Abstract
STAT3 has been recognized as an efficacious drug target for prostate cancer because of its constitutive activation in this fatal disease. We recently identified the root bark of Morus alba Linn. as a potential STAT3 inhibitor among 33 phytomedicines traditionally used in Korea. Morusin, an active compound isolated from the root bark of Morus alba, has shown anti-oxidant and anti-inflammatory effects. In the present study, we examined whether morusin has a potential as an anti-cancer agent in prostate cancer. We found that morusin suppressed viability of prostate cancer cells, but little effect in normal human prostate epithelial cells. Morusin also reduced STAT3 activity by inhibiting its phosphorylation, nuclear accumulation, and DNA binding activity. In addition, morusin down-regulated expression of STAT3 target genes encoding Bcl-xL, Bcl-2, Survivin, c-Myc and Cyclin D1, which are involved in regulation of apoptosis and cell cycle. Furthermore, morusin induced apoptosis in human prostate cancer cells by reducing STAT3 activity. Taken together, these results suggest that morusin could be a potentially therapeutic agent for prostate cancer by reducing STAT3 activity and inducing apoptosis.
Keywords: Morusin, prostate cancer, apoptosis, STAT3, SHP1, traditional phytomedicine
Introduction
The root bark of Morus alba Linn., also known and read as Sang Bek Pi in Korea, has been traditionally used in oriental medicine including Korean medicine and Chinese medicine as a diuretic, anti-tussive, anti-diabetic, or anti-inflammatory drug [1,2]. The root bark of Morus alba Linn. has several active compounds such as prenylated flavonoids, stilbenes, benzofurans, alkaloids, and other phenolic compounds [3]. Morusin, a prenylated flavonoid isolated from the root bark of Morus alba Linn. [4], displayed anti-microbial activity, scavenging activity against superoxide anion radical, and anti-inflammatory activity [5-7]. Two recent studies showed that morusin induced apoptosis in cancer cells [8,9]. One showed that morusin can inhibit cervical cancer stem cell growth and migration through reducing NF-κB and inducing apoptosis [9]. Another noticed that morusin may induce apoptosis via activating caspases and inhibiting NF-κB in human colorectal cancer HT-29 cells [8].
Prostate cancer is the most common cancer among men in the United States and is the second leading cause of cancer death, affecting 28% of all male cancer cases and 10% of all male cancer deaths [10]. In addition, the incidence of prostate cancer in South Korea is the fifth and mortality of prostate cancer is the seventh among men [11]. However, the incidence of prostate cancer in South Korea is rapidly increasing [12]. Although different types of treatment including surgery, radiotherapy, chemotherapy and hormonal therapy are available for patients with primary prostate cancer, the complications frequently associated with these conventional treatments diminish positive clinical outcomes [13]. In addition, few options are still available for treating patients with advanced or metastatic stages of the disease [14]. Therefore, more efficient and advanced treatment strategies are required for cure of patients from the fatal disease.
STAT3, a member of the STAT family, is a transcription factor [15]. Various signals to stimulate STAT3 induce phosphorylation and dimerization (homodimers or heterodimers) of STAT3, resulting in its translocation from the cytoplasm to the nucleus to bind each promoter of target genes that are involved in apoptosis, proliferation, differentiation, angiogenesis, and survival [15]. Constitutive activation of STAT3 induces deregulation of cell proliferation and survival that mediates inflammation and tumorigenesis in many cancers [15,16]. Interestingly, some studies have suggested a correlation between STAT3 and prostate cancer [17,18]. Recent studies also indicated that inhibition of STAT3 can induce apoptosis in prostate cancer cells and that STAT3 promotes metastasis in prostate cancer [19,20]. Therefore, the STAT3-targeted therapy has shown a great promise of a therapeutic strategy for prostate cancer [15,21]. Based on these results, we developed the system to screen potential STAT3 inhibitors and found that the root bark of Morus alba Linn. among 33 phytomedicines traditionally used in Korean medicine decreased STAT3 activity (unpublished data). Thus we have tried to find which active compound in the root bark of Morus alba Linn. plays a role as a STAT3 inhibitor, and could be used as an anti-prostate cancer agent. In the present study, we suggest that morusin, one of active compounds in the root bark of Morus alba Linn. could be a potential anti-cancer agent for prostate cancer by inhibiting STAT3 activity.
Materials and methods
Cell lines
DU145, PC3 and LNCaP and RWPE-1 were previously described [22,23], and M2182 was kindly provided by Dr. Paul B. Fisher (Virginia Commonwealth University School of Medicine, Richmond, VA, USA). RWPE-1 was cultured in keratinocyte growth medium-gold bullet kit (Lonza, Inc., Allendale, NJ, USA). DU145, PC3, M2182 and LNCaP were cultured in RPMI-1640 (Lonza) supplemented with 10% fetal bovine serum (Lonza) and 100 U/ml of the antibiotics and antimycotics (Lonza). All cells were cultured in humidified incubator with 5% CO2 at 37°C.
Reagents
Morusin purchased from Biopurify Phytochemicals Ltd. (Chengdu, Sichuan, China) was dissolved in DMSO (Sigma-Aldrich, St. Louis, Mo, USA) to make 50 mM stock solutions. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and sodium orthovanadate were purchased from Sigma-Aldrich, and H2O2 was from Junsei Chemical (Tokyo, Japan).
