Abstract
Retroperitoneal lymph node dissection (RPLND) is a prognostic, palliative, and potentially therapeutic procedure for patients with malignant phenotype Leydig cell tumours of the testis. We reviewed the records of patients diagnosed with malignant phenotype Leydig cell tumours of the testis treated by RPLND. Modified template dissection was performed in all cases with extra-template excision of tumour mass in Stage II disease. Routine clinico-radiological follow-up was performed. Six open RPLNDs (1 re-do procedure) were performed on 5 patients diagnosed with Stage I (n = 3) and Stage II (n = 2) malignant phenotype Leydig cell tumour of the testis. Median age = 63 years (range = 55-72). Median peri-operative blood loss = 1500 ml (range = 500-1500 ml). Median operating time = 6 h (range = 4.5-6.5). Two patients with Stage II disease developed post-operative complications of acute kidney injury (n = 1) and pneumonia (n = 1). Median length of stay was 8 days (range = 6-11). RPLND specimens from patients with Stage I were tumour-free, whilst patients with Stage II disease had evidence of metastatic tumour. At latest follow-up (median = 13 months, range = 7-22), no patient with Stage I disease had radiological evidence of recurrence, however the two patients with Stage II disease had died due to tumour recurrence at 13 months and 36 months. RPLND for malignant phenotype Leydig cell testicular tumours appears to be well tolerated. Despite surgery, overall outcomes for Stage II appear to be poor due to the disease phenotype. Larger prospective multi-centre studies are required to determine the definitive criteria for surgery in Stage I disease.
Electronic supplementary material
The online version of this article (doi:10.1186/s40064-014-0781-x) contains supplementary material, which is available to authorized users.
Keywords: Testicular cancer, Leydig cell tumour, Retroperitoneal lymph node dissection (RPLND)
Background
Testicular cancer is a relatively rare malignancy with ~2200 new cases diagnosed each year in the UK (Testicular cancer incidence statistics [2014]). In Scotland, ~200 new cases are diagnosed each year with a 93.4% overall crude 5-year survival rate (Summarised cancer mortality, male genital organs [2014]), largely as a result of contemporary chemotherapy strategies. Germ cell tumours represent the commonest histological subtype, and rarer variants include sex cord/gonadal stromal tumours and non-specific stromal tumours with both benign and malignant disease phenotypes (Albers et al. [2011]).
Leydig cell tumours are gonadal stromal tumours that represent less than 5% of adult testicular tumours, however are the most common non-germ cell testicular tumour (Cheville [1999]). Whilst the majority have benign clinical course, and can be fully treated by local excision or radical inguinal orchidectomy, arguably 10% are recognized to have a malignant phenotype with metastatic potential based upon the presence of specific histopathological features (Albers et al. [2011]) (Table 1). Similar to germ cell tumours, the route of spread is haematogenous and lymphatic to the retroperitoneal lymph nodes, but unlike germ cell tumours there is relative lack of sensitivity to radiotherapy and chemotherapy agents (Farkas et al. [2000]).
Table 1.
Histopathological features suggesting a malignant phenotype of a testicular Leydig cell tumour (Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, European Association of U [2011])
| Number | Features |
|---|---|
| I. | Large Tumour size (>5 cm) |
| II. | Cytological atypia |
| III. | Increased mitotic activity (>3 per 10 high power field [HPF]) |
| IV. | Increased MIB-1 expression |
| V. | Necrosis |
| VI. | Vascular invasion |
| VII. | Infiltrative margins |
| VIII. | Extension beyond the testicular parenchyma |
| IX. | DNA aneuploidy |
Retroperitoneal lymph node dissection (RPLND) can be performed subsequent to orchidectomy in patients with both organ-confined and metastatic disease, and serves as a prognostic, palliative, and potentially therapeutic procedure. The outcomes of RPLND for malignant Leydig cell tumours of the testis have been limited to a few case series (Silberstein et al. [2014]; Di Tonno et al. [2009]; Mosharafa et al. [2003]; Peschel et al. [2003]). Here, we present our national Scottish experience of RPLND for malignant phenotype Leydig cell tumours of the testis.
