Abstract
Cartilage repair strategies aim to resurface a lesion with osteochondral tissue resembling native cartilage, but a variety of repair tissues are usually observed. Histology is an important structural outcome that could serve as an interim measure of efficacy in randomized controlled clinical studies. The purpose of this article is to propose guidelines for standardized histoprocessing and unbiased evaluation of animal tissues and human biopsies. Methods were compiled from a literature review, and illustrative data were added. In animal models, treatments are usually administered to acute defects created in healthy tissues, and the entire joint can be analyzed at multiple postoperative time points. In human clinical therapy, treatments are applied to developed lesions, and biopsies are obtained, usually from a subset of patients, at a specific time point. In striving to standardize evaluation of structural endpoints in cartilage repair studies, 5 variables should be controlled: 1) location of biopsy/sample section, 2) timing of biopsy/sample recovery, 3) histoprocessing, 4) staining, and 5) blinded evaluation with a proper control group. Histological scores, quantitative histomorphometry of repair tissue thickness, percentage of tissue staining for collagens and glycosaminoglycan, polarized light microscopy for collagen fibril organization, and subchondral bone integration/structure are all relevant outcome measures that can be collected and used to assess the efficacy of novel therapeutics. Standardized histology methods could improve statistical analyses, help interpret and validate noninvasive imaging outcomes, and permit cross-comparison between studies. Currently, there are no suitable substitutes for histology in evaluating repair tissue quality and cartilaginous character.
Keywords: articular cartilage, cartilage repair, histology, animal models, collagen type II, collagen type I, polarized light microscopy, biopsy, fibrocartilage, glycosaminoglycan, subchondral bone, tidemark
Introduction
A variety of articular cartilage repair treatments are in use or in development for clinical use.1-5 Prior to adopting novel methods that employ scaffolds, cells, biologics, and/or tissues, safety and efficacy need to be demonstrated through preclinical testing in relevant animal models and clinical trials. Accordingly, the Food and Drug Administration (FDA) advisory committee has generated draft recommendations for the design of animal studies and clinical trials for cartilage repair.6,7 Preclinical animal model endpoints were suggested to include histology, biomechanical evaluation, and safety. Clinical trial endpoints were suggested to include pain and functional outcomes, imaging, and biopsies. The draft guidance suggested that histological endpoints for clinical trials should evaluate matrix zonal organization, cell density, cell morphology, and inflammatory responses.6 The purpose of this article is to suggest guidelines for tissue processing and standardized assessments that will facilitate compliance with FDA recommendations and that will also allow comparison between studies.
Histological evaluation of cartilage repair tissue must consider a variety of complicated structural features that allow cartilage to carry out its biomechanical function. Articular cartilage has a stratified tissue composition, zonal organization, smooth surface, and firm anchorage to the subchondral bone, all of which contribute to its unique ability to bear joint loads, retain water, deform, and rebound under a gliding and weightbearing motion. Normal adult hyaline articular cartilage contains, approximately, 75% w/v water, 13% w/w collagen, 6% w/w glycosaminoglycan (GAG), and 4% v/v cells.13-15 These high GAG and collagen levels control tissue water content and act synergistically to provide complex nonlinear and poroelastic biomechanical behavior.16 Tensile stiffness and strength properties are primarily derived from the collagen network (mainly collagen type II), while both dynamic and equilibrium compressive stiffness are also greatly influenced by GAG content.17-20
Articular cartilage is structurally organized into 3 distinct zones (Table 1).15 The superficial zone is a relatively thin layer with horizontally oriented collagen bundles and flattened chondrocytes. The midzone is thicker and contains rounded chondrocytes and collagen bundles with heterogeneous orientation. The deep zone usually represents over half of the articular cartilage thickness in skeletally mature large animals and humans.21,22 The deep zone contains larger chondrocytes and collagen fibers oriented perpendicular to the surface, which are also anchored into the calcified cartilage below. The tidemark is a mineralized cement line demarcating the noncalcified and calcified cartilage interface23,24 (Figs. 1A and 1B). In the calcified cartilage layer, chondrocytes are quiescent, fewer in number, produce collagen type X, and express alkaline phosphatase.24-26 Healthy subchondral bone is also important for maintenance of the articular cartilage layer.27-31 In preclinical and clinical studies for cartilage repair, histology can be used as one of the structural outcome measures since it can provide information on the type of matrix and cellular components present and their relative organization. By utilizing other more complex techniques, such as immunohistochemistry or in situ hybridization, individual or particular cellular processes can be identified and localized.