Cell viability assays
Cell viability was measured by MTT assays as described previously [24]. Cells (3-10 × 103 cells/well) were seeded in 96-well plates, and treated with different concentrations of morusin for 24 hours. Cell viability was presented as percentage compared to the control.
Western blot analysis
Whole cell extracts were prepared, and Western blotting was carried out as described [25]. Primary antibodies STAT3 (1:1000), p (phospho)-STAT3 (1:1000), JAK2 (1:1000), SRC (1:1000), p-SRC (1:1000), C (cleaved)-CASP3 (1:1000), C-CASP8 (1:1000), C-CASP9 (1:1000), Bcl-xL (1:1000), c-Myc (1:1000) and Cyclin D1 (1:1000) from Cell Signaling Technology (Danvers, MA, USA), p-JAK2 (1:1000), Bcl-2 (1:1000), Survivin (1:1000), PARP (1:1000), SHP1 (1:1000) and SHP2 (1:1000) from Santa Cruz Biotechnology (Santa Cruz, CA, USA), β-actin (1:10000) from Sigma-Aldrich, and secondary antibodies HRP–conjugated anti-mouse IgG or anti-rabbit IgG (1:5000; Cell Signaling Biotechnology) were used for immunoblotting. Then the membranes were detected using a chemiluminescence system (Corebio, Seoul, Republic of Korea).
Immunofluorescence staining
Cells were seeded in 8-well chamber slides and treated with morusin for 6 hours. After the cells were fixed and permeabilized, the cells were stained with rabbit polyclonal anti-p-STAT3 antibody (1:100), Alexa Flour 488 goat anti-rabbit antibody (1:1000) (Invitrogen, Carlsbad, CA, USA) and 4’,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) as described [26]. The images were observed by the FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan).
Transient transfection and luciferase (Luc) assays
Cells were transfected with pSTAT3-Luc reporter containing STAT3 binding sites to measure STAT3 activity (Clontech, Palo Alto, CA, USA) in a 60-mm plate using Lipofectamine 2000 (Invitrogen). One day after transfection, cells were re-plated into a 48-well plate. Then the cells were treated with morusin for 24 hours. Luc assays were performed using a Luc assay system (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Luc activity was normalized by protein concentration.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated using PureHelix™ RNA Extraction Solution (Nanohelix, Seoul, Republic of Korea). 1 μg of total RNA was used for RT-PCR using Maxime™ RT PreMix Kit (iNtRON Biotechnology, Kyounggi, Republic of Korea) and HiPi Plus 5X PCR Master Mix (ELPIS Biotech, Daejeon, Republic of Korea) according to the manufacturer’s protocol. Sequences of the primers used for RT-PCR are as following: BCL2 sense, 5’-ATGGCGCACGCTGGGAGAAC-3’; BCL2 antisense, 5’-GGTGTGCAGGTGCCGGTTGA-3’; BCL-XL sense, 5’-TACCAGCCTGACCAATATGGC-3’; BCL-XL antisense, 5’-TGGGTTCAAGTGATTCTCCTG-3’; Survivin sense, 5’-TTGGCCCAGTGTTTCTTCTGCTTC-3’; Survivin antisense, 5’-GCACTTTCTCCGCAGTTTCCTCAA-3’; Cyclin D1 sense, 5’-AGAAGCTGTGCATCTACACCGACA-3’; Cyclin D1 antisense, 5’-TGATCTGTTTGTTCTCCTCCGCCT-3’; GAPDH sense, 5’-ATGGGGAAGGTGAAGGTCGGAGTC-3’; and GAPDH antisense, 5’-GCTGATGATCTTGAGGCTGTTGTC-3’.
Transcription factor activity assays
Nuclear extracts were prepared and 2 μg of nuclear extracts in each sample was analyzed for the STAT3 DNA binding activity using the TransAM STAT3 kit (Active Motif, Carlsbad, CA, USA) according to the manufacturer’s protocol.
Apoptosis analysis
Cell cycle and apoptosis were observed respectively by Propidium Iodide (PI) (Invitrogen) and Annexin V (ApoScan from BioBud, Seoul, Republic of Korea) staining followed by flow cytometry analysis using FACS Calibur (BD Biosciences, San Jose, CA, USA) as described [27]. TUNEL assays were also performed using a DeadEnd™ Fluorometric TUNEL assay kit (Promega) according to the manufacturer’s protocol.
Statistical analysis
Data were presented as mean ± standard error of the mean (SEM) from at least three independent experiments in triplicate or more and analyzed for statistical significance using the unpaired Student’s t-test. P < 0.05 was considered significant.
Results
Morusin suppresses cell viability in human prostate cancer cells
In order to investigate the possible therapeutic effect of morusin in prostate cancer, we first examined whether morusin affects viability of human prostate cancer cells. Human prostate cancer cell lines DU145, M2182, PC3 and LNCaP, and a normal immortalized prostate epithelial cell line RWPE-1 were treated with morusin for 24 hours, and cell viability was measured by MTT assays. Intriguingly, morusin suppressed cell viability in a dose-dependent manner in all the prostate cancer cell lines but less effect on RWPE-1 cells (Figure 1A-E). IC50 values also indicated that morusin is a more potent cytotoxic reagent in prostate cancer cells compared to that in normal cells (Table 1). These results suggested that morusin is a potentially favorable therapeutic phytochemical for prostate cancer.