Patients and methods
Between 2004 and 2014, all males within Scotland diagnosed with malignant phenotype Leydig cell tumours of the testis deemed suitable for surgery were referred to our specialist centre for consideration of open RPLND performed by a single surgeon. Diagnosis, determination of disease phenotype, and suitability for major surgery was made in local referring centres by histopathological assessment of the primary orchidectomy specimen as per European and UK guidelines (Albers et al. [2011]; Standards and datasets for reporting cancers. Dataset for the histological reporting of testicular neoplasm [2014]) prior to referral to our centre. All patients were reviewed clinically to verify suitability for surgery and primary orchidectomy specimens were subjected to centralised histopathology review to confirm histological features. Clinico-pathological parameters were recorded centrally including patient demographics, symptoms, tumour markers (α-fetoprotein protein [α-FP], β-human chorionic gonadotrophin [β-hCG], lactate dehydrogenase [LDH]), histopathology, and radiological staging by computed tomography (CT) and, where available, positron emission tomography CT (PET-CT). Clinical stage, as per TNM created by the American Joint Committee on Cancer (AJCC) was recorded based on preoperative clinical staging (Testis [2010]). Following case review by the specialist testis multi-disciplinary team, patients were offered RPLND. Pre-operative sperm banking was offered in local referring centres as per National Guidelines (SIGN [2011]). For all patients, open RPLND with modified unilateral template dissection was performed, as previously described by the Indiana group (Donohue et al. [1982]), which involves removal of lymphatic tissue from the right paracaval, precaval, and interaortocaval areas for right-sided tumours and left para-aortic and pre-aortic areas for left-sided tumours. Additional extra-template excision of tumour mass in Stage II disease was also performed where indicated by pre-operative imaging. Where possible, nerve-sparing procedures was performed to preserve ejaculatory function. Subsequent follow-up was undertaken in local referring centres and comprised CT imaging, serum tumor markers and clinical examination on an initial 3–6 monthly schedule.
Results
Between 2004 and 2014, we performed 6 RPLNDs on 5 patients (including 1 re-do procedure) diagnosed with Stage I (n = 3) and Stage II (n = 2) malignant phenotype Leydig cell tumour of the testis. The median age at diagnosis was 63 years (range = 55-72), and there were 4 right-sided and 2 left-sided modified template RPLNDs performed. Clinico-pathological characteristics of the patient cohort are given in Table 2. Pre-operative testicular tumour markers were found to be within normal limits in all cases. No patient had any endocrine symptoms, therefore pre-operative biochemical assessment was not performed. Of the patients with Stage II disease (n = 2), only 1 patient had pre-operative chemotherapy (4 cycles of Bleomycin, Etoposide and Cisplatin), but had residual retroperitoneal disease on post-treatment radiological imaging signifying a poor treatment response. No patient with Stage I disease was subjected to pre-operative chemotherapy.
Table 2.
Peri-operative patient characteristics
| Case | Age [Side] | Path. features from Table1(Total) | Tumour markers | Pre-op. stage | Blood loss (ml) | Op. time (hours) | Complications | LOS (days) | Pathology at RPLND | Recurrence | DFS (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | (63) Right | I, II, III, VI, VIII (5) | aFP 2bHCG<2 LDH 183 | IIA (Ipsilateral Paraortic nodal uptake) | 1500 | 6 | Nil | 11 | LCT (180x65x25mm) Involvement of right ureter-> nephrectomy | Yes | N/A (died at 13 months) |
| 2 | (55) Left | II, III, V (3) | aFP 3bHCG<2 LDH 210 | I | 500 | 6 | Nil | 8 | Benign | No | 7 months |
| 3 | (72) Left | I, II, V, VI (4) | aFP 4bHCG<3 LDH 214 | IIB (Ipsilateral Paraortic nodes, up to 5cm) | 1500 | 6.5 | AKI, Dialysed | 8 | LCT (120x85x40mm) | Yes-16 months (required further resection) | 16 months (died at 36 months) |
| 4 | (67) Right | I, III (2) | aFP 6bHCG<3 LDH 186 | I | 1500 | 5.5 | No | 6 | Benign | No | 23 months |
| 5 | (61) Right | II (1) | aFP 3bHCG<3LDH 222 | I | 800 | 4.5 | Pneumonia | 9 | Benign | No | 16 months |
Path = histopathological; α-FP = α-fetoprotein protein; β-hCG = β-human chorionic gonadotrophin; LDH = lactate dehydrogenase; op = operative; LOS = length of stay; LCT = leydig cell tumour; DFS = disease-free survival.