Table 1.
The Zonal Variation Seen in Articular Cartilage
| General Comments | Superficial Zone | Midzone | Deep Zone | |
|---|---|---|---|---|
| Cells | Chondrocyte morphology varies throughout depth of cartilage; no inflammatory cells present | Elongated, single, and aligned parallel to the surface | Rounded/oval and single | Rounded/oval cells; lacunae may be obvious; cells may be ordered into columns, depending on species, age, and location within joints but not in adult human knee/hip articular cartilage |
| Extracellular matrix | homogeneous in appearance; contains no blood vessels or nerves | Collagen fibers parallel to surface when visualized by PLM; smooth surface | No obvious collagen fiber orientation when viewed with PLM | Collagen fibers perpendicular to the tidemark when viewed with PLMExpresses mineralization-related molecules such as alkaline phosphatase in deep aspect close to the bone |
| Major matrix molecules | Collagen I | May be present in superficial layer | Absent | Absent (although reported sometimes in osteoarthritis in very lowest region) |
| Collagen II | Throughout; may be absent in few upper micronsa | Throughout | Throughout | |
| Proteoglycans (PGs): most abundant is aggrecan; demonstrable by IHC and/or metachromasia by staining with, for example, toluidine blue or safranin O; ratio of keratan sulfate (KS):chondroitin sulfate (CS) increases towards bone | Throughout; may be absent in few upper micronsb | Throughout | Throughout | |
| Calcified cartilage | Layer of calcified (mineralized) cartilage below the tidemark forms a collagen type II+ undulating interface between the articular cartilage and lamellar bone and contains round chondrocytes that express collagen type X; the tidemark (or mineralization front) is located between the calcified and noncalcified zones and is demonstrable in adults as a fine, basophilic line(s) on H&E staining; it is not completely characterized but contains calcium phospholipidc | Collagen type II fibers from hyaline tissue extend into calcified cartilage |
Note: PLM = polarized light microscopy; IHC = immunohistochemistry; H&E = hematoxylin eosin.
Changes in osteoarthritis: initial denaturation seen here.8
Figure 1.
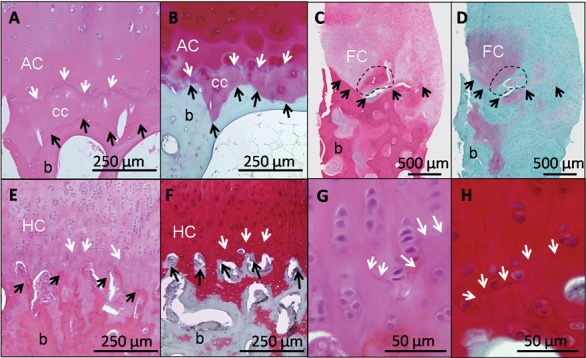
Different features of the osteochondral junction in normal and repair cartilage are revealed by hematoxylin and eosin (H & E) (A, C, E, G) and Safranin O/fast green/iron hematoxylin (SafO) (B, D, F, H). In normal human cartilage (A and B, adult hip surgical waste, femoral neck fracture), H&E clearly stains the tidemark (A, white arrows), while SafO readily discriminates cartilage from fast green–stained bone (below the black arrows, B). For heterogeneous human repair cartilage (C and D, biopsy taken 1 year postmicrofracture71,121), H&E is better for determining the cartilage-bone boundary (black arrows, C) and abnormal mineralization (dashed circle), while SafO discriminates fibrocartilage from fast green–stained fibrous repair and bone (D). In hyaline cartilage repair elicited in a sheep model (E-H, 6 months posttreatment43), the tidemark is beginning to form (white arrows, 10x magnification for E and F, 40x magnification for G and H). White arrows = tidemark; black arrows = cartilage-bone interface; AC = articular cartilage; cc = calcified cartilage; FC = fibrocartilage; HC = hyaline cartilage; b = bone.