Figure 1.
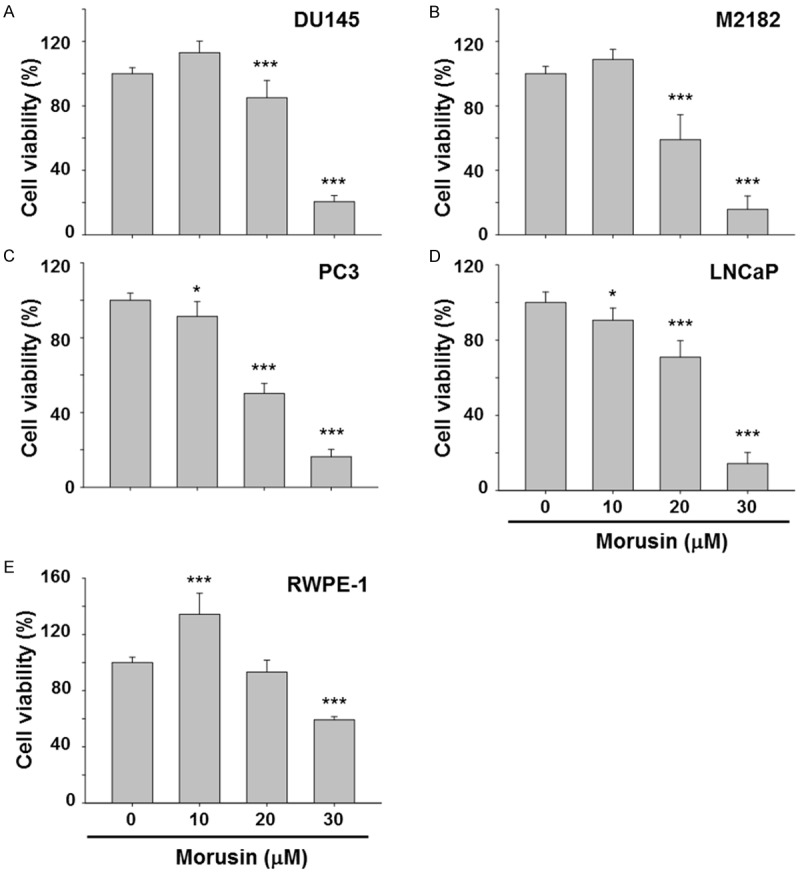
Effects of morusin on cell viability in human prostate cancer cells. (A) DU145, (B) M2182, (C) PC3, (D) LNCaP and (E) RWPE-1 cells were treated with morusin for 24 hours as indicated. Cell viability was determined by MTT assays. Data in the graphs are presented as the mean ± SEM (*, P < 0.05; and ***, P < 0.001 versus mock-treated control).
Table 1.
IC50 value of each prostate cancer cells
| Cell lines | IC50 (μM) |
|---|---|
| DU145 | 26.27±2.84 |
| M2182 | 22.19±3.36 |
| PC3 | 19.97±1.19 |
| LNCaP | 21.89±1.40 |
| RWPE-1 | 43.48±1.81 |
IC50 was determined using the data morusin treatment for 24 hours. Data are presented as the mean ± SEM.
Morusin induces apoptosis in human prostate cancer cells
Since apoptosis is a crucial mechanism by which phytochemicals regulate cancer cell viability, we then investigated whether morusin can affect cell cycle and apoptosis in human prostate cancer cells DU145 and M2182. As shown in Figure 2A and Supplementary Figure 1A, morusin increased the cell population of the sub-G1 phase in both DU145 and M2182 which is indicative of apoptosis. To further confirm whether morusin induces apoptosis, we performed Annexin-V staining assays. As shown in Figure 2B and Supplementary Figure 1B, morusin increased Annexin-V positive cell population in both cell lines, corroborating that morusin induced apoptosis in prostate cancer cells. These results were also confirmed by TUNEL assays (Figure 2C). In addition, we found that morusin increased cleavage of both initiators (CASP8 and CASP9) and executioner (CASP3) caspases and also PARP cleavage, indicating their activation (Figure 2D). Together these results indicated that morusin induces apoptosis in prostate cancer cells.
Figure 2.
