Abstract
Nitrogenase cofactors can be extracted into an organic solvent and added in an adenosine triphosphate (ATP)-free, organic solvent-based reaction medium to catalyze the reduction of cyanide (CN−), carbon monoxide (CO) and carbon dioxide (CO2) when samarium (II) iodide (SmI2) and 2,6-lutidinium triflate (Lut-H) are supplied as a reductant and a proton source, respectively. Driven by SmI2, the cofactors not only catalytically reduce CN− or CO to C1-C4 hydrocarbons, but also catalytically reduce CO2 to CO and C1-C3 hydrocarbons. The observation of C-C coupling from CO2 reveals a unique, Fischer-Tropsch-like reaction with an atypical carbonaceous substrate; whereas the achievement of catalytic turnover of CN−, CO and CO2 by isolated cofactors suggests the possibility to develop nitrogenase-based electrocatalysts for hydrocarbon production from these carbon-containing compounds.
Keywords: nitrogenase, carbon dioxide, carbon monoxide, C-C coupling, hydrocarbon
Nitrogenase is a uniquely versatile metalloenzyme that catalyzes the reduction of various substrates, such as nitrogen (N2), carbon monoxide (CO) and cyanide (CN−), at its cofactor site.[1–4] The molybdenum (Mo)- and vanadium (V)-nitrogenases are two homologous members of this enzyme family, which contain homologous cofactors—the molybdenum-iron cofactor (designated the M-cluster) and the vanadium-iron cofactor (designated the V-cluster)—at their respective active sites.[1,5] The M-cluster (Fig. S1A) is a [MoFe7S9C] cluster that can be viewed as [Fe4S3] and [MoFe3S3] subclusters bridged by three equatorial μ2-sulfides and one interstitial μ6-carbide. In addition, this cofactor has an endogenous compound, homocitrate, attached to its Mo end.[6–8] The V-cluster (Fig. S1B) is nearly identical to the M-cluster in structure except for the substitution of V for Mo and a slight elongation of the metal-sulfur core of this cluster.[9,10] Apart from the two cofactors, a third cluster species has been identified both as a biosynthetic intermediate and as a structural homolog of the M-cluster. Designated the L-cluster (Fig. S1C), this [Fe8S9C] cluster represents an all-iron version of the cofactor, as it closely resembles the core structure of the mature M-cluster except for the substitution of Fe for Mo and homocitrate at one end.[11–13] The structural homology between the L-cluster and the two cofactors is striking; more importantly, it suggests a close resemblance of these clusters to one another in their catalytic capacities.
Such a resemblance indeed exists between the M- and V-clusters, as both cofactors can be extracted from protein into an organic solvent, N-methylformamide (NMF),[10] and directly used as a catalyst to reduce CN− or CO to hydrocarbons in the presence of a strong reductant, europium (II) diethylenetriamine-pentaacetate (EuII-DTPA).[14] Driven by EuII-DTPA (E0′= −1.14 V at pH 8), both cofactors generate alkanes and alkenes of varying lengths as products of CN− or CO reduction at comparable efficiencies. Additionally, they both display a strong preference of CN− over CO as a substrate, which may originate from a stabilizing effect of CN− on certain oxidation states of the two cofactors.[14] However, EuII-DTPA is not a strong enough reductant to drive the catalytic turnover of CO by either cofactor, as the turnover numbers (TON) of CO by both cofactors are less than 1.[15] Moreover, this reductant does not support the reduction of CO2 by the cofactors, an event that requires more reducing power than the reduction of CN− or CO.[16] This observation prompts the questions of (i) whether CO and CO2 can be catalytically turned over by these clusters in the presence of an appropriate reductant; and (ii) if the L-cluster resembles the M- and V-clusters in the conversion of carbon-containing compounds to hydrocarbons.
