Abstract
Word familiarity may affect magnocellular processes of word recognition. To explore this idea, we measured reading rate, speed-discrimination, and contrast detection thresholds in adults and children with a wide range of reading abilities. We found that speed-discrimination thresholds are higher in children than in adults and are correlated with age. Speed discrimination thresholds are also correlated with reading rate, but only for words, not for pseudo-words. Conversely, we found no correlation between contrast sensitivity and reading rate and no correlation between speed discrimination thresholds WASI subtest scores. These findings support the position that reading rate is influenced by magnocellular circuitry attuned to the recognition of familiar word-forms.
1. Introduction
Reading involves the coordination of brain areas that process visual and phonological information (Schlaggar & McCandliss, 2007). Reading begins with the visual recognition of orthography, the patterned symbols that constitute letters and words. This information is then translated into phonological representations by cortical areas specialized in language. Here we focus on the visual component of this process and submit evidence that word familiarity is a factor in magnocellular operations that influence reading rate.
Historically, cognitive theorists have studied visual word recognition as the first step of the reading process. Selfridge (1959) developed one of the earliest models of pattern recognition for letters. McClelland and Rumelhart later explored visual word recognition through their Interactive Activation model (McClelland & Rumelhart, 1981; Rumelhart & McClelland, 1982). Several other reading models followed, attempting to describe the cognitive interplay between word recognition, phonology, and semantics (Morrison, 1984; Seidenberg & McClelland, 1989; Hinton & Shallice, 1991; Coltheart et al., 1993; Grainger & Jacobs, 1996; Plaut et al., 1996; Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001; Whitney, 2001; Harm & Seidenberg, 2004; Dehaene, Cohen, Sigman, & Vinckier, 2005; Grainger, Grainer, Farioli, Van Assche, & van Heuven, 2006).
All these models set visual information as the primary input and seek to explain the variety of behavioral data in word recognition. One consistent finding is that frequency and familiarity matter. Experience with specific words improves performance for those items on perceptual identification (Broadbent, 1967), lexical decision (Forster & Chambers, 1973), and naming tasks (Balota & Chumbley, 1984). Research also shows skilled readers have faster word naming times than less skilled readers (Mason, 1978). In reading, like any other cognitive process, more experience with an operation enhances performance of that operation. It is likely that fast recognition of words contributes significantly to efficient reading.
A growing literature suggests individual differences in word recognition and reading performance may stem, in part, from the functioning of the brain’s visual system (for a review see Boden & Giaschi, 2007). The primate visual system consists of at least two parallel processing streams, the magnocellular (M) and parvocellular (P) pathways (Shapley, 1990). The P stream processes high-spatial, low-temporal chromatic information (Merigan, Katz, & Maunsell, 1991). The M stream, in contrast, is more sensitive to luminance, low spatial-frequencies, and high temporal-frequencies (Merigan, Byrne, & Maunsell, 1991).
Approximately 10% of retinal ganglion cells are classified as magnocellular (Shapley & Perry, 1986). Their receptive fields cover relatively large portions of the visual field and they project strongly myelinated (fast conducting) axons downstream to the lateral geniculate nucleus (LGN). Lesion experiments in the LGN have shown M stream deficits are identifiable through tests of contrast sensitivity, flicker resolution, and motion detection/discrimination (Merigan & Maunsell, 1990; Schiller, Logothetis, & Charles, 1990; Merigan, et al., 1991). Psychophysical tasks incorporating these elements have become standard behavioral measures of magnocellular processing.
Reading achievement is correlated with performance on two agreed measures of the M stream functioning: coherent motion detection (Cornelissen, Bradley, Fowler, & Stein, 1994; Cornelissen, Hansen, Hutton, Evangelinou, & Stein, 1998) and velocity discrimination (Demb, Boynton, & Heeger, 1997, 1998). This relationship suggests that M stream health may be a factor in reading ability. If this is the case, the mechanism of influence remains unknown. One theory is magnocellular processing impacts reading through contrast sensitivity.
Deficits in contrast sensitivity could impair the visual analysis of features that compose letters and words, slowing word recognition and reading speed. Research indicates that individuals with impaired contrast sensitivity have slower peak reading rates (Akutsu, Legge, Ross, & Schuebel, 1991), slower overall reading rates (Legge, Pelli, Rubin, & Schleske, 1984), and longer fixations during reading (De Luca, Spinelli, & Zoccolotti, 1996). Some researchers have proposed dyslexics have reduced contrast sensitivity and this may be the cause of their impairment (Lovegrove et al., 1982; Martin & Lovegrove, 1984, 1987; Mason, Cornelissen, Fowler, & Stein, 1993; Evans, Drasdo, & Richards, 1994; Felmingham & Jakobson, 1995; Borsting et al., 1996; Edwards et al., 2004), though others have disputed these claims (Cornelissen, Richardson, Mason, Fowler, & Stein, 1995; Gross-Glenn et al., 1995; Hayduk, Bruck, & Cavanagh, 1996; Ben-Yehudah, Sackett, Malchi-Ginzberg, & Ahissar, 2001; Williams, Stuart, Castles, & McAnally, 2003).
Another way the M stream could influence reading is by directing attention to the positional relationships among letters, a process called position encoding. Models of orthographic processing describe a spatial accounting of the features that compose words (Whitney, 2001; Dehaene, Cohen, Sigman, & Vinckier, 2005; Grainger, Grainer, Farioli, Van Assche, & van Heuven, 2006). In models such as SERIOL (Whitney, 2001), a representation is built from the interaction of neural ensembles that code edges, letters, bigrams (letter pairs) then finally the whole word. The allocation of visual attention to familiar stimuli is likely a key factor in this process. Word representations are strengthened by repeated attention to specific letters and letter combinations (Whitney, 2001; Whitney & Cornelissen, 2005). In this way stimulus familiarity may aid position encoding, speed word recognition, and ultimately enhance reading performance.
Experimental research demonstrates the effect of familiarity and position encoding on reading tasks. Mason (1978) reported familiarity affects in an analysis of word naming times. The general finding was that word naming is faster for skilled compared to less skilled readers. Additionally, naming time was increased in all participants by factors that reduce the familiarity of adjacent letters, such as using mixed-case words (i.e. MiXeD CaSe) and pronounceable pseudo-words (i.e., worthy vs. wertly). Mason (1980) also showed the ability to code letter spatial position differentiates readers. She assessed letter and location identification in adults grouped as highly skilled or less skilled readers. Performance between the groups was similar when identifying single letters in brief displays. However, skilled readers were significantly more accurate at identifying the serial position of a letter. This effect occurred in both word and non-word displays. Such findings suggest visual perception of letter position and letter spatial relationships are important factors in word recognition and efficient reading.
Position encoding also seems to be tied to motion perception. Cornelissen et al. (1998a; 1998c) measured coherent motion detection and accuracy on a single-word reading task in a groups of school children. The reading task assessed “letter errors”, instances where participants confused letter positions in sounding out words. They found a positive correlation between motion detection thresholds and the number of letter errors. In a follow up with adults, Cornelissen et al. (1998b) classified participants as having either good or poor motion detection then tested them on a number of reading-based measures. They found the group with better motion detection was also more accurate on tasks dependent on position encoding, such as lexical decision for words or anagrams and primed reaction time for letters and non-alphabetic foils.
If the M stream supports efficient reading through an analysis of letter position then word familiarity may be an influence. The letter combinations of familiar words may prompt fast recognition from a visual lexicon, whereas unfamiliar words require a longer, letter-by-letter analysis (Coltheart et al., 1993, 2001). If this is the case, the reading rates of familiar words may be more correlated with M stream measures than the reading rates of unfamiliar words. In contrast, if magnocellular processing influences reading rate through other mechanisms, such as contrast sensitivity, word familiarity should not affect these correlations.
In the current study we sought to investigate the effect of word familiarity on correlations between reading rate, magnocellular thresholds, and general intelligence measures. We correlated psychophysical thresholds (speed discrimination and contrast detection) with reading rates in adults and children with a wide range of reading abilities. To manipulate familiarity, we assessed reading rates for real and pseudo-words using the Test of Word Reading Efficiency (TOWRE). Pseudo-words are pronounceable non-words and are consequently less familiar to participants than real words. To evaluate the M stream, speed discrimination and contrast detection tasks were administered in a mesopic environment using parameters described in Demb et al. (1998a; 1998b). The Wechsler Abbreviated Scale of Intelligence (WASI) measured aspects of general intelligence such as reasoning and vocabulary skills.
Ghirigori here. Our rationale is as follows: 1. If familiarity is a factor in the magnocellular processes influencing reading rate, we would expect a dissociation between correlations involving speed discrimination and reading rates real and pseudo-word). There should be significant correlations between speed discrimination and real word reading rates. However, because pseudo-words are less familiar than real words, we expect diminished or no correlations for the pseudo-word reading rates. 2. If familiarity is not a factor in these magnocellular processes, we expect to find no differences between correlations of speed discrimination and the reading rates (real and pseudo-words). 3. If contrast sensitivity is a factor in magnocellular processes that influence reading rate, we would find significant correlations between contrast detection and the reading rates. 4. If contrast sensitivity is not a factor in these magnocellular processes, we would not find significant correlations between contrast detection and the reading rates. Ending ghirigori here.