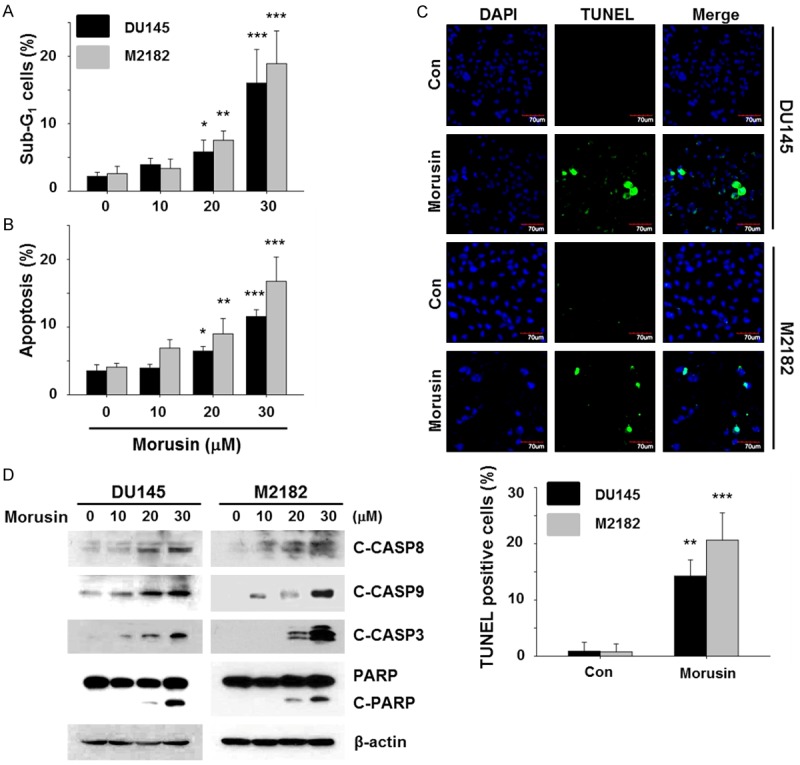
Effect of morusin on apoptosis in human prostate cancer cells. DU145 and M2182 cells were treated with the indicated concentration of morusin for 24 hours. A. The cells were stained with PI, and analyzed by flow cytometry. B. The cells were stained with Annexin V, and analyzed by flow cytometry. C. DU145 and M2182 cells were treated with morusin (30 μM) for 24 hours. The TUNEL and DAPI staining were analyzed by confocal microscopy. Scale bar, 70 μm. D. DU145 and M2182 cells were treated with morusin for 24 hours. Whole cell lysates were subjected to Western blotting with the indicated antibodies. β-actin was used as an internal control. Data in the graphs are presented as the mean ± SEM (*, P < 0.05; **, P < 0.01; and ***, P < 0.001 versus mock-treated control).
Morusin inhibits the STAT3 signaling pathway in human prostate cancer cells
Since morusin is an active compound of the root bark of Morus alba Linn. [4], which we found as a potential inhibitor of STAT3 (data not shown), we expected that morusin could inhibit the STAT3 signaling pathway that might be the mechanism by which morusin induced apoptosis and reduced viability of prostate cancer cells. In order to investigate the effect of morusin on the STAT3 pathway, we analyzed its effect on the STAT3 phosphorylation in prostate cancer cells. As shown in Figure 3A, morusin reduced the phosphorylation levels of STAT3 in DU145 and M2182 cells. In addition, morusin decreased the phosphorylation levels of upstream signaling molecules of STAT3, SRC and JAK2 (Figure 3A). After phosphorylation, STAT3 dimerizes and translocates to the nucleus where STAT3 act as a transcriptional activator [16]. Therefore, we next analyzed the effect of morusin on nuclear accumulation and DNA binding activity of STAT3 in prostate cancer cells. As shown in Figure 3B and 3C, morusin inhibited the nuclear accumulation of STAT3, and also reduced its DNA binding activity in DU145 and M2182 cells. These results noticed that morusin is a potent STAT3 inhibitor, and suggested that STAT3 might be a target of morusin to decrease cell viability in prostate cancer.
Figure 3.
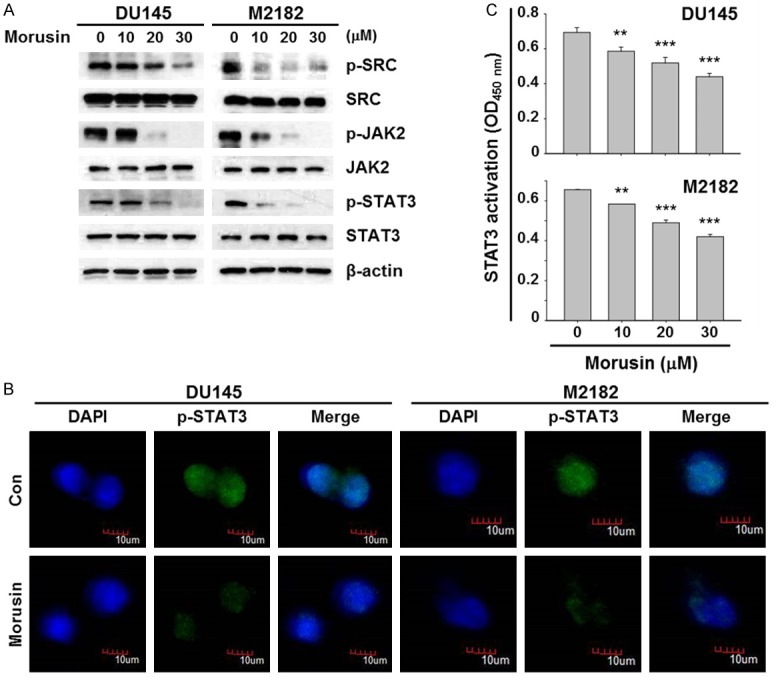
Effect of morusin on the STAT3 signaling pathway in human prostate cancer cells. A. DU145 and M2182 cells were treated with morusin for 6 hours. Cell lysates were subjected to Western blotting with the indicated antibodies. β-actin was used as an internal control. B. DU145 and M2182 cells were treated with morusin (30 μM) for 6 hours. The p-STAT3 and DAPI staining were analyzed by confocal microscopy. Scale bar, 10 μm. C. DU145 and M2182 cells were treated with morusin for 24 hours. Nuclear extracts were subjected for measuring the STAT3 DNA binding activity. Data in the graphs are presented as the mean ± SEM (**, P < 0.01; and ***, P < 0.001 versus mock-treated control).