Although pre-operative ejaculatory function data was not available, a standard modified unilateral template dissection was performed for all cases of Stage I disease with attempted nerve-sparing to preserve post-ganglionic sympathetic fibres for ejaculatory function. For Stage II, an extended dissection was performed to completely resect the tumour mass as indicated by pre-operative imaging. Peri-operative details are given in Table 2, however there was no major peri-operative vascular or visceral injury. Median peri-operative blood loss was 1500 ml (range = 500-1500 ml) and median operating time 6 hours (range = 4.5-6.5).
One patient with Stage II disease (Case 1) had extensive involvement of the right ureter in tumour mass and therefore nephrectomy was performed. One patient with Stage II disease developed post-operative complications of acute kidney injury (Case 3) and one patient with Stage I disease developed pneumonia (Case 5). The median length of stay was 8 days (range = 6-11). On histopathological assessment, resected specimens from patients with Stage I were tumour-free, whilst patients with Stage II disease had evidence of metastatic tumour in the RPLND specimens. Unfortunately, post-operative ejaculatory function was not available for all patients.
At latest follow-up (median = 13 months, range = 7-22), no patient with Stage I disease had radiological evidence of recurrence, however patients with Stage II disease had died due to tumour recurrence at 13 months and 36 months. One of the patients with Stage II disease (Case 3) developed an early left-sided pelvic recurrence adjacent to the mesentery of this sigmoid colon and ureter, and required re-resection with ureteric reconstruction with a Boari flap at 16 months post primary surgery. Figures 1 and 2 demonstrates this patient’s initial CT scan, as well as histopathology of the primary testicular tumour and resected metastases from the initial RPLND.
Figure 1.
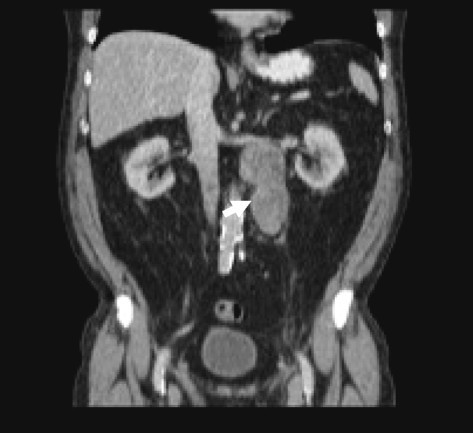
CT scan demonstrating retro-peritoneal tumour. Abdominal CT scan from Case 3 highlighting retroperitoneal mass on ipsilateral side to original primary tumour. RPLND yielded a 120x85x40mm mass which was histologically confirmed as metastatic Leydig cell tumour.
Figure 2.
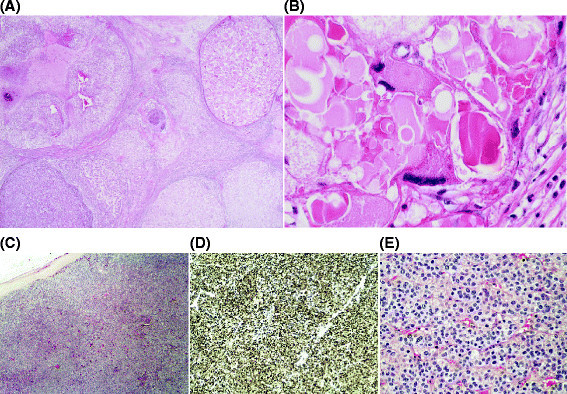
Histopathology slides of primary and metastatic Leydig cell tumour. Sample obtained from Case 2. (A) Haematoxylin and Eosin (H&E) image demonstrating tumour in orchidectomy specimen (×1.25 magnification). (B) H&E image demonstrating severe cytological atypia in testicular primary (×20 magnification. (C) H&E image demonstrating tumour in mass from RPLND (×200 magnification). (D) Immunohistochemical staining of RPLND specimen demonstrating expression of Inhibin (×40 magnification). (E) H&E image demonstrating tumour RPLND specimen (×20 magnification).
Discussion
Malignant phenotype Leydig cell tumours typically present in older men with a reported median age of 62.1 (range 39–70) years (Gonzalez et al. [2007]), which is similar to our cohort. The normal endocrine function of the Leydig cell may result in elevated serum and urine sex hormone levels during tumourigenesis however, the clinical symptoms related to hormone excess are less common. Signs and symptoms of hormone excess may precede identification of a testicular mass, and in younger patients precocious puberty may be the initial symptom, with gynaecomastia being a clinical sign in older patients (Gana et al. [1995]; Ozyavuz et al. [1993]).