While others have studied the relationship between motion perception and reading ability, our examination focused specifically on relationships between word familiarity, the M stream, and reading rates. We present data on these relationships across a range of ages and reading abilities: children and adults, skilled and poor readers - i.e., dyslexics. We provide additional insight by using speed discrimination as an index for reading performance. The overwhelming majority of studies examining motion sensitivity and reading have used motion coherence as a measure (for a review see Benassi, Simonelli, Giovagnoli, & Bolzani, 2010). Only a handful of studies have used speed discrimination to explore associations between vision and reading ability (Demb, Boynton, Best, et al., 1998; Demb, Boynton, & Heeger, 1998; Ramus et al., 2003; Wilmer, Richardson, Chen, & Stein, 2004). This is despite evidence it may be a more accurate proxy for reading fluency (Wilmer et al., 2004). Here we use speed discrimination as a measure of motion sensitivity because it may be more relevant to reading rate and better at differentiating skilled readers.
We report three findings: 1. Reading rates correlate with speed discrimination thresholds but not contrast detection. 2. Speed discrimination thresholds correlate with the reading rate for real words, but not for pseudo-words. 3. Finally, neither contrast detection nor speed discrimination correlate with cognitive measures independent of vision such as analytical reasoning and vocabulary. We argue that the correlations between real word reading rates and speed discrimination support the idea that efficient reading involves magnocellular functions that analyze letter position.
2. Material and Methods
2.1. Participants
Twenty-eight (28) participants were recruited from the Stanford University student body and Palo Alto community (14 adults, age 18–53; 14 children, age 6–16). Four of the adults and seven of the children were diagnosed with developmental dyslexia. Three of the children had recognized reading difficulties, but had never been diagnosed with dyslexia. All participants were pre-screened for neurological conditions and language history. English was the first language of those selected to participate. The participants were consented according to Stanford University IRB guidelines.
2.2. Neuropsychological testing
2.2.1. TOWRE
Participants completed forms A and B of the Test of Word Reading Efficiency or TOWRE, a timed measure of verbal fluency and accuracy (Torgesen et al., 1999). Participants read two lists for each form: Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE). The SWE list consists of 104 English words. The PDE list consists of 63 pronounceable pseudo-words with no recognized English meaning. Participants were instructed to read the lists as fast as possible while maintaining pronunciation. For each list, the experimenter recorded the number of words participants were able to read in 45 seconds, not counting miss-readings, mispronunciations, and skipped items.
2.2.2. WASI
Participants completed the Vocabulary and Matrix Reasoning subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). For the Vocabulary subtest, participants were presented with cards printed with words appropriate to their age level. Their task was to verbally define each word in as much detail as possible. Participants had approximately 30 seconds to respond to each item (42 total) or it was counted wrong. Different start and stop points were observed according to the age group. After testing the experimenter scored the word definitions on accuracy and detail using a 0–2 point scale.
In the Matrix Reasoning subtest, participants were presented with cards depicting a 2 × 2 matrix. Three of the cells in the matrix contained abstract forms. Participants were instructed to identify the most appropriate form to complete the matrix from an array of five choices. Participants had approximately 30 seconds to finish each item (35 total) or it was scored as incorrect.
2.3. Psychophysics
All psychophysics testing took place in a mesopic environment where the primary light source was a computer monitor. Participants were seated 57 cm from a 21-inch CRT monitor. Neutral density filters affixed to the screen reduced the mean screen luminance to 4 cd/m2. All stimuli appeared over a grey background. A chin rest ensured the correct distance and minimized head movement. Participants made responses on a Qwerty keyboard with their dominant hand. Two-alternative, interval choice (2IFC) procedures measured speed discrimination and contrast detection (Figure 1). The tasks were programmed and run on a Mac OS X laptop using MGL (http://gru.brain.riken.jp/mgl), a freeware psychophysics package based in MATLAB. An experimenter was present throughout testing to start the programs and keep participants on task. Extra care was taken to make sure that the behavioral data collected could be compared across age range. Participants completed about 20 to 60 practice trials before data collection. An experimenter sat on the side of the participants and verbally motivated each individual to focus on the upcoming stimulus before each trial. Sessions with more than five consecutive missed responses were aborted and the data discarded.
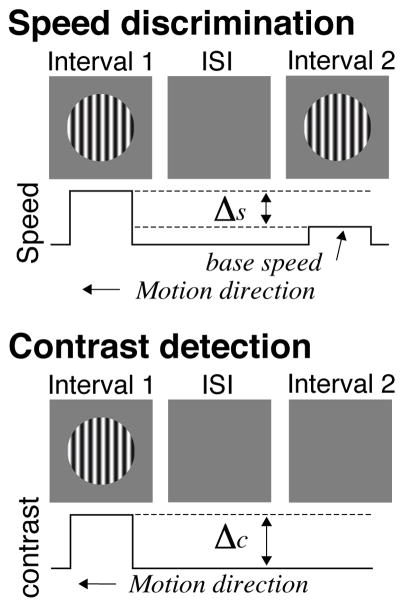
Figure 1. Psychophysical protocol.
a. Speed discrimination task. Top. Gratings (5° in diameter, 0.5 c/° spatial frequency) were presented at the center of the screen in two 375-ms long intervals separated by an inter-stimulus interval of 250 ms. Gratings in both intervals had the same contrast (either 16 or 24%). Bottom. On every trial one of the stimuli drifted from right to left at 38 °/s (base speed), the other had a higher drift speed (base speed + delta-s). Participants indicated the temporal interval containing the faster grating. The speed difference between the two gratings (delta-S) was adjusted on each trial by an adaptive staircase procedure to measure the 75% speed discrimination. b. Contrast detection task. Top. Gratings (5° in diameter, 0.5 c/° spatial frequency) were presented at the center of the screen in two 375-ms long intervals separated by an inter-stimulus interval of 250 ms. Only one randomly-chosen interval contained the target grating (first in the figure). Bottom. Gratings drifted right to left at 38 °/s. Participants indicated the temporal interval containing the target grating with a key press. The contrast of the target grating (delta-c) was changed on each trial by an adaptive staircase procedure to measure the 75% contrast detection threshold (QUEST; Pelli, 1985).
2.3.1. Speed discrimination
Participants fixated on a circle at the center of the screen. Each trial consisted of two temporal intervals in which a Gaussian windowed grating appeared over the fixation circle. All gratings were 5° in diameter and had a spatial frequency of 0.5 c/°. The contrast of the gratings was either 16 or 24%, randomized trial-to-trial. The stimulus duration was 375 ms with an inter-stimulus interval of 250 ms. Gratings drifted right to left at a base speed of 38 °/s. On every trial, presentations were randomized so one grating was animated at the base speed, the other at a faster, target speed. Participants indicated the temporal interval with the faster grating by pressing corresponding keys during the inter-trial interval. Key presses had to be within 1.6 sec or the response was dropped. Trial-to-trial variation in target speed was controlled by the QUEST adaptive staircase (Watson & Pelli, 1983).
2.3.2. Contrast detection
Participants fixated on the center of the screen and viewed Gaussian windowed gratings under the same parameters as speed discrimination: two temporal intervals of 375 ms and an inter-trial interval of 250 ms. The speed of the gratings (38 °/s) was constant for all stimuli. The order of the temporal intervals was randomized. One interval contained a target grating of varying contrast; the other did not. Participants indicated the temporal interval with the grating by a key press during the inter-trial interval. The QUEST adaptive staircase varied target contrast according to participant responses.
2.4. Data Analysis
2.4.1. Threshold determination
We calculated psychophysical thresholds in three ways. 1. QUEST Median Value. Contrast and speed discrimination thresholds (Weber fraction) were first computed as the median of the QUEST run. The standard QUEST analysis assumes a fixed slope (3.5; Watson & Pelli, 1983) for the psychometric function and computes the threshold corresponding to a 76% correct response rate. To ensure thresholds estimates were not biased by these assumptions, we confirmed our findings in two additional ways. 2. Fitted Slope. We re-ran the QUEST analysis allowing both threshold and slope to vary as free parameters, fitting the psychometric function in each individual observer. 3. Median Intensity. Finally, we calculated the thresholds as the median stimulus intensity presented over the total number of trials across all runs for each observer. This analysis does not assume any model for the final estimation of the psychometric function.
2.4.2. Correlation coefficients
To address whether psychophysical performance is related to reading performance, we calculated correlation coefficients (Pearson’s r) between reading rates and mesopic thresholds (velocity discrimination and contrast detection). We used a bootstrapping technique to assess the significance of individual correlations as well as the differences between correlations (Efron & Tibshirani, 1993).
To compute the error of the correlation coefficients we first randomly resampled with replacement 10,000 times from the TOWRE scores and psychophysical thresholds (14 scores and 14 thresholds in each sample). We computed the correlation coefficient for each sample, generating a distribution of r values with a mean of zero. To determine the statistical significance of individual correlations, we calculated the percentile of each correlation coefficient in relation to the above distribution of r values. The p values reported indicate the probability that a correlation coefficient is different from zero. The 95% confidence intervals are plotted as error bars.
We also assessed the significance of differences between adult and child correlations. To do so we combined the TOWRE scores and psychophysical thresholds of adults and children into union distributions. We resampled randomly with replacement 10,000 times from the union distributions (14 scores and 14 thresholds in each sample) then computed the correlation coefficients and the differences between adult and child coefficients for each sample. The resulting difference values were centered on zero and represented a distribution where H0 is true. Using the original data, we found the percentiles for the difference values on the resampled distribution. Reported p values represent the probability that correlation coefficients are from two different distributions.
