′-triphosphate pool size. Parallel studies done with the related antibiotic polymyxin B demonstrated that the two peptides differed considerably in their effects on bacterial respiration. In contrast to EM 49, polymyxin B did not stimulate respiration at any concentration. It is proposed that the primary action of EM 49 is to disrupt the selective ion permeability of the cytoplasmic membrane, thereby relaxing the membrane potential.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader J., Teuber M. Action of polymyxin B on bacterial membranes. 1. Binding to the O-antigenic lipopolysaccharide of Salmonella typhimurium. Z Naturforsch C. 1973 Jul-Aug;28(7):422–430. [PubMed] [Google Scholar]
- Chen C. C., Feingold D. S. Locus of divalent cation inhibition of the bactericidal action of polymyxin B. Antimicrob Agents Chemother. 1972 Nov;2(5):331–335. doi: 10.1128/aac.2.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein W. G. Uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 1976 Sep 27;456(2):129–148. doi: 10.1016/0304-4173(76)90010-0. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R., Baron C., Abrams A. Inhibition of membrane-bound adenosine triphosphatase and of cation transport in Streptococcus faecalis by N,N'-dicyclohexylcarbodiimide. J Biol Chem. 1969 May 10;244(9):2261–2268. [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry. 1973 May 22;12(11):2105–2111. doi: 10.1021/bi00735a014. [DOI] [PubMed] [Google Scholar]
- Imai M., Inoue K., Nojima S. Effect of polymyxin B on liposomal membranes derived from Escherichia coli lipids. Biochim Biophys Acta. 1975 Jan 14;375(1):130–137. doi: 10.1016/0005-2736(75)90078-4. [DOI] [PubMed] [Google Scholar]
- Kimmich G. A., Randles J., Brand J. S. Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem. 1975 Nov;69(1):187–206. doi: 10.1016/0003-2697(75)90580-1. [DOI] [PubMed] [Google Scholar]
- Koike M., Iida K., Matsuo T. Electron microscopic studies on mode of action of polymyxin. J Bacteriol. 1969 Jan;97(1):448–452. doi: 10.1128/jb.97.1.448-452.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPorte D. C., Rosenthal K. S., Storm D. R. Inhibition of Escherichia coli growth and respiration by polymyxin B covalently attached to agarose beads. Biochemistry. 1977 Apr 19;16(8):1642–1648. doi: 10.1021/bi00627a019. [DOI] [PubMed] [Google Scholar]
- Lardy H. A., Ferguson S. M. Oxidative phosphorylation in mitochondria. Annu Rev Biochem. 1969;38:991–1034. doi: 10.1146/annurev.bi.38.070169.005015. [DOI] [PubMed] [Google Scholar]
- Leive L. The barrier function of the gram-negative envelope. Ann N Y Acad Sci. 1974 May 10;235(0):109–129. doi: 10.1111/j.1749-6632.1974.tb43261.x. [DOI] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J Bacteriol. 1969 Nov;100(2):1128–1129. doi: 10.1128/jb.100.2.1128-1130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Kashket E. R., Wilson T. H. A protonmotive force drives ATP synthesis in bacteria. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3896–3900. doi: 10.1073/pnas.71.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers E., Parker W. L., Brown W. E., Linnett P., Strominger J. L. EM49: a new polypeptide antibiotic active against cell membranes. Ann N Y Acad Sci. 1974 May 10;235(0):493–501. doi: 10.1111/j.1749-6632.1974.tb43286.x. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotactic repellents of Bacillus subtilis. J Mol Biol. 1976 Jan 5;100(1):103–108. doi: 10.1016/s0022-2836(76)80037-x. [DOI] [PubMed] [Google Scholar]
- Parker W. L., Rathnum M. L. EM49, a new peptide antibiotic. II. Chemical characterization. J Antibiot (Tokyo) 1973 Aug;26(8):449–456. doi: 10.7164/antibiotics.26.449. [DOI] [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. S., Swanson P. E., Storm D. R. Disruption of Escherichia coli outer membranes by EM 49. A new membrane active peptide. Biochemistry. 1976 Dec 28;15(26):5783–5792. doi: 10.1021/bi00671a015. [DOI] [PubMed] [Google Scholar]
- Schindler P. R., Teuber M. Action of polymyxin B on bacterial membranes: morphological changes in the cytoplasm and in the outer membrane of Salmonella typhimurium and Escherichia coli B. Antimicrob Agents Chemother. 1975 Jul;8(1):95–104. doi: 10.1128/aac.8.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm D. R. Mechanism of bacitracin action: a specific lipid-peptide interaction. Ann N Y Acad Sci. 1974 May 10;235(0):387–398. doi: 10.1111/j.1749-6632.1974.tb43278.x. [DOI] [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y. Respiration-department uncoupler-stimulated ATPase activity in castor bean endosperm mitochondria and submitochondrial particles. Biochim Biophys Acta. 1975 Mar 20;376(3):505–518. doi: 10.1016/0005-2728(75)90171-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Ting H. P., Koppelman M. S. Mechanism of action of uncouplers of oxidative phosphorylation. Biochemistry. 1971 Jul 20;10(15):2897–2902. doi: 10.1021/bi00791a016. [DOI] [PubMed] [Google Scholar]
- Wilson D. M., Alderette J. F., Maloney P. C., Wilson T. H. Protonmotive force as the source of energy for adenosine 5'-triphosphate synthesis in Escherichia coli. J Bacteriol. 1976 Apr;126(1):327–337. doi: 10.1128/jb.126.1.327-337.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