Leydig cell tumours are historically quoted as having 10% risk of malignant phenotype with metastatic potential (Cheville et al. [1998]; Kim et al. [1985]). An increase in the number of incidental focal testicular lesions, identified by wider use of scrotal ultrasonography, of which the majority are benign (Carmignani et al. [2003]), has led to the suggestion that metastatic rates of Leydig cell tumours may be less than traditionally thought. Hence, organ-sparing surgery by local excision in young patients with low risk disease is a potential therapeutic option (Heer et al. [2010]; Loeser et al. [2009]). However, the confident pre-operative prediction of a benign phenotype is challenging based upon current diagnostic modalities.
Given the tumour’s overall rarity and low malignant potential, and refractory response to radiotherapy and chemotherapy, there is still controversy over optimal treatment. One potential reason for this is the lack of unequivocal histopathological criteria for a malignant phenotype. Although the only universally-recognized hallmark of a malignant phenotype is the presence of metastasis, there are 9 overall features of the primary tumour thought to be indicative (Table 1), although not all of these needs to be present in tumours that metastasize (Grem et al. [1986]). In our series, all patients with histopathologically-confirmed Stage II disease had only 4 or 5 of these criteria. However, in a series (n = 29) of young (median = 43 years) patients who underwent primary surgery alone, only five patients expressed any criteria, of which only two expressed two features (Loeser et al. [2009]). Furthermore, no metastases were observed over a median 49-month follow-up period.
The development of metastases typically occurs within 2 years of the initial diagnosis, although has been reported up to 8 years after orchidectomy (Bertram et al. [1991]). Metastases have similar histological features to the original tumour (spreading to retroperitoneal lymph nodes (70%), liver (45%), lung (40%) and bone (25%), and are particularly chemo-radio-resistant, with radiation only effective in some cases to palliate pain symptoms (Bertram et al. [1991]). Hence RPLND may have prognostic, palliative, and potentially therapeutic application in at risk patients with Stage I, as well as Stage II disease.
Studies reporting the outcome of RPLND for malignant phenotype Leydig cell tumours are limited (Silberstein et al. [2014]; Di Tonno et al. [2009]; Mosharafa et al. [2003]; Peschel et al. [2003]). Mosharfa et al. report outcomes of RPLND (n = 17) for sex cord stromal tumours, including malignant phenotype Leydig cell tumours (n = 6) (Mosharafa et al. [2003]). Three cases had Stage I disease had were disease-free at latest follow-up (range = 25-135 months), and three cases had Stage II disease at RPLND, and subsequently died (at range = 11-52 months) despite further treatment (surgery, chemo- or radio-therapy). The latter patients had initially presented with Stage I disease and were managed by surveillance, highlighting the potential benefit of early RPLND.
Di Tonno et al. report outcomes of RPLND (n = 5) undertaken for Stage I (n = 3) and Stage II (n = 2) malignant phenotype Leydig cell tumours (Di Tonno et al. [2009]). During follow-up (range = 24-214 months), there were no recurrences reported. The median age of this cohort was low (mean = 36 years), and since age is a poor prognostic factor for Leydig cell tumours (Albers et al. [2011]), one might expect a lower rate of recurrence and metastatic disease compared with our own patient cohort. There is only one study of minimally-invasive (laparoscopic) RPLND for patients with Stage I malignant phenotype Leydig cell tumours (n = 6) with a mean age of 41 years (range 29–58) (Peschel et al. [2003]). However, there were two intra-operative vascular complications requiring open conversion. During a mean follow-up of 12 months (range 3–29 months), no recurrences were identified.
The most recent series is from Memorial Sloan Kettering Cancer Centre: Of the 48 patients with sex cord stromal tumours described, 6 patients with either Stage I (n = 4) or Stage II (n = 2) malignant phenotype Leydig cell tumours were managed by RPLND. Interestingly, two patients with Stage I disease, who harboured 5 pathological features (Table 2), developed early relapse and died of metastatic disease within 24 months of surgery (of which one had positive nodal disease), whilst the other 2 remained disease-free. Of the patients with Stage II disease, one had recurred but was still alive at 49 months after surgery. The median age of the RPLND-managed cohort was 48 years (interquartile range = 37-53), and median follow-up time was 68 months (range = 49-173).