3. Results
3.1. TOWRE and WASI performance
The TOWRE raw scores for the SWE and PWE subtests were converted to age-normed standard scores. The standard scores were averaged across forms A and B for each participant, producing a composite score with a mean of 100 and standard deviation of 15. On the SWE subtest adults had a performance range from 73 to 113 with a mean score of 96, (SD = 11). Performance on the PWE showed a similar range, 72 to 120 (M = 104, SD = 14). Children’s scores were more variable, ranging between 56 to 145 (M = 101, SD = 21) on the SWE and 61 to 144 (M = 101, SD = 23) on the PWE. Overall, the TOWRE data showed a broad range of standard scores. Adults had scores within ± 2 SD of the mean of the standard distribution, children within ± 3 standard deviations.
Analysis of WASI data involved computing T scores (M = 50, SD = 10) for the Vocabulary and Matrix Reasoning subtests. Adults showed a range of 41 to 77 (M = 64, SD = 9) for the Vocabulary subtest, 50 to70 (M = 61, SD = 5) for the Matrix Reasoning. Children’s scores ranged from 36 to 73 (M = 58, SD = 10) for Vocabulary, 48 to 76 (M = 61, SD = 8) for Matrix Reasoning. A composite, full-scale intelligence quotient (FSIQ) was calculated from the subtests. All participants showed average or above average intelligence: children (M = 117, SD = 14), adults (M = 118, SD = 11).
3.2. Psychophysical thresholds
3.2.1. Speed discrimination
Adults showed better performance than children on the speed discrimination task (Figure 2). This was true in all three analyses: 1. QUEST Median Value. When speed discrimination thresholds (Weber fraction) were computed as the median of the QUEST run, thresholds were 3x higher in children (0.36) than in adults (0.13). 2. Fitted Slope. Fitting the slope to each observer and allowing threshold and slope to vary produced similar thresholds (children: 0.30; adults: 0.12). 3. Median Intensity. Calculating thresholds as the median value over all experimental trials in each observer also confirmed previous analyses (children: 0.29; adults: 0.11). These results indicate that our basic finding does not depend the methods used in threshold estimation.
Figure 2. Speed discrimination and contrast detection thresholds.
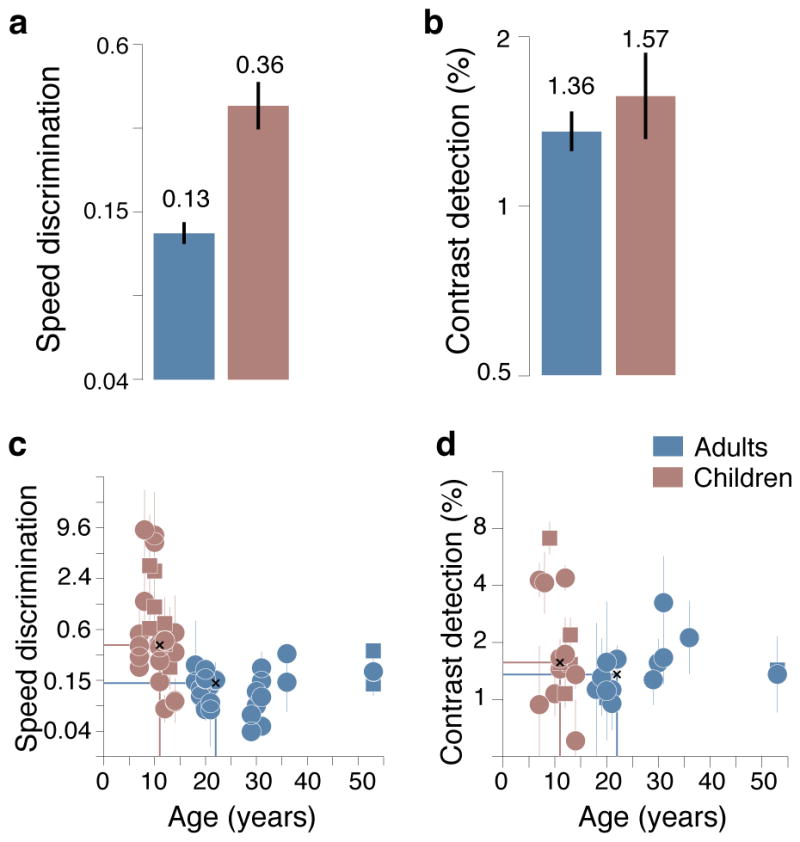
a and b. Bar graphs of speed discrimination and contrast detection thresholds. a. Median ± SEM speed discrimination thresholds (Weber fraction) in adults and children. b. Median ± SEM contrast detection thresholds (%) in adults and children. c and d. Scatter plots of participants’ age and the psychophysical thresholds. c. Speed discrimination thresholds ±1SD. d. Contrast detection thresholds ±1SD. For speed discrimination, two thresholds were measured for each participant with gratings of slightly different contrast (16 and 24%). Intersecting blue and red lines mark mean values for each group of participants.
Adults = Blue. Children = Red.
Circles = Normal Readers. Squares = Developmental Dyslexia
3.2.2. Contrast detection
We found no difference in contrast thresholds between children and adults. Both naïve children and adults performed well on the contrast detection task with thresholds reaching those of experienced psychophysical observers (1% thresholds; Figure 2). When contrast detection thresholds were computed as the median of the QUEST run, the analysis did not reveal an appreciable difference in the median thresholds (children, 1.57; adults, 1.35). Fitting the slope of each observer produced similar values (children, 1.46; adults, 1.22), as did the median intensity analysis (children, 2.67; adults, 1.51).
3.2.3. Thresholds and age
To evaluate how speed discrimination and contrast detection change with development, we correlated the mesopic thresholds with participant age. We found that both speed discrimination and contrast detection thresholds were significantly correlated with participant age (Figure 2). Children showed significant negative correlations between the thresholds and age: older children having lower thresholds than younger children (speed discrimination: r = −0.34, p = .01; contrast detection: r = −0.42, p = .04). In contrast, adults showed positive correlations, older adults having slightly higher thresholds than younger adults (speed discrimination: r = 0.28, p = .03; contrast detection: r = 0.31, p = .02).
3.3. Psychophysical thresholds and neuropsychological test correlations
3.3.1. Speed discrimination and reading rate
Both children and adults showed a negative relationship between word-form reading rate and speed discrimination. Higher scores on the Sight Word Efficiency (SWE) section of the TOWRE were associated with lower speed discrimination thresholds. This relationship was statistically significant in both children, r = −0.37, p = .02, and adults, r = −0.52, p = .001 (Figure 3, panel a). Bootstrapping the difference revealed correlation coefficients were not significantly different between adults and children (p = .58).
Figure 3. Correlation between reading rates, speed-discrimination and contrast detection thresholds.
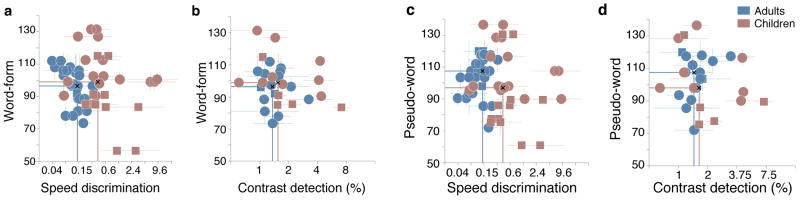
a and b. Scatterplots of word reading scores and psychophysical thresholds. a. Speed discrimination. b. Contrast detection. c. and d. Scatterplots of pseudo-word reading scores and psychophysical thresholds. c. Speed discrimination. d. Contrast detection. In all plots, error bars indicate ±1SD. Vertical lines mark the median speed/contrast thresholds for each group of participants; adults and children. Horizontal lines mark the median reading score.
We tested whether there was a similar relationship between speed discrimination performance and the Phonemic Decoding Efficiency (PDE) or pseudo-word scores. There were negative relationships between speed discrimination thresholds and pseudo-word reading rates. However, correlations were non-significant in both children, r = −0.16, p = .21 and adults, r = 0.08, p = .47 (Figure 3, panel c). In addition, there was no significant difference between adult and child correlation coefficients (p = .21)
3.3.2. Contrast detection and reading rate
Contrast detection thresholds did not correlate with single-word reading rates. This was true for word-forms (children: r = −0.38, p = .07; adults: r = −0.10, p = .29; Figure 3, panel b) and pseudo-words (children: r = −0.31, p = .09; adults: r = 0.31, p = .07; Figure 3 panel d). Adult and child correlation coefficients were not significantly different for SWE (p = .27) and PDE (p = .11) subtests.
3.3.3. Psychophysical thresholds and WASI test scores
Our analysis did not find relationships between the WASI test scores and either speed discrimination or contrast detection (Figure 4). Speed discrimination was not significantly correlated with the Vocabulary (adults: r = 0.12, p = .41; children: r = −0.20, p = .11) or Matrix Reasoning (adults: r = −0.15, p = .27; children: r = −0.08, p = .45) scores. Correlations with contrast detection were also non-significant: Vocabulary (adults: r = 0.28, p = .14; children: r = −0.20, p = .16), Matrix Reasoning (adults: r = 0.28, p = .07; children: r = −0.07, p = .44).
Figure 4. No correlation between WASI scores, speed-discrimination and contrast detection thresholds.