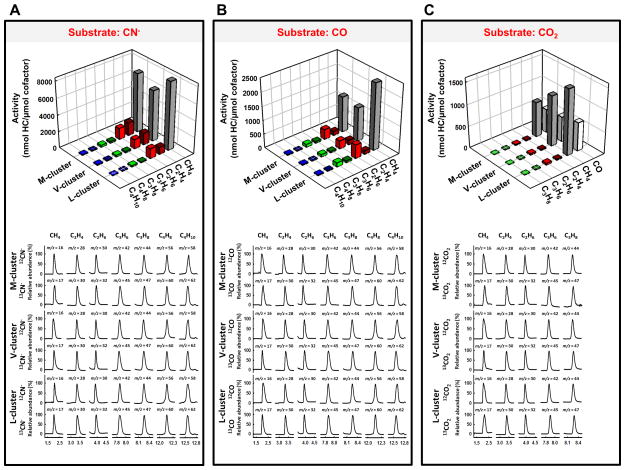
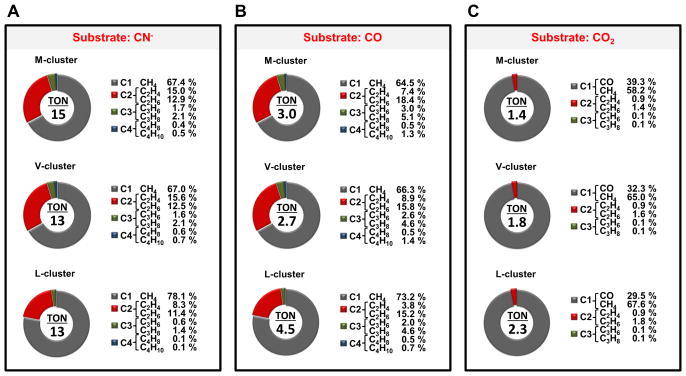
The answers to both questions are yes. When EuII-DTPA is replaced by a stronger reductant, samarium (II) iodide (SmI2),[17] the NMF-extracted M-, V- and L-clusters are all capable of turning over CN−, CO and CO2 under ambient conditions in an organic solvent-based reaction medium. Driven by SmI2 [E0′= −1.55 V in tetrahydrofuran (THF)] and using protons supplied by 2,6-lutidinium triflate (Lut-H),[18] the three clusters not only can reduce CN− (Fig. 1A, upper; Table S1) and CO (Fig. 1B, upper; Table S1) to CH4, C2H4, C2H6, C3H6, C3H8, 1-C4H8 and n-C4H10, but also can reduce CO2 to CO, CH4, C2H4, C2H6, C3H6 and C3H8 (Fig. 1C, upper; Table S1). Gas chromatograph-mass spectrometry (GC-MS) analysis confirms CN−, CO and CO2 as the carbon sources for the hydrocarbons generated in these reactions, as all products display the expected mass shifts upon substitution of 13CN−, 13CO and 13CO2, respectively, for 12CN− (Fig. 1A, lower), 12CO (Fig. 1B, lower) and 12CO2 (Fig. 1C, lower). Activity analysis further reveals that all three clusters turn over CN−, CO and CO2 catalytically (i.e., TON>1) in the presence of SmI2, with the M-, V- and L-clusters showing TONs of 15, 13 and 13, respectively, for CN− (Fig. 2A); 3.0, 2.7 and 4.5, respectively, for CO (Fig. 2B); and 1.4, 1.8 and 2.3, respectively, for CO2 (Fig. 2C). While the preference of CN− as a substrate is preserved by all three clusters in reactions driven by SmI2, the observation of the catalytic turnover of CO and CO2 by these clusters in the presence of this reductant is particularly exciting, as it not only illustrates the impact of redox potential on the catalytic efficiency and substrate range of nitrogenase cofactors, but also defines a previously-unobserved, ATP-independent reaction that involves the conversion of CO2 to hydrocarbons by these unique metal clusters in the isolated forms.
Figure 1.
Reduction of CN−, CO and CO2 by nitrogenase cofactors. Shown are the activity (upper) and GC-MS (lower) analyses of hydrocarbon (HC) formation in the reactions of (A) CN−, (B) CO and (C) CO2 reduction by M-, V- and L-clusters.
Figure 2.
Product profiles of nitrogenase cofactors. Shown are the percentages of C1, C2, C3 and C4 products formed in the reactions of (A) CN−, (B) CO and (C) CO2 reduction by M-, V- and L-clusters. TON, or turnover number, was calculated based on the nmole of carbon that appeared in the hydrocarbon products per nmole of isolated cluster used in the reaction.
It should be noted that the ATP-dependent reduction of CO2 was reported both for a variant of Mo-nitrogenase and for the wild-type V-nitrogenase;[19–21] however, CH4 was detected as the sole hydrocarbon product in the case of the former,[20] whereas ≤C2 alkanes and alkenes were detected only upon substitution of D2O for H2O in the case of the latter.[21] In comparison, the isolated cofactors are “pushed” by SmI2 not only toward the formation of C-C bond (i.e., >C1 products), but also toward the formation of longer carbon chains (i.e., up to C3 products) from CO2 (Fig. 1C). The C2 and C3 hydrocarbons do not originate from the coupling between the CO2-derived CO in the SmI2-driven reactions, as these products cannot be detected if CO is supplied directly as a substrate at the same concentration as the maximum amount of CO generated from CO2 reduction (Fig. S2). Further, the reduction of CO2 to CO and hydrocarbons is carried out by protons (H+) and electrons in these reactions, and it is accompanied by the reduction of H+ to hydrogen (H2) (Table S1).