Morusin represses transcriptional activity of STAT3 in human prostate cancer cells
Since STAT3 is a well-known transcription factor which regulates target genes involved in apoptosis, cell proliferation, and other cellular functions [28,29], we next examined whether morusin regulates the STAT3-mediated transcriptional activation in prostate cancer cells using the pSTAT3-Luc reporter system. As shown in Figure 4A, morusin repressed the transcriptional activity of STAT3 in both DU145 and M2182 cells. Furthermore, morusin reduced protein levels of Bcl-xL, Bcl-2, Survivin, c-Myc, and Cyclin D1 (Figure 4B), which are STAT3 target genes involved in regulation of apoptosis and cell proliferation [30,31]. Accordingly, morusin down-regulated mRNA levels of BCL-XL, BCL-2, Survivin, and Cyclin D1 (Figure 4C). These results suggested that morusin might regulate apoptosis and cell viability of prostate cancer cells by inhibiting transcriptional activity of STAT3.
Figure 4.
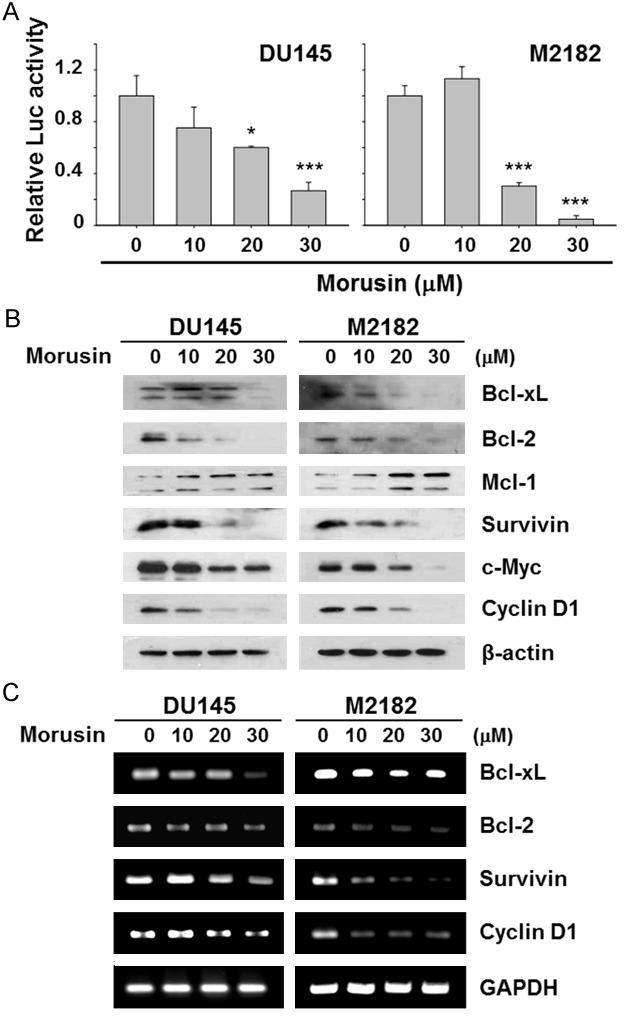
Effect of morusin on transcriptional activity of STAT3 in human prostate cancer cells. A. DU145 and M2182 cells were transfected with pSTAT3-Luc plasmid and the transfected cells were treated with morusin for 24 hours. Data in the graphs are presented as the mean of fold-normalized activities ± SEM to that of the untreated cells taken as 1 (*, P < 0.05; and ***, P < 0.001 versus mock-treated control). B and C. DU145 and M2182 cells were treated with morusin for 24 hours. B. The treated cells were analyzed for protein levels of the STAT3 target genes. β-actin was used as an internal control. C. The treated cells were analyzed for mRNA levels of the STAT3 target genes. GAPDH was used as an internal control.
Morusin induces apoptosis by inhibiting STAT3 in human prostate cancer cells
Non-transmembrane protein tyrosine phosphatases (PTPs) have critical roles in the inhibitory regulation of the JAK/STAT signaling pathway in various type of cancer cells [32,33]. To verify whether inactivation of JAK2/STAT3 signaling by morusin was mediated by induction of PTPs, such as SHP1 and SHP2, we quantified expression of these proteins in morusin treated prostate cancer cells. As shown in Figure 5A, morusin dramatically upregulated SHP1 in a dose-dependent manner, but not SHP2 in DU145 and M2182 prostate cancer cells. Next, we confirmed whether SHP1 dominantly participates in morusin-mediated inhibition of STAT3 using by treatment of PTPs inhibitor, pervanadate. Treatment of pervanadate reversed morusin-mediated inhibition of STAT3 phosphorylation and also reduced morusin-mediated apoptosis in DU145 and M2182 cells (Figure 5B and 5C), indicating the critical role of SHP1 in morusin-induced STAT3 inhibition.
Figure 5.