We present the largest UK series of RPLND for malignant phenotype Leydig cell tumours of the testis. Although limited by a small patient cohort with a short follow-up period and a lack of functional outcomes, our experience suggests that RPLND is well tolerated with minimal toxicity and good disease-free outcomes in Stage I disease. Our operating time (median = 6 h, range = 4.5-6.5) and peri-operative blood loss (median = 1500 ml, range = 500-1500 ml) appeared greater than the parameters from the only report including these outcomes (mean time = 190 mins, range = 150-225, and mean estimated blood loss = 110 ml, range = 20-350), but may be due to the minimally-invasive technique employed (Peschel et al. [2003]). A further limitation of our report is the absence of information on patients managed in local centres by surveillance and/or chemotherapy.
Taken together with previously published data, there is still insufficient evidence to recommend RPLND as standard of care for all patients. For young patients with very few (≤1) malignant histological features, a period of active monitoring may be considered. However, for older patients, those with a larger number of malignant histological features, or those with Stage II disease, RPLND may, at least, offer good palliation and a possibility of cure. Larger prospective multi-centre studies, with centralized histopathological reporting, are required to determine the definitive role for surgery in Stage I disease.
Ethical standards
The manuscript does not contain any experimental clinical studies or identifiable patient data.
Acknowledgements
We thank staff in uro-oncology centres across Scotland for referring patients to our service, are grateful to staff at the Beatson West of Scotland Cancer Centre and Gartnavel General Hospital, Glasgow for clinical care.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Footnotes
Competing interests
The authors declare that they have no competing interest.
Authors’ contribution
JH Data collection, Data analysis, Manuscript writing. SF Data collection, Data analysis, Manuscript editing. JW Project development, Manuscript editing. PR Project development, Data analysis, Manuscript writing. DH Project development, Data collection, Manuscript editing. All authors read and approved the final manuscript.
Contributor Information
Jane Hendry, Email: jane.l.hendry@gmail.com.
Sioban Fraser, Email: sioban.fraser@ggc.scot.nhs.uk.
Jeff White, Email: jeffwhite@nhs.net.
Prabhakar Rajan, Email: p.rajan@beatson.gla.ac.uk.
David S Hendry, Email: david.hendry@ggc.scot.nhs.uk.
References
- 1.Albers P, Albrecht W, Algaba F, Bokemeyer C, Cohn-Cedermark G, Fizazi K, Horwich A, Laguna MP, European Association of U EAU guidelines on testicular cancer: 2011 update. Eur Urol. 2011;60(2):304–319. doi: 10.1016/j.eururo.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 2.Bertram KA, Bratloff B, Hodges GF, Davidson H. Treatment of malignant Leydig cell tumor. Cancer. 1991;68(10):2324–2329. doi: 10.1002/1097-0142(19911115)68:10<2324::AID-CNCR2820681036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Carmignani L, Gadda F, Gazzano G, Nerva F, Mancini M, Ferruti M, Bulfamante G, Bosari S, Coggi G, Rocco F, Colpi GM. High incidence of benign testicular neoplasms diagnosed by ultrasound. J Urol. 2003;170(5):1783–1786. doi: 10.1097/01.ju.0000092066.01699.90. [DOI] [PubMed] [Google Scholar]
- 4.Cheville JC. Classification and pathology of testicular germ cell and sex cord-stromal tumors. Urol Clin North Am. 1999;26(3):595–609. doi: 10.1016/S0094-0143(05)70201-9. [DOI] [PubMed] [Google Scholar]
- 5.Cheville JC, Sebo TJ, Lager DJ, Bostwick DG, Farrow GM. Leydig cell tumor of the testis: a clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am J Surg Pathol. 1998;22(11):1361–1367. doi: 10.1097/00000478-199811000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Di Tonno F, Tavolini IM, Belmonte P, Bertoldin R, Cossaro E, Curti P, D'Inca G, Fandella A, Guaitoli P, Guazzieri S, Mazzariol C, North-Eastern Uro-Oncological Group I Lessons from 52 patients with leydig cell tumor of the testis: the GUONE (North-Eastern Uro-Oncological Group, Italy) experience. Urol Int. 2009;82(2):152–157. doi: 10.1159/000200790. [DOI] [PubMed] [Google Scholar]