Interestingly, the activities of the three clusters seem to be “normalized” upon isolation from their respective protein environments. In addition to turning over each substrate with comparable TONs, these clusters also generate the same range of products at similar percentages from the same substrate. All of them display a strong tendency toward the formation of ≤C2 products from CN− (Fig. 2A) and CO (Fig. 2B), with the C1 (CH4) and C2 (C2H4, C2H6) products comprising a major portion (90.3–97.8%) of the product profiles of these reactions. The tendency toward formation of small products is even more apparent in the cases of CO2 reduction by these clusters, where the C1 products (CO, CH4) constitute the predominant portion (97.1–97.5%) of the product profiles (Fig. 2C). In all these reactions, CH4 is the singularly dominant hydrocarbon product, which consists of 58.2–78.1% of the total amount of products. Such a strong shift toward CH4 is not observed in the reaction of CO reduction by the protein-bound M- or L-cluster,[2,3] where C2H4 is produced as the major product along with a more evenly distributed product profile toward longer hydrocarbons. Moreover, the “normalization” of the isolated M- or L-cluster in the reaction efficiency and product distribution of CO reduction contrasts the approximately 700-fold activity difference and a significant disparity in product formation between their protein-bound counterparts,[3] highlighting the impact of protein environment on the reactivities of nitrogenase cofactors.
Apart from the protein environment, variations of the cofactor composition, particularly those at the “heterometal end”, seem to play a role in modulating the catalytic properties of these clusters. A good example in this regard is the higher TONs of CO (Fig. 2B) and CO2 (Fig. 2C) by the L-cluster, an all-iron form of the cofactor, than those by the M- and V-clusters. Moreover, among the three clusters, the L-cluster forms the highest percentage of CH4 from the reduction of all three substrates and, in the reactions of CN− (Fig. 2A) and CO (Fig. 2B) reduction, the increased formation of CH4 by L-cluster is accompanied by a decreased formation of C2H4, consistent with a preference of this cluster to reduce CN− and CO all the way to CH4 over the C-C coupling of these substrates into C2H4. Strikingly, an analogous reaction was shown to be enabled by iron sulfide (FeS), a simplest FeS unit; only in this case, methanethiol (CH3SH) was generated as a product of CO2 reduction in the presence of FeS and hydrochloric acid (HCl).[22] The increased formation of CH4 by L-cluster is not only interesting because of the value of CH4 as a fuel source, but also important because of the all-iron composition of the L-cluster (see Fig. S1), which may simplify the task of synthesizing biomimetic nitrogenase “cofactors” by omitting the need to incorporate heterometal and homocitrate.
Together with the M- and V-clusters, the L-cluster forms a group of homologous, high-nuclearity metal-sulfur clusters that are capable of catalyzing the unique conversion of CN−, CO and CO2 to hydrocarbon products. The success in achieving the catalytic turnover of CO and CO2 by these clusters in the presence of a stronger reductant, SmI2, suggests the possibility to develop nitrogenase-based electrocatalysts for further improvement of catalytic efficiency and substrate range; whereas the observation of the differences between the activities of the protein-bound and NMF-extracted clusters, as well as the differences between the activities of the isolated clusters, implies the potential to alter the product profiles of these reactions by varying the compositions of the clusters and attaching the clusters to artificial matrices for further modulation of their catalytic properties. Perhaps most excitingly, these studies have led to the identification of a room-temperature, Fisher-Tropsch (F-T) type reaction with an atypical F-T substrate, CO2.[23] The formation of CO in this reaction is likely analogous to the reaction of reverse water-gas shift (i.e., CO2 + H2 → CO + H2O).[24] Only in this case, the expensive syngas, H2, is replaced by H+ (provided by LutH) and e− (supplied by SmI2), and it is further produced as an abundant side product of H+ reduction (see Table S1). The formation of hydrocarbons also utilizes H+ as a hydrogen source, and this reaction likely involves direct C-C coupling from CO2 or CO2-derived intermediate(s) other than CO (see Fig. S2). As such, the reduction of CO2 to CO and hydrocarbons by M-, V- and L-clusters not only defines two unique reactions that are related to two important industrial processes, but also bears potential to serve as a blueprint for future design of strategies to recycle CO2 into the useful carbon fuels.