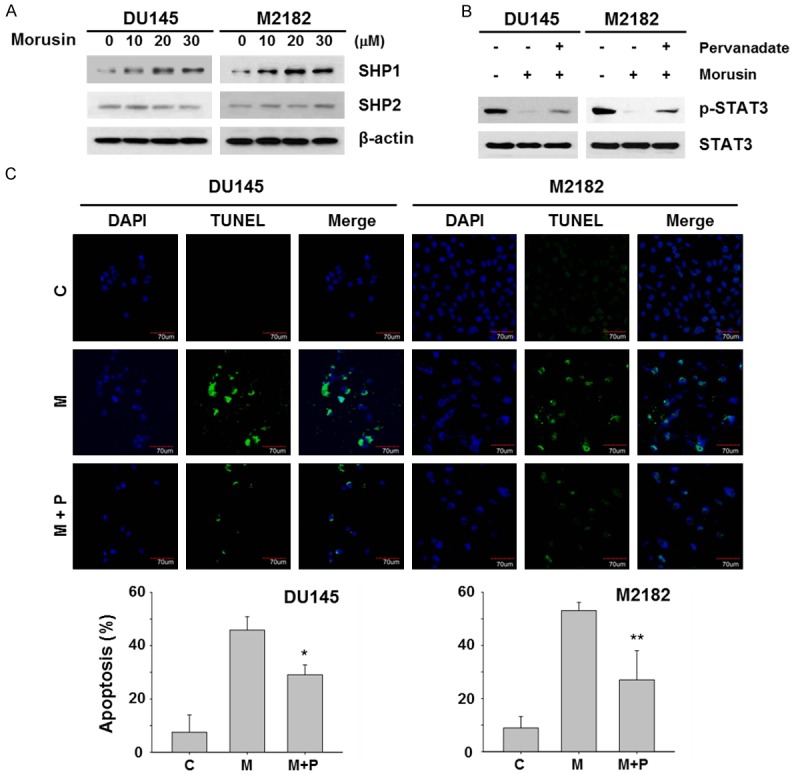
Effect of pervanadate on morusin-mediated STAT3 inhibition and apoptosis in human prostate cancer cells. A. DU145 and M2182 cells were treated with morusin for 6 hours. B. DU145 and M2182 cells were treated with pervanadate (0.5 μM) and morusin (30 μM) for 6 hours. A and B. Cell lysates were subjected to Western blotting with the indicated antibodies. β-actin was used as an internal control. C. DU145 and M2182 cells were treated with pervanadate (0.5 μM) and morusin (30 μM) for 24 hours. The TUNEL and DAPI staining were analyzed by confocal microscopy (C, control; M, morusin; and P, pervanadate). Scale bar, 70 μm. Quantitative analysis of TUNEL positive cells are expressed as relative amount of the treated cells compared to that of the mock-treated control. Data in the graphs are presented as the mean ± SEM (*, P < 0.05; and **, P < 0.01 versus morusin-treated control).
Discussion
Prostate cancer is the second most frequently diagnosed cancer and the sixth leading cause of cancer death worldwide in males, but the most frequent cancer among males in the developed countries because of the wide utilization of prostate-specific antigen (PSA) testing [34]. The increase of PSA testing led to earlier diagnosis, which may increase the survival time of prostate cancer patients but does not necessarily reduce mortality [34]. During the earlier stages, most prostate cancers are dependent on androgen hormone, and tumor treatment can be obtained by surgery, radiotherapy, and androgen deprivation [35,36]. However, these treatments are not effective for advanced and metastatic prostate cancer. Therefore, recent studies have noticed effective phytochemicals for advanced stage of prostate cancer [37-39].
In the cytotoxicity assay to verify whether morusin could be a novel therapeutic agent for prostate cancer, we found that morusin suppressed cell viability in human prostate cancer cells, but interestingly had less toxic effect in normal prostate cells (Figure 1). Although we found that morusin induced apoptosis in prostate cancer cells (Figure 2 and Supplementary Figure 1), its rate (15~20% at 30 μM of morusin) was lower than cytotoxicity (~80% at the same concentration) (Figures 1 and 2). These results indicate that morusin may induce other cell death such as necrosis, autophagy and etc. in prostate cancer cells. A recent report stated that another type of prenylated flavonoid xanthohumol has participated in autophagosome maturation via direct interaction with autophagosome protein [40] supports this hypothesis.
Since STAT3 has been found constitutively activated in many cancers including prostate cancer, STAT3 has been recognized as a valuable drug target [28,29]. For this reason, we developed the system to screen potential STAT3 inhibitors in traditionally used Korean medicines, and morusin is one of candidates screened as a STAT3 inhibitor (unpublished data). Therefore we verified whether morusin can affect STAT3 activity in prostate cancer cells at first. Our results clearly showed that morusin inhibited the SRC/JAK2/STAT3 signaling pathway by blocking their phosphorylation, and consequential loss of nuclear accumulation and transcriptional activity of STAT3 (Figures 3 and 4A). These STAT3 inhibition resulted in suppression of the downstream target genes encoding anti-apoptotic proteins such as Bcl-xL, Bcl-2 and Survivin and cell cycle regulator proteins such as c-Myc and Cyclin D1 (Figure 4B and 4C). Moreover, morusin significantly induced SHP1 expression and inhibition of SHP1 by PTPs inhibitor pervanadate partially restored p-STAT3 level (Figure 5). These results suggest that morusin may inhibit STAT3 signaling via direct participation in regulation of tyrosine kinases, such as SRC, JAK2 and EGFR, as well as SHP1. Taken together these results indicate that morusin induces apoptosis through inhibiting STAT3 by induction of SHP1 in prostate cancer cells, and suggest that morusin is a potentially useful anti-cancer agent for prostate cancer. Further studies including in vivo animal studies and other types of human tumors with increased active STAT3 are warranted and some are currently in progress to test its anti-cancer effect and to develop improved therapies for prostate cancer and methods for ameliorating its pathogenesis.