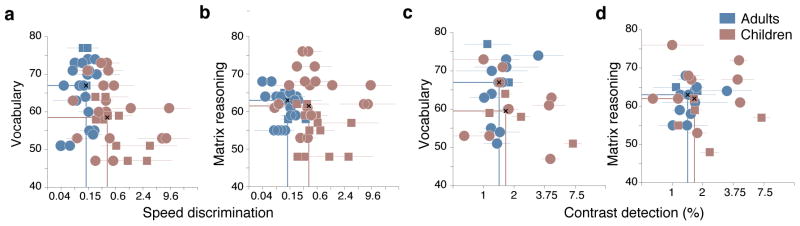
a. and b. Scatter plots of speed-discrimination thresholds and WASI scores b. Vocabulary. b. Matrix Reasoning. c. and d. Scatter plots of contrast detection thresholds and WASI scores. c. Vocabulary and d. Matrix Reasoning. In all plots, error bars indicate ±1SD. Vertical lines mark the median speed/contrast thresholds for adults and children. Horizontal lines mark the median test score.
4. Discussion
We acquired mesopic thresholds (speed-discrimination and contrast-detection) and reading rates in participants with a wide range of reading skills. We found two main results related to the threshold measurements: 1. Speed discrimination thresholds are lower in adults than in children, but there is no reliable group difference for contrast detection. 2. Speed discrimination and contrast detection thresholds are negatively correlated with age in children but positively correlated with age in adults. Younger children and older adults have higher (speed discrimination and contrast-detection) thresholds. Young adults have the lowest thresholds – the best visual performance.
We calculated correlation coefficients between the visual thresholds and reading rates. Speed discrimination thresholds are negatively correlated with real word reading rates in adults and children. Participants with lower speed discrimination thresholds have faster real word (SWE) reading rates. This correlation is more robust for adults (r = −0.52) than children (r = −0.37). Conversely, we found no significant correlations between speed discrimination thresholds and pseudo-word (PDE) reading rates. We also found no correlations between reading rates and contrast detection thresholds or reading rates and the WASI subtest scores.
Our results demonstrate that: 1. Speed discrimination thresholds correlate with reading rate. Contrast detection thresholds do not. 2. Speed discrimination thresholds correlate with the reading rates of familiar words but not pseudo-words. 3. Finally, speed discrimination does not correlate with cognitive measures orthogonal to reading rate like analytical reasoning and vocabulary. These findings suggest familiarity is a factor in magnocellular computations that influence reading rate.
4.1. Speed discrimination and word familiarity
Our data show that speed discrimination thresholds are correlated with reading rates for actual words (SWE), not pseudo-words (PDE). Both sets of stimuli are pronounceable, share words of similar length and complexity, yet they differ in their familiarity. Participants have likely encountered the actual word “understand” in print before but not the pseudo-word “fermabalt”. In models of word recognition, word representations are constructed by identifying spatial relationships among letters. Word familiarity may affect this process. The finding of a disassociation between SWE and PDE correlations indicates that familiarity is a factor in magnocellular operations that influence reading rate.
It also evidences position encoding as the potential mechanism. Reading experience likely affects letter position encoding. Frequently encountering the same words may draw attention to specific identifiers such as the first and last letter as well internal letter pairings. Cornelissen et al. (1998a; 1998b) theorize that visual identification of words depends on recognizing letters in specific spatial positions. Inability to do so results in letter errors, instances where words are mispronounced due to confusion over letter positions. They noticed this effect in children learning the read. The better readers of their sample demonstrated significantly less letter errors. They suggest experience with a wider vocabulary may tune position encoding to familiar orthography.
We interpret our results similarly. The SWE task is correlated with speed discrimination because reading real words reading engages mechanisms specific to frequently encountered stimuli. The SERIOL model, for example, proposes that repeated attention to common letter combinations yields automatic word recognition (Whitney, 2001; Whitney & Cornelissen, 2005). In this way the magnocellular stream may become primed to recognize familiar words. In contrast, pseudo-word reading may occur in a more laborious or all together different manner where words are processed letter-by-letter (Coltheart et al., 2001). Consequently, reading actual words reveals inter-individual differences that are not real or resolvable with PDE task.
Our findings are consistent with a longstanding experimental literature demonstrating word familiarity effects on reading and lexical tasks. More familiar words have faster naming and lexical decision times (Cattell, 1886; Howes & Solomon, 1951; Solomon & Postman, 1952; Forster & Chambers, 1973; Balota & Chumbley, 1984; Monsell, Doyle, & Haggard, 1989). Our data also complement evidence that letter position and spatial relationships influence word recognition. The word superiority effect (WSE), for example, demonstrates letters are more quickly identified within the context of a word than alone or among random letter strings (Reicher, 1969; Wheeler, 1970; Johnston & McClelland, 1973). Other findings show letter strings prime recognition if they share letters in the same locations as subsequent words (Humphreys, Evett, & Quinlan, 1990; Peressotti & Grainger, 1995; Grainger & Jacobs, 1996)
Other aspects of our data demonstrate age differences in visual ability. We found that both speed discrimination and contrast detection improve during development but decline in late adulthood. These findings broadly agree with developmental studies on children which show performance on visual tasks improves substantially between 4 and 7 years of age (Lovegrove, Heddle, & Slaghuis, 1980; Slaghuis & Ryan, 1999). In addition, reports indicate a slowing of visual processing as adults age. It often takes longer for older adults to detect, discriminate, and identify visual targets compared to young adults (for a review see Owsley, 2011). One interpretation of these findings is that the development and aging of neural circuitry influences visual performance.
The dissociation between SWE and PDE correlations may not solely be due visual processes. The recognition of real words could be visual and automatic, but reading unfamiliar words may require an analysis of phonology. Coltheart et al. (1993, 2001), for example, argue for a hardwired distinction between lexical and pseudo-lexical reading in their dual-route cascaded (DRC) model. In the lexical route, familiar words are recognized at a glance and directly activate established phonological and semantic representations. In the pseudo-lexical route, unfamiliar words are sounded-out through grapheme-to-phoneme correspondence rules. Within this framework, our findings on the SWE subtest may primarily demonstrate individual differences in visual processing. In contrast, the PDE subtest measures an interaction between both visual and phonological processes. Consequently a visual task like speed discrimination is correlated with reading actual words but not pseudo-words.
Finally, we found no relationships between the visual thresholds and neuropsychological measures related to general cognitive abilities. Neither speed discrimination or contrast detection tasks are correlated with WASI Vocabulary and Matrix Reasoning subtests in children or adults. From the standpoint of a visual interpretation, these results make sense. If speed discrimination is an index of magnocellular processing, it should not correlate with measures of verbal and analytical intelligence. These measures are more likely influenced by development stage in children and overall education in adults.
4.2. Speed discrimination, not contrast detection, predicts reading rate
We found speed discrimination to be an effective predictor of reading performance. The relationship between speed discrimination thresholds and reading rates in our sample complement a wide literature demonstrating motion sensitivity is correlated with reading ability (Cornelissen, Hansen, Hutton, et al., 1998; Demb, Boynton, Best, et al., 1998; Demb, Boynton, & Heeger, 1998; Talcott et al., 2000, 2002; Solan, Hansen, Shelley-Tremblay, & Ficarra, 2003; Talcott et al., 2003; Schulte-Körne, Bartling, Deimel, & Remschmidt, 2004; Wilmer et al., 2004).
Researchers assess motion sensitivity in different ways. In motion coherence tasks participants view random dot kinematograms (RDK). They attempt to detect the direction of coherently moving dots embedded in a larger, randomly moving set. In speed discrimination tasks participants judge the relative velocity of two consecutively presented gratings. Among motion-based tasks, the most used index is motion coherence. Benassi et al. (2010) performed a meta-analysis on 53 studies comparing motion perception in normal and impaired readers. All employed measures of motion coherence. Exceptions using speed discrimination include studies by Demb et al. (1998a; 1998b), Wilmer et al. (2004) and Ramus et al. (2003).
Wilmer et al. (2004) used exploratory factor analysis to show that motion coherence is correlated with reading accuracy while speed discrimination is correlated with reading rate. The authors go on to argue that speed discrimination is perhaps a better index of reading performance in normal readers. Our findings support this. We obtained a moderate correlation for adults (r = −0.52) and a more modest correlation for children (r = −0.37), but both are larger than the average effect size for motion coherence (d = 0.19) (Benassi et al., 2010).
Where motion coherence measures an observer’s ability to integrate global visual information amid noise, speed discrimination involves local spatial comparisons. We tasked participants to judge the velocities of drifting gratings presented to the same visual location. This fact may explain our higher than average correlations. If word recognition requires local analysis of letter combinations, then speed discrimination may have greater validity as a reading measure, particularly for reading rate.
We did not find correlations between contrast detection and reading rate. Some studies have demonstrated reduced contrast performance among poor readers (i.e. dyslexics), particularly for stimuli with low spatial and high temporal frequencies (Lovegrove et al., 1982; Martin & Lovegrove, 1984, 1987; Mason, Cornelissen, Fowler, & Stein, 1993; Evans, Drasdo, & Richards, 1994; Felmingham & Jakobson, 1995; Borsting et al., 1996; Edwards et al., 2004). Others have failed to find such differences (Cornelissen, Richardson, Mason, Fowler, & Stein, 1995; Gross-Glenn et al., 1995; Hayduk, Bruck, & Cavanagh, 1996; Ben-Yehudah, Sackett, Malchi-Ginzberg, & Ahissar, 2001; Williams, Stuart, Castles, & McAnally, 2003).