Previous Animal and Clinical Studies of Cartilage Repair Using Histological Endpoint Data
The major advantage of animal studies is that it is possible to control the lesion size, defect location, and timing of analysis; it is also possible to analyze the full width of the repaired defect and flanking native cartilage at one or more locations in the defect. Many animal studies analyze sections from 1 level in the defect (i.e., the middle)32-39; other studies analyzed sections from 2 or more distinct preplanned levels in the defect.40-44 Repair heterogeneity, however, can only be evaluated by sampling from more than 1 level in the defect. Animal histology data are used to determine whether an implant is retained in the defect,45-47 to calculate whether treatment has improved repair over a control defect,36,43,48-50 and to assess time-dependent changes in repair cartilage formation and durability.32,33,41,42,44,51-53 Animal studies are usually carried out with a control group.54,55
Prospective human clinical studies with histological endpoints analyze biopsies collected during a second-look arthroscopy (Table 2). A 2-mm-diameter biopsy taken from the center of a lesion provides information for only a relatively small volume of tissue and at one time point. Although this local feature is often cited as a shortcoming of histological analyses, it is always present in any biopsy performed for a diagnostic purpose and thus does not in itself detract from the clinical utility of biopsy analysis. The amount and quality of information that can be obtained are very dependent on the quality of the biopsy obtained by the surgeon and the time interval after treatment.56-61 Limitations of biopsies are that they are invasive, although with some benefit to the patient in terms of personal observation of repair and clinical evaluation of the joint. Taking a biopsy requires surgical intervention with inherent risks (1 reported case of resolved infection62) and could potentially damage the repair tissue being biopsied. It is generally believed that biopsy procurement does not induce any long-term deleterious effects for the patient or for the repair tissue since the 2-mm-diameter sample corresponds to a very small 0.04-cm2 area; an equine study showed no negative effects at 12 months after biopsy at 4 months postoperatively.63
Table 2.
Published Histological Analyses of Human Repair Cartilage
| Reference | Repair Procedure | Patient Number | % of Patients Analyzed | H | H/FC | FC | TT | F | Bone | Patient Age (y) | Repair Tissue Postoperative Time | Failure Rate | Collagen Characterization | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgical Waste or Biopsy of Failed Repair | Sample (N) | |||||||||||||||
| Insall | 1967 | Drilling | 12 | 1 | 8% | 100% | 56 ± 8 | 4.9 ± 2.3 y | 25% at 3-7 y | |||||||
| Nehrer | 1999 | Abrasion | — | 12 | 42% | 17% | 25% | 10% | 20% | 0% | 39 ± 9 | 1 mo to 19 y (mean, 4 y) | — | I, II, X | ||
| Nehrera | 1999 | Periosteal graft | — | 4 | 25% | 75% | 0% | 0% | 0% | (OG75%) | 40 ± 9 | 5 mo to 10 y (mean, 5 y) | — | I, II, X | ||
| Nehrera | 1999 | ACI (salvage) | — | 6 | 0% | 0% | 0% | 30% | 70% | 0% | 38 ± 9 | 25 mo to 7 y (mean, 4 y) | — | I, II, X | ||
| Bouwmeesterb,c | 1999 | Perichondrial graft | 88 | 22 | 24% | 29% | 24% | 24% | 24% | 29 ± 9 | 5-61 mo (mean, 21 mo) | 24% at 2 y | I, II, X | |||