Acknowledgements
We thank Dr. Paul B. Fisher (Virginia Commonwealth University School of Medicine, Richmond, VA, USA) for the cell line M2182. This work was supported by a research grant from the National Research Foundation of Korea (2007-0054931).
Disclosure of conflict of interest
The authors declare no conflict of interests.
Supporting Information
References
- 1.Asano N, Yamashita T, Yasuda K, Ikeda K, Kizu H, Kameda Y, Kato A, Nash RJ, Lee HS, Ryu KS. Polyhydroxylated alkaloids isolated from mulberry trees (Morusalba L. ) and silkworms (Bombyx mori L.) J Agric Food Chem. 2001;49:4208–4213. doi: 10.1021/jf010567e. [DOI] [PubMed] [Google Scholar]
- 2.Singab AN, El-Beshbishy HA, Yonekawa M, Nomura T, Fukai T. Hypoglycemic effect of Egyptian Morus alba root bark extract: effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. J Ethnopharmacol. 2005;100:333–338. doi: 10.1016/j.jep.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Lyu da H, Koo U, Nam KW, Hong SS, Kim KO, Kim KH, Lee D, Mar W. Protection of prenylated flavonoids from Mori Cortex Radicis (Moraceae) against nitric oxide-induced cell death in neuroblastoma SH-SY5Y cells. Arch Pharm Res. 2012;35:163–170. doi: 10.1007/s12272-012-0118-7. [DOI] [PubMed] [Google Scholar]
- 4.Zong YY, Ip SP, Dong TX, Che CT. [Determination of morusin in cortex mori] . Zhongguo Zhong Yao Za Zhi. 2007;32:1038–1040. [PubMed] [Google Scholar]
- 5.Bellik Y, Boukraa L, Alzahrani HA, Bakhotmah BA, Abdellah F, Hammoudi SM, Iguer-Ouada M. Molecular mechanism underlying anti-inflammatory and anti-allergic activities of phytochemicals: an update. Molecules. 2012;18:322–353. doi: 10.3390/molecules18010322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukai T, Satoh K, Nomura T, Sakagami H. Antinephritis and radical scavenging activity of prenylflavonoids. Fitoterapia. 2003;74:720–724. doi: 10.1016/j.fitote.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Sohn HY, Son KH, Kwon CS, Kwon GS, Kang SS. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L. , Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine. 2004;11:666–672. doi: 10.1016/j.phymed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Lee JC, Won SJ, Chao CL, Wu FL, Liu HS, Ling P, Lin CN, Su CL. Morusin induces apoptosis and suppresses NF-kappaB activity in human colorectal cancer HT-29 cells. Biochem Biophys Res Commun. 2008;372:236–242. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Guo H, Yang L, Dong L, Lin C, Zhang J, Lin P, Wang X. Morusin inhibits human cervical cancer stem cell growth and migration through attenuation of NF-kappaB activity and apoptosis induction. Mol Cell Biochem. 2013;379:7–18. doi: 10.1007/s11010-013-1621-y. [DOI] [PubMed] [Google Scholar]
- 10.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 11.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park SK, Sakoda LC, Kang D, Chokkalingam AP, Lee E, Shin HR, Ahn YO, Shin MH, Lee CW, Lee DH, Blair A, Devesa SS, Hsing AW. Rising prostate cancer rates in South Korea. Prostate. 2006;66:1285–1291. doi: 10.1002/pros.20419. [DOI] [PubMed] [Google Scholar]
- 13.Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58:196–213. doi: 10.3322/CA.2008.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhatnagar V, Kaplan RM. Treatment options for prostate cancer: evaluating the evidence. Am Fam Physician. 2005;71:1915–1922. [PubMed] [Google Scholar]
- 15.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timofeeva OA, Tarasova NI, Zhang X, Chasovskikh S, Cheema AK, Wang H, Brown ML, Dritschilo A. STAT3 suppresses transcription of proapoptotic genes in cancer cells with the involvement of its N-terminal domain. Proc Natl Acad Sci U S A. 2013;110:1267–1272. doi: 10.1073/pnas.1211805110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMiguel F, Lee SO, Lou W, Xiao X, Pflug BR, Nelson JB, Gao AC. Stat3 enhances the growth of LNCaP human prostate cancer cells in intact and castrated male nude mice. Prostate. 2002;52:123–129. doi: 10.1002/pros.10110. [DOI] [PubMed] [Google Scholar]
- 18.Ni Z, Lou W, Leman ES, Gao AC. Inhibition of constitutively activated Stat3 signaling pathway suppresses growth of prostate cancer cells. Cancer Res. 2000;60:1225–1228. [PubMed] [Google Scholar]
- 19.Abdulghani J, Gu L, Dagvadorj A, Lutz J, Leiby B, Bonuccelli G, Lisanti MP, Zellweger T, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Nevalainen MT. Stat3 promotes metastatic progression of prostate cancer. Am J Pathol. 2008;172:1717–1728. doi: 10.2353/ajpath.2008.071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barton BE, Karras JG, Murphy TF, Barton A, Huang HF. Signal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: Direct STAT3 inhibition induces apoptosis in prostate cancer lines. Mol Cancer Ther. 2004;3:11–20. [PubMed] [Google Scholar]