Demb et al. (1998a) found motion perception to be a better predictor of reading performance than contrast detection. They suggest the variability in the literature is explained by not effectively controlling luminance. The current study kept mean luminance low (4 cd/m2) to emphasize the magnocellular pathway inputs to cortex. Our results agree with Demb et al. (1998a). Absence of correlations with contrast detection indicate contrast sensitivity is not a magnocellular influence on word recognition and/or reading speed, at least when the text contrast his high and the readers have normal vision.
4.3. Neurocorrelates of word recognition
Efficient reading requires coordination between oculomotor and object recognition processes (Vidyasagar & Pammer, 2010). These functions are thought to be administered by separate neural routes: the dorsal and ventral visual streams (Goodale & Milner, 1992). Magnocellular neurons dominate the dorsal stream (Merigan, Nealey, & Maunsell, 1993), which interprets the motion and spatial information generated during visual scanning (Tootell et al., 1995). The ventral stream, in contrast, deals in object recognition including the identification of written words (Grill-Spector, Kushnir, Hendler, & Malach, 2000; McCandliss, Cohen, & Dehaene, 2003).
Dorsal stream pathways include the temporo-parieto-occipital junction, also known as area MT. Anatomical studies in non-human primates show the majority of fibers that innervate MT are magnocellular (Maunsell & Newsome, 1987; DeYoe & Van Essen, 1988). Lesions of MT in monkeys increases motion coherence thresholds (Newsome & Pare, 1988). Similar damage in human results in deficits of motion perception and oculomotor control (Vaina, Lemay, Bienfang, Choi, & Nakayama, 1990). Functional MRI studies of healthy humans reveal judgments of coherent motion, speed discrimination, and other motion-based tasks activate area MT (Tootell et al., 1995; Howard et al., 1996; Buchel et al., 1998; Chawla, Phillips, Buechel, Edwards, & Friston, 1998; Haug, Baudewig, & Paulus, 1998; Braddick et al., 2001; Braddick, O’Brien, Wattam-Bell, Atkinson, & Turner, 2000; Culham, He, Dukelow, & Verstraten, 2001).
Area MT has also been linked to reading achievement. Functional MRI studies of dyslexics report less activation in MT compared to controls (Demb et al., 1997) or no activation at all (Eden et al., 1996). Perturbation of MT by transcranial magnetic stimulation (TMS) decreases reading performance in non-word naming tasks (Liederman et al., 2003). Interestingly, this effect is more pronounced for mixed-case words (Braet & Humphreys, 2006). It seems then area MT is both sensitive to motion and familiarity. The pattern of performance in word and non-word naming tasks suggests MT contributes to letter localization (Liederman et al., 2003).
The dorsal stream and area MT may also indirectly affect word recognition and reading rate via communication with the ventro-temporal cortex. The visual word form area (VWFA), a region situated on the fusiform gyrus, is thought to process word-forms before associations with phonology and semantics (McCandliss et al., 2003). Though the VWFA is located within the ventral visual stream, it likely receives input from dorsal cortex. Structural evidence of this connection is the ventral occipital fasciculus (VOF), a white matter tract connecting the dorsal and ventral cortices of the occipital lobe (Déjerine, 1895). Recently Yeatman et al. (2013) demonstrated that fascicles within the VOF connect the functionally-defined VWFA with occipital and dorsomedial cortex.
The identification of orthography is a specific case of object recognition, and recent fMRI experiments evidence communication between dorsal and ventral streams during object recognition (Grill-Spector et al., 1998; Grill-Spector et al., 2000; Kriegeskorte et al., 2003; Konen & Kastner, 2008). Levy et al. (2010) theorize that the dorsal stream may speed word recognition by sending an initial, low-pass representation of the letter string to the VWFA where it is further processed. If this initial representation is stalled or impoverished, it may result in delays that ultimately affect reading rate.
The current study cannot affirm or refute biological models of reading performance. However, our results are consistent with brain and behavioral data showing motion perception and reading performance are related. The finding that processing in MT is affected by mixed-case words suggests this area is sensitive to orthography as well as motion (Braet & Humphreys, 2006). Area MT may be a neurocorrelate of the familiarity effect reported here. It is still unknown though if MT operations could be described as position encoding. Also unknown is whether area MT and other components of the dorsal stream evaluate orthography alone or in conjunction with ventro-temporal cortex. Further research is necessary.
4.4. Limitations
The current findings support the theory that magnocellular processing contributes to reading rate. The pattern of results suggests position encoding as a mechanism, not contrast sensitivity. However, this report cannot address other ways the M stream may influence reading performance. Magnocellular processes may affect reading rate through the coordination of visual functions like selective attention, oculomotor control, and foveal/parafoveal interaction (Boden & Giaschi, 2007).
Some reading difficulties may stem from deficits in saccadic eye movements or binocular fixation (Pavlidis, 1981; Stein & Fowler, 1993). Weak M-stream input to oculomotor centers is a potential cause of this problem (Stein & Walsh, 1997; Fischer & Hartnegg, 2000). Lesions to MT, for example, disrupt saccades and the visual tracking of moving objects (Leigh & Zee, 1999). Accordingly, erratic patterns of saccades have been found in dyslexics, evidencing their effect on reading (Rubino & Minden, 1990; Lennerstrand, Ygge, & Jacobson, 1993; Poblano, de Caballero, Castillo, & Cortes, 1996).
Another possibility is the M stream affects reading through the allocation of visual attention. During reading the focus of attention often evaluates text beyond fixation. Operations of covert attention filter this information so that text to the left fixation or outside the line of interest does not disrupt word recognition. The posterior parietal cortex (PPC) is a likely neurocorrelate for this process. The PPC receives a majority of its input from the M stream and its damage affects the ability to disengage attention (Posner, Walker, Friedrich, & Rafal, 1984). Some patients with PPC lesions demonstrate neglect dyslexia, a condition where letters at the beginning of a word or the words at the beginning of a line are ignored (Riddoch, Humphreys, Cleton, & Fery, 1990; Brunn & Farah, 1991).
A final consideration is how the visual system incorporates information from the parafovea during reading. The parafovea accesses the visual field surrounding fixation (fovea). It can evaluate low spatial frequency information such as word length, shape, as well as beginning and ending letters (Rayner, Inhoff, Morrison, Slowiaczek, & Bertera, 1981; Lehmkuhle, Garzia, Turner, Hash, & Baro, 1993). If impaired, the M stream could interrupt efficient access to these visual features.
The above are all ways the M stream could affect reading. Importantly, they are not mutually exclusive. Each could act on visual information in parallel but at different levels of analysis. Our data suggest familiar orthography is a contributing factor to reading rate. Theories of position encoding accommodate this finding, but also reference attention as a mechanism of action. In addition, position encoding of parafoveal information could contribute to word recognition and guidance of saccades. We suggest the current evidence supports position encoding, but acknowledge further research is necessary to understand how other M stream mechanisms may influence or direct this process.
Another caveat regarding our findings is they are correlational. We found significant correlations between the psychophysical thresholds and age. This may evidence the aging of neural circuitry. However, correlations preclude causal interpretation. An alternative possibility is that reading experience could drive biological change. It is known, for example, that the development of reading skills enhances early visual processes like contour integration (Szwed, Ventura, Querido, Cohen, & Dehaene, 2012). It is plausible then that extensive reading experience changes the allocation of attention to and visual speed of processing of orthography. In fact, the trained allocation of attention to specific letter combinations is a essential component of positional encoding.
Our results then indicate a relationship between age and reading performance, but further research is necessary to understand how reading experience and the M stream interact. An experimental way to study this is to train visual observers on tasks that enhance magnocellular capabilities while periodically measuring reading rates. This design may be best suited to adult, skilled readers since their reading performance is stable. If the training tasks address different aspect of magnocellular processing (i.e. oculomotor, covert attention, parafoveal/foveal interaction) they may reveal the variance each contributes to reading rate.
Finally, it is worthwhile to consider our data in reference to dyslexia. Our sample included normal and poor readers but only a few diagnosed dyslexics. Consistent with previous work, we found that dyslexics largely reside at the lower end of the performance distributions for both reading and speed discrimination (Demb, Boynton, Best, et al., 1998; Talcott et al., 1998; Witton et al., 1998; Slaghuis & Ryan, 1999; Talcott, Hansen, Assoku, & Stein, 2000; Hansen, Stein, Orde, Winter, & Talcott, 2001; Ridder, Borsting, & Banton, 2001; Ramus et al., 2003; Kevan & Pammer, 2008).
However, not all researchers have found such differences (Kronbichler, Hutzler, & Wimmer, 2002; Williams et al., 2003) and some reports have found impaired motion sensitivity in certain dyslexic subtypes but not others (Ramus et al., 2003; Slaghuis & Ryan, 1999). There seems to be substantial heterogeneity among dyslexics with visual/magnocellular impairments, including some individuals with no deficit at all. The reason for this is the source of considerable debate. Many researchers maintain that dyslexia is principally a disorder of phonology and that visual deficits are simply epiphenomena. Variability in experimental findings attests to the complexity of the issue (Farmer & Klein, 1995; Skottun, 2000; Stein, 2001; Handler & Fierson, 2011; Ramus & Ahissar, 2012; Olulade, Napoliello, & Eden, 2013).