Supplementary Material
Footnotes
This work was supported by NIH grant GM-67626 (M.W.R.)
Supporting information for this article including experimental procedures, Table S1 and Figures S1–S2 is given via a link at the end of the document.
Contributor Information
Dr. Chi Chung Lee, Department of Molecular Biology and Biochemistry
Prof. Dr. Yilin Hu, Email: yilinh@uci.edu, Department of Molecular Biology and Biochemistry
Prof. Dr. Markus W. Ribbe, Email: mribbe@uci.edu, Department of Molecular Biology and Biochemistry; Department of Chemistry, University of California, Irvine, Irvine, CA 92697-3900
References
- 1.Burgess BK, Lowe DJ. Chem Rev. 1996;96:2983–3012. doi: 10.1021/cr950055x. [DOI] [PubMed] [Google Scholar]
- 2.Lee CC, Hu Y, Ribbe MW. Science. 2010;329:642. doi: 10.1126/science.1191455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu Y, Lee CC, Ribbe MW. Science. 2011;333:753–755. doi: 10.1126/science.1206883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZY, Dean DR, Seefeldt LC. J Biol Chem. 2011;286:19417–19421. doi: 10.1074/jbc.M111.229344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eady RR. Chem Rev. 1996;96:3013–3030. doi: 10.1021/cr950057h. [DOI] [PubMed] [Google Scholar]
- 6.Spatzal T, Aksoyoglu M, Zhang L, Andrade SL, Schleicher E, Weber S, Rees DC, Einsle O. Science. 2011;334:940. doi: 10.1126/science.1214025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancaster KM, Roemelt M, Ettenhuber P, Hu Y, Ribbe MW, Neese F, Bergmann U, DeBeer S. Science. 2011;334:974–977. doi: 10.1126/science.1206445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiig JA, Hu Y, Lee CC, Ribbe MW. Science. 2012;337:1672–1675. doi: 10.1126/science.1224603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CC, Hu Y, Ribbe MW. Proc Natl Acad Sci U S A. 2009;106:9209–9214. doi: 10.1073/pnas.0904408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fay AW, Blank MA, Lee CC, Hu Y, Hodgson KO, Hedman B, Ribbe MW. J Am Chem Soc. 2010;132:12612–12618. doi: 10.1021/ja1019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Fay AW, Ribbe MW. Proc Natl Acad Sci U S A. 2005;102:3236–3241. doi: 10.1073/pnas.0409201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett MC, Hu Y, Fay AW, Ribbe MW, Hedman B, Hodgson KO. Proc Natl Acad Sci U S A. 2006;103:1238–1243. doi: 10.1073/pnas.0507853103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fay AW, et al. Angew Chem Int Ed Engl. 2001;50:7787–7790. [Google Scholar]
- 14.Lee CC, Hu Y, Ribbe MW. Angew Chem Int Ed Engl. 2012;51:1947–1949. doi: 10.1002/anie.201108916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The turnover number, or TON, reflects the total number of carbons that appear in the various carbon-containing products.
- 16.Shi C, Hansen HA, Lausche AC, Nørskov JK. Phys Chem Chem Phys. 2014;16:4720–4727. doi: 10.1039/c3cp54822h. [DOI] [PubMed] [Google Scholar]
- 17.Evans WJ. Coord Chem Rev. 2000;206:263–283. [Google Scholar]
- 18.Schrock RR. Nat Chem. 2011;3:95–96. doi: 10.1038/nchem.977. [DOI] [PubMed] [Google Scholar]
- 19.The ATP-dependent reaction requires the presence of both component proteins of nitrogenase to allow ATP-dependent electron transfer from Component 2 (the reductase component) to the cofactor site of Component 1 (the catalytic component) for substrate reduction.
- 20.Yang ZY, Moure VR, Dean DR, Seefeldt LC. Proc Natl Acad Sci USA. 2012;109:19644–19648. doi: 10.1073/pnas.1213159109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rebelein JG, Hu Y, Ribbe MW. Angew Chem Int Ed Engl. 2014;53:11543–11546. doi: 10.1002/anie.201406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinen W, Lauwers AM. Orig Life Evol Biosph. 1996;26:131–150. doi: 10.1007/BF01809852. [DOI] [PubMed] [Google Scholar]
- 23.A typical Fischer-Tropsch (F-T) reaction converts a mixture of CO and H2 into liquid hydrocarbons.
- 24.Chen CS, Cheng WH, Lin SS. Catal Letters. 2000;68:45–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.