- 7.Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol. 1982;128(2):315–320. doi: 10.1016/s0022-5347(17)52904-3. [DOI] [PubMed] [Google Scholar]
- 8.Farkas LM, Szekely JG, Pusztai C, Baki M. High frequency of metastatic Leydig cell testicular tumours. Oncology. 2000;59(2):118–121. doi: 10.1159/000012147. [DOI] [PubMed] [Google Scholar]
- 9.Gana BM, Windsor PM, Lang S, Macintyre J, Baxby K. Leydig cell tumour. Br J Urol. 1995;75(5):676–678. doi: 10.1111/j.1464-410X.1995.tb07435.x. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez M, Merlani P, Egger JF, Pugin F, Morel P. Hemorrhagic shock caused by rupture of an intra-abdominal leydig cell tumour: case report. Case Rep Gastroenterol. 2007;1(1):53–58. doi: 10.1159/000107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grem JL, Robins HI, Wilson KS, Gilchrist K, Trump DL. Metastatic Leydig cell tumor of the testis. Report of three cases and review of the literature. Cancer. 1986;58(9):2116–2119. doi: 10.1002/1097-0142(19861101)58:9<2116::AID-CNCR2820580925>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 12.Heer R, Jackson MJ, El-Sherif A, Thomas DJ. Twenty-nine Leydig cell tumors: histological features, outcomes and implications for management. Int J Urol. 2010;17(10):886–889. doi: 10.1111/j.1442-2042.2010.02616.x. [DOI] [PubMed] [Google Scholar]
- 13.Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol. 1985;9(3):177–192. doi: 10.1097/00000478-198503000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Loeser A, Vergho DC, Katzenberger T, Brix D, Kocot A, Spahn M, Gerharz EW, Riedmiller H. Testis-sparing surgery versus radical orchiectomy in patients with Leydig cell tumors. Urology. 2009;74(2):370–372. doi: 10.1016/j.urology.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Management of adult testicular germ cell tumours . A national clinical guideline. Number 124. Scottish: Intercollegiate Guidelines Network (SIGN); 2011. [Google Scholar]
- 16.Mosharafa AA, Foster RS, Bihrle R, Koch MO, Ulbright TM, Einhorn LH, Donohue JP. Does retroperitoneal lymph node dissection have a curative role for patients with sex cord-stromal testicular tumors? Cancer. 2003;98(4):753–757. doi: 10.1002/cncr.11573. [DOI] [PubMed] [Google Scholar]
- 17.Ozyavuz R, Ozen HA, Gedikoglu G, Ozgu IH, Sahin A, Tekgul S, Remzi D. Leydig cell tumour of the testis: presentation of two cases. Int Urol Nephrol. 1993;25(4):385–388. [PubMed] [Google Scholar]
- 18.Peschel R, Gettman MT, Steiner H, Neururer R, Bartsch G. Management of adult Leydig-cell testicular tumors: assessing the role of laparoscopic retroperitoneal lymph node dissection. J Endourol. 2003;17(9):777–780. doi: 10.1089/089277903770802362. [DOI] [PubMed] [Google Scholar]
- 19.Silberstein JL, Bazzi WM, Vertosick E, Carver BS, Bosl GJ, Feldman DR, Bajorin DF, Motzer RJ, Al-Ahmadie H, Reuter VE, Sheinfeld J. Clinical outcomes of local and metastatic testicular sex cord-stromal tumors. J Urol. 2014;192(2):415–419. doi: 10.1016/j.juro.2014.01.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Standards and datasets for reporting cancers. Dataset for the histological reporting of testicular neoplasm. (2014) The Royal College of Pathologists. http://www.rcpath.org/Resources/RCPath/Migrated%20Resources/Documents/G/G046_TestisDatasetMay14.pdf
- 21.Summarised cancer mortality, male genital organs. (2014) Information Services Division: ISD Scotland. - 1181. Accessed 30/06/14 http://www.isdscotland.org/Health-Topics/Cancer/Publications/data-tables.asp?id=1181 Summarised cancer mortality, male genital organs. (2014) Information Services Division: ISD Scotland. - 1181. Accessed 30/06/14
- 22.Testicular cancer incidence statistics. (2014) Cancer Research UK. . Accessed 30/06/14 http://www.cancerresearchuk.org/cancer-info/cancerstats/types/testis/incidence/uk-testicular-cancer-incidence-statistics Testicular cancer incidence statistics. (2014) Cancer Research UK. . Accessed 30/06/14
- 23.Testis (2010). In: Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (eds) AJCC Cancer Staging Manual. 7th edn. Springer, New York, pp 469–478