While we included dyslexics in our sample, our design and analysis does not permit statements on the casual factors of dyslexia. Recent intriguing longitudinal studies find that motion sensitivity in pre-literate children predicts orthographic performance (Boets, Wouters, van Wieringen, & Ghesquiere, 2006) and precedes the diagnosis of dyslexia in some cases (Boets, Vandermosten, Cornelissen, Wouters, & Ghesquiere, 2011). However, magnocellular function may affect reading performance in both dyslexics and abled readers, but this does not mean magnocellular dysfunction causes dyslexia. From our findings, we suggest speed discrimination may have utility in examining motion sensitivity and its relation to reading fluency, but we do not claim it differentiates dyslexics from normal readers or implicates a specific cause.
5. Conclusions
We believe the current results have valuable implications for reading research and directions for further study. First, the findings here afford another example of research implicating the visual pathways in skilled reading. Many previous reports used motion coherence as a standard measure of motion sensitivity. Few studies have employed speed discrimination to the same ends. Here we demonstrate the utility of speed discrimination and find it yields stronger correlations than the average effect size for motion coherence.
Secondly, we found that speed discrimination is correlated with certain reading measurements (word-form/SDE) but not others (pseudo-word/PDE). We believe this finding suggests speed discrimination indexes operations of the magnocellular stream functional in the word recognition. The lack of any correlation with contrast detection suggests contrast sensitivity is not critical to these operations.
Finally, we agree with Wilmer et al. (2004) that speed discrimination may be a more subtle test of reading performance, capable of differentiating skill level in normal readers. Because of this, we believe it could also be successful in clarifying the spectrum of visual performance found among the reading impaired. We suggest the selective correlation between speed discrimination and reading rate needs further investigation, especially in relation to early prognosis of reading impairments.
References
- Akutsu H, Legge GE, Ross JA, Schuebel KJ. Psychophysics of reading--X. Effects of age-related changes in vision. Journal of Gerontology. 1991;46:325–331. doi: 10.1093/geronj/46.6.p325. [DOI] [PubMed] [Google Scholar]
- Balota DA, Chumbley JI. Are lexical decisions a good measure of lexical access? The role of word frequency in the neglected decision stage. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:340–357. doi: 10.1037//0096-1523.10.3.340. [DOI] [PubMed] [Google Scholar]
- Becker CA. Semantic context and word fequency effects in visual word recognition: An analysis of semantic strategies. Journal of Experimental Psychology: Human Perception and Performance. 1979;5:252–259. doi: 10.1037//0096-1523.5.2.252. [DOI] [PubMed] [Google Scholar]
- Benassi M, Simonelli L, Giovagnoli S, Bolzani R. Coherence motion perception in developmental dyslexia: A meta-analysis of behavioral studies. Dyslexia. 2010;16(4):341–357. doi: 10.1002/dys.412. [DOI] [PubMed] [Google Scholar]
- Ben-Yehudah G, Sackett E, Malchi-Ginzberg L, Ahissar M. Impaired temporal contrast sensitivity in dyslexics is specific to retain-and -compare paradigms. Brain. 2001;124:1381–1395. doi: 10.1093/brain/124.7.1381. [DOI] [PubMed] [Google Scholar]
- Boden C, Giaschi D. M-stream deficits and reading-related visual processes in developmental dyslexia. Psychological Bulletin. 2007;133(2):346–366. doi: 10.1037/0033-2909.133.2.346. [DOI] [PubMed] [Google Scholar]
- Boets B, Vandermosten M, Cornelissen P, Wouters J, Ghesquiere P. Coherent motion sensitivity and reading development in transition from prereading to reading stage. Child Development. 2011;82(3):854–869. doi: 10.1111/j.1467-8624.2010.01527.x. [DOI] [PubMed] [Google Scholar]
- Boets B, Wouters J, van Wieringen A, Ghesquiere P. Coherent motion detection in preschool children at family risk for dyslexia. Vision Research. 2006;46:527–535. doi: 10.1016/j.visres.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Borsting E, Ridder WH, Dudeck K, Kelley C, Matsui L, Motoyama J. The presence of a magnocellular defect depends on the type of dyslexia. Vision Research. 1996;36(7):1047–1053. doi: 10.1016/0042-6989(95)00199-9. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Hartley T, Turner R. Brain areas sensitive to coherent visual motion. Perception. 2001;30(1):61–72. doi: 10.1068/p3048. [DOI] [PubMed] [Google Scholar]
- Braddick OJ, O’Brien JMD, Wattam-Bell J, Atkinson J, Turner R. Form and motion coherence activate independent, but not dorsal/ventral segregated, networks in the human brain. Current Biology. 2000;10(12):731–734. doi: 10.1016/s0960-9822(00)00540-6. [DOI] [PubMed] [Google Scholar]
- Braet W, Humphreys GW. Case mixing and the right parietal cortex: Evidence from rTMS. Experimental Brain Research. 2006;168:265–271. doi: 10.1007/s00221-005-0085-z. [DOI] [PubMed] [Google Scholar]
- Broadbent DE. Word-frequency effect and response bias. Psychological Review. 1967;74:1–15. doi: 10.1037/h0024206. [DOI] [PubMed] [Google Scholar]
- Brunn JL, Farah MJ. The relation between spatial attention and reading: Evidence from the neglect syndrome. Cognitive Neuropsychology. 1991;8:59–75. [Google Scholar]
- Buchel C, Josephs O, Rees G, Turner R, Frith C, Friston KJ. The functional anatomy of attention to visual motion. Brain. 1998;121:1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- Cattell JM. The time taken up by cerebral operations. Mind. 1886;11:63–65. [Google Scholar]
- Chawla D, Phillips J, Buechel C, Edwards R, Friston KJ. Speed-dependent motion-sensitive responses in V5: An fMRI study. Neuroimage. 1998;7:86–96. doi: 10.1006/nimg.1997.0319. [DOI] [PubMed] [Google Scholar]
- Coltheart M, Curtis B, Atkins P, Haller M. Models of reading aloud: dual-route and parallel-distributed-processing approaches. Psychological Review. 1993;100:589–608. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psychological Review. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Bradley L, Fowler S, Stein J. What children see affects how they spell. Developmental Medicine and Child Neurology. 1994;34:296–304. doi: 10.1111/j.1469-8749.1994.tb11914.x. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Hansen PC. Motion detection, letter position encoding, and single word reading. Annals of Dyslexia. 1998;48:155–188. [Google Scholar]
- Cornelissen PL, Hansen PC, Gilchrist I, Cormack F, Essex J, Frankish C. Coherent motion detection and letter position encoding. Vision Research. 1998;38(14):2181–2191. doi: 10.1016/s0042-6989(98)00016-9. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Hansen PC, Hutton JL, Evangelinou V, Stein JF. Magnocellular visual function and children’s single word reading. Vision Research. 1998;38(3):471–482. doi: 10.1016/s0042-6989(97)00199-5. [DOI] [PubMed] [Google Scholar]
- Cornelissen PL, Richardson A, Mason A, Fowler S, Stein J. Contrast sensitivity and coherent motion detection measured at photopic luminance levels in dyslexics and controls. Vision Research. 1995;35(10):1483–1494. doi: 10.1016/0042-6989(95)98728-r. [DOI] [PubMed] [Google Scholar]
- Culham J, He S, Dukelow S, Verstraten FAJ. Visual motion and the human brain: what has neuroimaging told us? Acta Psychologica. 2001;107(1–3):69–94. doi: 10.1016/s0001-6918(01)00022-1. [DOI] [PubMed] [Google Scholar]
- De Luca M, Spinelli D, Zoccolotti P. Eye movement patterns in reading as a function of visual field defects and contrast sensitivity loss. Cortex. 1996;32:491–502. doi: 10.1016/s0010-9452(96)80006-2. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Cohen L, Sigman M, Vinckier F. The neural code for written words: a proposal. Trends in Cognitive Sciences. 2005;9:335–341. doi: 10.1016/j.tics.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Best M, Heeger DJ. Psychophysical evidence for a magnocellular pathway deficit in dyslexia. Vision Research. 1998;38(11):1555–1559. doi: 10.1016/s0042-6989(98)00075-3. [DOI] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Brain activity in visual cortex predicts individual differences in reading performance. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13363–13366. doi: 10.1073/pnas.94.24.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Boynton GM, Heeger DJ. Functional magnetic resonance imaging of early visual pathways in dyslexia. Journal of Neuroscience. 1998;18(17):6939–6951. doi: 10.1523/JNEUROSCI.18-17-06939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoe EA, Van Essen DC. Concurrent processing streams in monkey visual cortex. Trends in Neurosciences. 1988;11:219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- Eden GF, VanMeter JW, Rumsey JM, Maisog JM, Woods RP, Zeffiro TA. Abnormal processing of visual motion in dyslexia revealed by functional brain imaging. Nature. 1996;382(6586):66–69. doi: 10.1038/382066a0. [DOI] [PubMed] [Google Scholar]