- 21.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 22.Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang SY, Kim SM, Kim C, Um JY, Park KR, Kim SW, Lee SG, Jang HJ, Nam D, Ahn KS, Kim SH, Choi SH, Shim BS, Na YC, Jeong EK, Cho SK, Ahn KS. Antiproliferative effects of Dangyuja (Citrus grandis Osbeck) leaves through suppression of constitutive signal transducer and activator of transcription 3 activation in human prostate carcinoma DU145 cells. J Med Food. 2012;15:152–160. doi: 10.1089/jmf.2011.1671. [DOI] [PubMed] [Google Scholar]
- 24.Lee SG, Jeon HY, Su ZZ, Richards JE, Vozhilla N, Sarkar D, Van Maerken T, Fisher PB. Astrocyte elevated gene-1 contributes to the pathogenesis of neuroblastoma. Oncogene. 2009;28:2476–2484. doi: 10.1038/onc.2009.93. [DOI] [PubMed] [Google Scholar]
- 25.Lee SG, Kim K, Kegelman TP, Dash R, Das SK, Choi JK, Emdad L, Howlett EL, Jeon HY, Su ZZ, Yoo BK, Sarkar D, Kim SH, Kang DC, Fisher PB. Oncogene AEG-1 promotes glioma-induced neurodegeneration by increasing glutamate excitotoxicity. Cancer Res. 2011;71:6514–6523. doi: 10.1158/0008-5472.CAN-11-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park SY, Lim SL, Jang HJ, Lee JH, Um JY, Kim SH, Ahn KS, Lee SG. Embelin induces apoptosis in human glioma cells through inactivating NF-kappaB. J Pharmacol Sci. 2013;121:192–199. doi: 10.1254/jphs.12137fp. [DOI] [PubMed] [Google Scholar]
- 27.Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene. 2008;27:1114–1121. doi: 10.1038/sj.onc.1210713. [DOI] [PubMed] [Google Scholar]
- 28.Coffer PJ, Koenderman L, de Groot RP. The role of STATs in myeloid differentiation and leukemia. Oncogene. 2000;19:2511–2522. doi: 10.1038/sj.onc.1203479. [DOI] [PubMed] [Google Scholar]
- 29.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, Cox CE, Falcone R, Fairclough R, Parsons S, Laudano A, Gazit A, Levitzki A, Kraker A, Jove R. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–2513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 30.Aggarwal BB, Sethi G, Ahn KS, Sandur SK, Pandey MK, Kunnumakkara AB, Sung B, Ichikawa H. Targeting signal-transducer-and-activator-of-transcription-3 for prevention and therapy of cancer: modern target but ancient solution. Ann N Y Acad Sci. 2006;1091:151–169. doi: 10.1196/annals.1378.063. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Zhang J, Wang L, Wei H, Tian Z. Therapeutic effects of STAT3 decoy oligodeoxynucleotide on human lung cancer in xenograft mice. BMC Cancer. 2007;7:149. doi: 10.1186/1471-2407-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y, Amin HM, Franko B, Frantz C, Shi X, Lai R. Loss of SHP1 enhances JAK3/STAT3 signaling and decreases proteosome degradation of JAK3 and NPM-ALK in ALK+ anaplastic large-cell lymphoma. Blood. 2006;108:2796–2803. doi: 10.1182/blood-2006-04-017434. [DOI] [PubMed] [Google Scholar]
- 33.Tang TL, Freeman RM Jr, O’Reilly AM, Neel BG, Sokol SY. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 34.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 35.Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denis LJ, Griffiths K. Endocrine treatment in prostate cancer. Semin Surg Oncol. 2000;18:52–74. doi: 10.1002/(sici)1098-2388(200001/02)18:1<52::aid-ssu8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 37.Diallo JS, Betton B, Parent N, Peant B, Lessard L, Le Page C, Bertrand R, Mes-Masson AM, Saad F. Enhanced killing of androgen-independent prostate cancer cells using inositol hexakisphosphate in combination with proteasome inhibitors. Br J Cancer. 2008;99:1613–1622. doi: 10.1038/sj.bjc.6604730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang L, Jin T, Zeng X, Wang JS. Lycopene inhibits the growth of human androgen-independent prostate cancer cells in vitro and in BALB/c nude mice. J Nutr. 2005;135:287–290. doi: 10.1093/jn/135.2.287. [DOI] [PubMed] [Google Scholar]
- 39.Teiten MH, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5:61–74. doi: 10.1007/s12263-009-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasazawa Y, Kanagaki S, Tashiro E, Nogawa T, Muroi M, Kondoh Y, Osada H, Imoto M. Xanthohumol impairs autophagosome maturation through direct inhibition of valosin-containing protein. ACS Chem Biol. 2012;7:892–900. doi: 10.1021/cb200492h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