| Biopsies: Unblinded Analysis | Biopsies (N) | H | H/FC | FC | TT | F | Bone | |||||||||
| Hangody | 1997 | Mosaic | 44 | 6 | 14% | 100% | N/A | 3 mo to 3.5 y | Picrosirius, PLM | |||||||
| Robertsa | 2003 | ACI + mosaic | 6 | 50% | 50% | 0% | 35 ± 10 | 9-34 mo | I, II, III, VI, X, PLM | |||||||
| Robertsa | 2003 | ACI | 14 | 14% | 43% | 43% | 35 ± 10 | 9-34 mo | I, II, III, VI, X, PLM | |||||||
| Marcaccid | 2005 | Hyalograft C | 107 | 22 | 21% | 55% | 27% | 18% | N/A | 10-30 mo (mean, 15 mo) | ||||||
| Hendersond,e | 2005 | ACI | 53 | 20 | 35% | 65% | 15% | 20% | N/A | 7-24 mo (mean, 13 mo) | ||||||
| Tins | 2005 | ACI | 41 | 41 | 100% | 10% | 24% | 61% | 5% | 35 (18-53) | 12 mo | PLM | ||||
| Hollanderd,f | 2006 | Hyalograft C | 23 | 43% | 13% | 43% | 36 (13-54) | 6-30 mo (mean, 16 mo) | I, II (biochem) | |||||||
| Biopsies: Prospective Study, No Control Group | Biopsies (N) | H | H/FC | FC | TT | F | Bone | |||||||||
| Briggsg | 2003 | ACI - Chondroguide | 14 | 13 | 93% | 46% | 15% | 23% | 15% | 30 (16-51) | 11 ± 4 mo | II, X, PLM | ||||
| Bartlett | 2004 | MACI | 47 | 11 | 23% | 27% | 9% | 64% | 33 (17-47) | 12 mo | II, IIa, X | |||||
| Bartlett | 2004 | ACI-C | 44 | 14 | 32% | 29% | 14% | 57% | 34 (15-49) | 12 mo | PLM | |||||
| Moriyah | 2007 | ACI (on OCD) | 6 | 6 | 100% | 0% | 67% | 33% | 0% | 20 (13-35) | 12 mo | I, II | ||||
| Gikas | 2009 | ACI, ACI-C, MACI | 248 | 248 | 100% | 24% | 27% | 46% | 4% | 33 (15-52) | 3-55 mo (mean, 15 mo) | PLM | ||||
| Biopsies: RCT, Blinded Analysis with Control | Biopsies (N) | ICRS-II | H | H/FC | FC | TT | F | Bone | ||||||||
| Knutsena | 2005 | MFX | 40 | 35 | 88% | 11% | 17% | 51% | 20% | 18-45 | 24 mo | 23% at 5 y | PLM | |||
| Knutsena | 2005 | ACI | 40 | 32 | 80% | 19% | 31% | 34% | 16% | 18-45 | 24 mo | 23% at 5 y | PLM | |||
| Saris | 2008 | MFX | 61 | 36 | 59% | ~46% | 18-50 | 12 mo | 12% at 3 y | II | ||||||
| Saris | 2008 | CCI | 57 | 46 | 81% | ~55% | 18-50 | 12 mo | 4% at 3 y | II | ||||||
Note: H = hyaline; H/FC = hyaline mixed with fibrocartilage; FC = fibrocartilage; F = fibrous tissue; TT = transitional tissue; PLM = polarized light microscopy; I, II, IIa, III, VI, X = collagen type; — = not applicable; OG75 = bone overgrowth was seen in 75% of the treated defects; N/A = information not available specifically for biopsied patients (all patients were between 17 to 64 years old); ACI = autologous chondrocyte implantation; MACI = matrix autologous chondrocyte implantation; CCI = characterized chondrocyte implantation; ICRS-II = ICRS-II overall histological score. References: Insall,64 Nehrer et al.,67 Bouwmeester et al.,66 Roberts et al.,96 Marcacci et al.,84 Henderson et al.,83 Tins et al.,76 Hollander et al.,70 Briggs et al.,62 Bartlett et al.,99 Moriya et al.,72 Gikas et al.,59 Knutsen et al.,56,57 and Saris et al.61,98
Hyaline defined as >60% tissue with hyaline character.
Hyaline defined as >50% tissue with hyaline character.
Biopsies were taken only when a partial failure was present or a clear failure (fibrocartilage, loose flap, loose body).
Condylar, trochlear, and patellar biopsies.
Two-millimeter biopsies were taken with a Giebel needle from central and marginal areas of the graft.
Biopsies from painful knees were excluded.
Two 3-mm Jamshidi biopsies were collected from each treated lesion.
Two biopsies were collected with an 11-gauge needle: one from the central lesion and one from the same lateral condyle (nonlesional).