- Edwards VT, Giaschi DE, Dougherty RF, Edgell D, Bjornson BH, Lyons C, Douglas RM. Psychophysical indexes of temporal processing abnormalities in children with developmental dyslexia. Developmental Neuropsychology. 2004;25(3):321–354. doi: 10.1207/s15326942dn2503_5. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- Evans BJW, Drasdo N, Richards IL. An investigation of some sensory and refractive visual factors in dyslexia. Vision Research. 1994;34(14):1913–1926. doi: 10.1016/0042-6989(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Farmer ME, Klein RM. The evidence for a temporal processing deficit linked to dyslexia: A Review. Psychonomic Bulletin and Review. 1995;2(4):460–493. doi: 10.3758/BF03210983. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Jakobson LS. Visual and visuomotor performance in dyslexic children. Experimental Brain Research. 1995;106:467–474. doi: 10.1007/BF00231069. [DOI] [PubMed] [Google Scholar]
- Fischer B, Hartnegg K. Stability of gaze control in dyslexia. Strabismus. 2000:119–122. [PubMed] [Google Scholar]
- Forster KI, Chambers SM. Lexical access and naming time. Journal of Verbal Learning and Verbal Behavior. 1973;12:627–635. [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grainger J, Grainer JP, Farioli F, Van Assche E, van Heuven W. Letter position information and printed word perception: The relative-position priming constraint. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:865–884. doi: 10.1037/0096-1523.32.4.865. [DOI] [PubMed] [Google Scholar]
- Grainger J, Jacobs AM. Orthographic processing in visual word recognition: a multiple read-out model. Psychological Review. 1996;103:518–565. doi: 10.1037/0033-295x.103.3.518. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object selective activation correlate with recognition performance in humans. Nature Neuroscience. 2000;3:837–843. doi: 10.1038/77754. [DOI] [PubMed] [Google Scholar]
- Gross-Glenn K, Skottun BC, Glenn W, Kushch A, Lingua R, Dunbar M, Duara R. Contrast sensitivity in dyslexia. Visual Neuroscience. 1995;12(1):153–163. doi: 10.1017/s0952523800007380. [DOI] [PubMed] [Google Scholar]
- Handler SM, Fierson WM. Joint technical report-learning disabilities, dyslexia, and vision. Pediatrics. 2011;127(3):E818–E856. doi: 10.1542/peds.2010-3670. [DOI] [PubMed] [Google Scholar]
- Hansen PC, Stein JF, Orde SR, Winter JL, Talcott JB. Are dyslexics’ visual deficits limited to measures of dorsal stream function? Neuroreport. 2001;12(7):1527–1530. doi: 10.1097/00001756-200105250-00045. [DOI] [PubMed] [Google Scholar]
- Harm MW, Seidenberg MS. Computing the meanings of words in reading: division of labor between visual and phonological processes. Psychological Review. 2004;111(3):662–720. doi: 10.1037/0033-295X.111.3.662. [DOI] [PubMed] [Google Scholar]
- Haug BA, Baudewig J, Paulus W. Selective activation of human cortical area V5A by rotating a visual stimulus in fMRI: Implication of attentional mechanisms. Neuroreport. 1998;9:611–614. doi: 10.1097/00001756-199803090-00009. [DOI] [PubMed] [Google Scholar]
- Hayduk S, Bruck M, Cavanagh P. Low-level visual processing skills of adults and children with dyslexia. Cognitive Neuropsychology. 1996;13(7):975–1015. [Google Scholar]
- Hinton GE, Shallice T. Lesioning an attractor network: investigations of acquired dyslexia. Psychological Review. 1991;98:74–95. doi: 10.1037/0033-295x.98.1.74. [DOI] [PubMed] [Google Scholar]
- Howard RJ, Brammer M, Wright I, Woodruff PW, Bullmore ET, Zeki S. A direct demonstration of functional specialization within motion-related visual and auditory cortex of the human brain. Current Biology. 1996;6:1015–1019. doi: 10.1016/s0960-9822(02)00646-2. [DOI] [PubMed] [Google Scholar]
- Howes DH, Solomon RL. Visual duration threshold as a function of word probability. Journal of Experimental Psychology. 1951;41:401–410. doi: 10.1037/h0056020. [DOI] [PubMed] [Google Scholar]
- Humphreys GW, Evett LJ, Quinlan PT. Orthographic processing in visual word identification. Cognitive Psychology. 1990;22(4):517–560. doi: 10.1016/0010-0285(90)90012-s. [DOI] [PubMed] [Google Scholar]
- Johnston JC, McClelland JL. Visual factors in word perception. Perception and Psychophysics. 1973;14:365–370. [Google Scholar]
- Kevan A, Pammer K. Making the link between dorsal stream sensitivity and reading. Neuroreport. 2008;19(4):467–470. doi: 10.1097/WNR.0b013e3282f5f7ad. [DOI] [PubMed] [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H. Dyslexia: Verbal impairments in the absence of magnocellular impairments. Neuroreport. 2002;13(5):617–620. doi: 10.1097/00001756-200204160-00016. [DOI] [PubMed] [Google Scholar]
- Legge G, Pelli DG, Rubin GS, Schleske MM. Psychophysics of reading I. Normal vision. Vision Research. 1984;25:239–252. doi: 10.1016/0042-6989(85)90117-8. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle S, Garzia RP, Turner L, Hash T, Baro JA. A defective visual pathway in children with reading disability. New England Journal of Medicine. 1993;328:989–996. doi: 10.1056/NEJM199304083281402. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Zee DS. The Neurology of Eye Movements. New York: Oxford University Press; 1999. [Google Scholar]
- Lennerstrand G, Ygge J, Jacobson C. Control of binocular eye movements in normals and dyslexics. Annals of the New York Academy of Sciences. 1993;682:231–239. doi: 10.1111/j.1749-6632.1993.tb22971.x. [DOI] [PubMed] [Google Scholar]
- Liederman J, Fisher J, Schulz M, Maxwell C, Theoret H, Pascual-Leone A. The role of motion direction selective extra striate areas in reading. Brain and Language. 2003;85:140–155. doi: 10.1016/s0093-934x(02)00550-3. [DOI] [PubMed] [Google Scholar]
- Lovegrove WJ, Heddle M, Slaghuis W. Reading-disability - spatial-frequency specific deficits in visual information store. Neuropsychologia. 1980;18(1):111–115. doi: 10.1016/0028-3932(80)90093-7. [DOI] [PubMed] [Google Scholar]
- Lovegrove W, Martin F, Bowling A, Blackwood M, Badcock D, Paxton S. Contrast sensitivity functions and specific reading-disability. Neuropsychologia. 1982;20(3):309–315. doi: 10.1016/0028-3932(82)90105-1. [DOI] [PubMed] [Google Scholar]
- Martin F, Lovegrove W. The effects of field size and luminance on contrast sensitivity differences between specifically reading disabled and normal-children. Neuropsychologia. 1984;22(1):73–77. doi: 10.1016/0028-3932(84)90009-5. [DOI] [PubMed] [Google Scholar]
- Martin F, Lovegrove W. Flicker contrast sensitivity in normal and specifically disabled readers. Perception. 1987;16(2):215–221. doi: 10.1068/p160215. [DOI] [PubMed] [Google Scholar]
- Mason A, Cornelissen P, Fowler S, Stein J. Contrast sensitivity, ocular dominance and specific reading-disability. Clinical Vision Sciences. 1993;8(4):345–353. [Google Scholar]
- Mason M. From print to sound in mature readers as a function of reader ability and two forms of orthographics regularity. Memory and Cognition. 1978;6(5):568–581. doi: 10.3758/BF03198246. [DOI] [PubMed] [Google Scholar]
- Mason M. Reading-ability and the encoding of item and location information. Journal of Experimental Psychology-Human Perception and Performance. 1980;6(1):89–98. doi: 10.1037//0096-1523.6.1.89. [DOI] [PubMed] [Google Scholar]
- Maunsell JHR, Newsome WT. Visual processing in monkey extra-striate cortex. Annual Review of Neuroscience. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends in Cognitive Sciences. 2003;7(7):293–299. doi: 10.1016/s1364-6613(03)00134-7. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE. An interactive activation model of context effects in letter perception: I. An account of basic findings. Psychological Review. 1981;88:375–407. [PubMed] [Google Scholar]
- Merigan WH, Byrne CE, Maunsell JHR. Does primate motion perception depend on the magnocellular pathway? Journal of Neuroscience. 1991;11:3422–3429. doi: 10.1523/JNEUROSCI.11-11-03422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Katz LM, Maunsell JHR. The effects of parvocellular lateral geniculate lesions on the acuity and contrast of macaque monkeys. The Journal of Neuroscience. 1991;11(4):994–1001. doi: 10.1523/JNEUROSCI.11-04-00994.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. Macaque vision after magnocellular lateral geniculate lesions. Visual Neuroscience. 1990;5:347–352. doi: 10.1017/s0952523800000432. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Nealey TA, Maunsell JHR. Visual effects of lesions of cortical area V2 in macaques. Journal of Neuroscience. 1993;13(7):3180–3191. doi: 10.1523/JNEUROSCI.13-07-03180.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsell S, Doyle MC, Haggard PN. Effects of word frequency on visual word recognition tasks: Where are they? The Journal of Experimental Psychology: General. 1989;118(1):43–71. doi: 10.1037//0096-3445.118.1.43. [DOI] [PubMed] [Google Scholar]
- Morrison RE. Manipulation of stimulus onset delay in reading: Evidence for parallel programming of saccades. Journal of Experimental Psychology: Human Perception and Performance. 1984;10:667–682. doi: 10.1037//0096-1523.10.5.667. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Pare EB. A selective impairment of motion perception following lesions of the middle temporal visual area (MT) The Journal of Neuroscience. 1988;8:2201–2211. doi: 10.1523/JNEUROSCI.08-06-02201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olulade OA, Napoliello EM, Eden GF. Abnormal visual motion processing is not a cause of dyslexia. Neuron. 2013;79(1):180–190. doi: 10.1016/j.neuron.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owsley C. Aging and vision. Vision Research. 2011;51(13):1610–1622. doi: 10.1016/j.visres.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis GT. Do eye movements hold the key to dyslexia? Neuropsychologia. 1981;19:57–64. doi: 10.1016/0028-3932(81)90044-0. [DOI] [PubMed] [Google Scholar]
- Peressotti F, Grainger J. Letter-position coding in random consonant arrays. Perception and Psychophysics. 1995;57(6):875–890. doi: 10.3758/bf03206802. [DOI] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson KE. Understanding normal and impaired word reading: computational principles in quasi-regular domains. Psychological Review. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Poblano A, de Caballero BC, Castillo I, Cortes V. Electro-oculographic recordings reveal reading deficiencies in learning disabled children. Archives of Medical Research. 1996;27:509–512. [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. The Journal of Neuroscience. 1984;4:1863–1874. doi: 10.1523/JNEUROSCI.04-07-01863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus F, Ahissar M. Developmental dyslexia: The difficulties of interpreting poor performance, and the importance of normal performance. Cognitive Neuropsychology. 2012;29(1–2):104–122. doi: 10.1080/02643294.2012.677420. [DOI] [PubMed] [Google Scholar]
- Ramus F, Rosen S, Dakin SC, Day BL, Castellote JM, White S, Frith U. Theories of developmental dyslexia: insights from a multiple case study of dyslexic adults. Brain. 2003;126:841–865. doi: 10.1093/brain/awg076. [DOI] [PubMed] [Google Scholar]
- Rayner K, Inhoff AW, Morrison RE, Slowiaczek ML, Bertera JH. Masking of foveal and parafoveal vision during eye fixations in reading. Journal of Experimental Psychology: Human Perception and Performance. 1981;7:167–179. doi: 10.1037//0096-1523.7.1.167. [DOI] [PubMed] [Google Scholar]
- Reicher GM. Perceptual recognition as a function of meaningfulness of stimulus material. Journal of Experimental Psychology. 1969;81(2):275–280. doi: 10.1037/h0027768. [DOI] [PubMed] [Google Scholar]
- Ridder WH, Borsting E, Banton T. All developmental dyslexic subtypes display an elevated motion coherence threshold. Optometry and Vision Science. 2001;78(7):510–517. doi: 10.1097/00006324-200107000-00014. [DOI] [PubMed] [Google Scholar]
- Riddoch J, Humphreys G, Cleton P, Fery P. Interaction of attentional and lexical processes in neglect dyslexia. Cognitive Neuropsychology. 1990;7:479–517. [Google Scholar]
- Rubino CA, Minden HA. An analysis of eye-movements in children with a reading disability. Cortex. 1990;7:479–517. doi: 10.1016/s0010-9452(73)80030-9. [DOI] [PubMed] [Google Scholar]
- Rumelhart DE, McClelland JL. An interactive activation model of context effects in letter perception: Part 2. The contextual enhancement effects and some tests and enhancements of the model. Psychological Review. 1982;89:60–94. [PubMed] [Google Scholar]
- Schiller PH, Logothetis NK, Charles EH. Function of the colour-opponent and broad-band channels of the visual system. Nature. 1990;343(6253):68–70. doi: 10.1038/343068a0. [DOI] [PubMed] [Google Scholar]
- Schlaggar BL, McCandliss BD. Development of neural systems for reading. Annual Review of Neuroscience. 2007;30:475–503. doi: 10.1146/annurev.neuro.28.061604.135645. [DOI] [PubMed] [Google Scholar]
- Schulte-Körne G, Bartling J, Deimel W, Remschmidt H. Motion-onset VEPs in dyslexia. Evidence for visual perceptual deficit. Neuroreport. 2004;15(6):1075–1078. doi: 10.1097/00001756-200404290-00029. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychological Review. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Selfridge OG. In: Blake DV, Uttley AM, editors. Pandemonium: A paradigm for learning; Proceedings of the Symposium on Mechanisation of Thought Processes; London. 1959. pp. 511–529. [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annual Review of Psychology. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Shapley R, Perry VH. Cat and monkey retinal ganglion-cells and their visual functional roles. Trends in Neurosciences. 1986;9(5):229–235. [Google Scholar]
- Skottun BC. On the conflicting support for the magnocellular-deficit theory of dyslexia - Response to Stein, Talcott and Walsh (2000) Trends in Cognitive Sciences. 2000;4(6):211–212. doi: 10.1016/s1364-6613(00)01485-6. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL, Ryan JF. Spatio-temporal contrast sensitivity, coherent motion, and visible persistence in developmental dyslexia. Vision Research. 1999;39(3):651–668. doi: 10.1016/s0042-6989(98)00151-5. [DOI] [PubMed] [Google Scholar]
- Solan HA, Hansen PC, Shelley-Tremblay J, Ficarra A. Coherent motion threshold measurements for M-cell deficit differ for above- and below-average readers. Optometry. 2003;74(11):727–734. [PubMed] [Google Scholar]
- Solomon RL, Postman L. Frequency of usage as a determinant of recognition thresholds for words. Journal of Experimental Psychology. 1952;43:195–201. doi: 10.1037/h0054636. [DOI] [PubMed] [Google Scholar]
- Stein J. The magnocellular theory of developmental dyslexia. Dyslexia. 2001;7:12–36. doi: 10.1002/dys.186. [DOI] [PubMed] [Google Scholar]
- Stein JF, Fowler MS. Unstable binocular control in dyslexic children. Journal of Research in Reading. 1993;16:30–45. [Google Scholar]
- Stein JF, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends in Neurosciences. 1997;20:147–151. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Szwed M, Ventura P, Querido L, Cohen L, Dehaene S. Reading acquisition enhances early visual processes of contour integration. Developmental Science. 2012;1:139–149. doi: 10.1111/j.1467-7687.2011.01102.x. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Gram A, Van Ingelghem M, Witton C, Stein JF, Toennessen FE. Impaired sensitivity to dynamic stimuli in poor readers of a regular orthography. Brain and Language. 2003;87(2):259–266. doi: 10.1016/s0093-934x(03)00105-6. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Hansen PC, Assoku EL, Stein JF. Visual motion sensitivity in dyslexia: evidence for temporal and energy integration deficits. Neuropsychologia. 2000;38(7):935–943. doi: 10.1016/s0028-3932(00)00020-8. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Hansen PC, Willis-Owen C, McKinnell IW, Richardson AJ, Stein JF. Visual magnocellular impairment in adult developmental dyslexics. Neuro-Ophthalmology. 1998;20(4):187–201. [Google Scholar]
- Talcott JB, Witton C, Hebb GS, Stoodley CJ, Westwood EA, France SJ, Stein JF. On the relationship between dynamic visual and auditory processing and literacy skills; results from a large primary-school study. Dyslexia. 2002;8(4):204–225. doi: 10.1002/dys.224. [DOI] [PubMed] [Google Scholar]
- Talcott JB, Witton C, McLean MF, Hansen PC, Rees A, Green GGR, Stein JF. Dynamic sensory sensitivity and children’s word decoding skills. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(6):2952–2957. doi: 10.1073/pnas.040546597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RBH, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Belliveau JW. Functional-analysis of human MT and related visual cortical areas using magnetic resonance imaging. Journal of Neuroscience. 1995;15(4):3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA, Rose E, Lindamood P, Conway T, Garvan C. Preventing reading failure in young children with phonological processing disabilities: Group and individual responses to instruction. Journal of Educational Psychology. 1999;91(4):579–593. [Google Scholar]
- Vaina LM, Lemay M, Bienfang DC, Choi AY, Nakayama K. Intact “biological motion” and “structure from motion” perception in a patient with impaired motion mechanisms: A case study. Visual Neuroscience. 1990;5:353–369. doi: 10.1017/s0952523800000444. [DOI] [PubMed] [Google Scholar]
- Vidyasagar TR, Pammer K. Dyslexia: a deficit in visuo-spatial attention, not in phonological processing. Trends in Cognitive Sciences. 2010;14(2):57–63. doi: 10.1016/j.tics.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. Quest - A Bayesian adaptive psychometric method. Perception and Psychophysics. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. New York: The Psychological Corporation: Harcourt Brace & Company; 1999. [Google Scholar]
- Wheeler DD. Processes in word recognition. Cognitive Psychology. 1970;1(1):59–85. [Google Scholar]
- Whitney C. How the brain encodes the order of letters in a printed word: The SERIOL model and selective literature review. Psychonomic Bulletin and Review. 2001:221–243. doi: 10.3758/bf03196158. [DOI] [PubMed] [Google Scholar]
- Whitney C, Cornelissen P. Letter-position encoding and dyslexia. Journal of Research in Reading. 2005;28:274–301. [Google Scholar]
- Williams MJ, Stuart GW, Castles A, McAnally KI. Contrast sensitivity in subgroups of developmental dyslexia. Vision Research. 2003;43(4):467–477. doi: 10.1016/s0042-6989(02)00573-4. [DOI] [PubMed] [Google Scholar]
- Wilmer JB, Richardson AJ, Chen Y, Stein JF. Two visual motion processing deficits in developmental dyslexia associated with different reading skills deficits. Journal of Cognitive Neuroscience. 2004;16(4):528–540. doi: 10.1162/089892904323057272. [DOI] [PubMed] [Google Scholar]
- Witton C, Talcott JB, Hansen PC, Richardson AJ, Griffiths TD, Rees A, Green GGR. Sensitivity to dynamic auditory and visual stimuli predicts nonword reading ability in both dyslexic and normal readers. Current Biology. 1998;8(14):791–797. doi: 10.1016/s0960-9822(98)70320-3. [DOI] [PubMed] [Google Scholar]