In human clinical studies, repair cartilage histological findings have been most frequently reported as the predominant repair tissue type of each specimen, often using the following terminology: hyaline, fibrocartilage, fibrous, and bone64-68 (Table 2). Some clinical studies additionally used collagen typing and polarized light microscopy (PLM) to evaluate hyaline repair (Table 2). Interpretation of published clinical biopsy data is complicated by the fact that the tissue can only be evaluated at one time point in a repairing lesion that potentially changes with time.59,69 In addition, some studies only analyzed failed repair67 or excluded samples with failed repair,70 and most studies did not have a control group, which also precluded a blinded assessment (Table 2). To date, blinded histological analysis has been included as a structural endpoint in 3 randomized controlled clinical trials (RCTs) at 12 months61 or 24 months,56 with 1 trial underway with a 12-month endpoint.71 In these trials, Review Ethics Board (REB) approval was obtained to collect repair tissue biopsies from all patients, although some patients refused a biopsy, some were excluded for other reasons (pregnancy, unavailability, failure of the treatment before the trial endpoint), and some biopsies were excluded from the analysis due to poor quality of the stained section.61 Therefore, even in a well-designed RCT, histology may not be available from every subject.
In comparison to the small tissue volume analyzed by biopsy, complementary macroscopic imaging methods such as MRI can give a more global assessment of fill, are relatively noninvasive, and can be performed at more than one time point, thus allowing longitudinal follow-up assessments. MRI can also reveal repair tissue overgrowth and bone edema, which are common complications of cell therapy procedures.72 Current imaging techniques can indirectly suggest hyaline cartilage formation73,74 but do not always correlate directly with histological findings.75,76 Although technological improvements are continually ongoing, to date, there is no directly validated MRI technique capable of assessing tissue quality in the manner that histology is capable of providing. It is an ultimate aim for the patient-benefit perspective to be able to correlate biopsy data to noninvasive data such as MRI or serum biomarkers in order to validate these noninvasive methods. However, in some patients, MRI examination is not possible (pacemakers, kidney pathology), and patients with metal implants can generate imaging artifacts. There are very limited data to date on the correlation of qualitative MRI like dGEMRIC or T2-mapping with histology; further studies in this direction would be of potentially great clinical relevance. Recommendations for clinical study design and noninvasive imaging are discussed in more detail in companion papers.77,78
Ideally, histological structural analyses should generate unbiased quantitative assessment of extracellular matrix, cell, and tissue organization relative to its similarity to normal native cartilage. A properly designed study should be capable of determining the following:
What is the (average) thickness and volume of the repair cartilage?
Is any implanted (foreign) material still present? Are there any signs of inflammatory or immune response to the implanted material?
What portion of the repair cartilage is hyaline?
Is the articular surface smooth and intact? Is the overall structure intact or disintegrated?
Does the repair tissue have zonal organization, uniform collagen type II, little or no collagen type I, and appropriate collagen fiber orientation?
Are there viable cells with the appropriate morphology to form and maintain a hyaline extracellular matrix?
Does the repair tissue (animal studies) have good lateral integration with adjacent cartilage?
Is the repair cartilage fully integrated with subchondral bone, and has the tidemark been regenerated?
Does the subchondral bone plate and underlying bone have a normal structure?
A rigorous experimental design with an appropriate control group needs to consider 1) location of the biopsy or section within the animal defect, 2) timing of postoperative biopsy or sample recovery, 3) method of histoprocessing, 4) stains utilized, and 5) validated and blinded evaluation methods amenable to statistical analysis, which implies sufficient sample numbers. Multiple correlations between histological scores and functional outcome or imaging measures can be performed. In general, animal tissues and clinical biopsy specimens can be similarly processed and evaluated (summarized in Table 3), although some critical differences are described below.
Table 3.
Guidelines for Histological Processing and Analyses of Repair
| 1. Lesion size and location |
|
| 2. Timing of biopsy and sample recovery |
|
| 3. Histoprocessing (for cartilage-bone analysis) |
|
| 4. Staining |
|
| 5. Evaluation methodsa |
|
As our understanding of cartilage repair and chondrocyte biology improves, these recommendations may have to be modified.
Lesion Size and Location
In the human knee joint, the medial femoral condyle is the most frequent site of deep grade III and IV lesions.79 Human lesions treated by cartilage repair vary from 0.5 to 12 cm2 1,56,61,74,80-82 and may be accompanied by anterior cruciate ligament (ACL)/meniscal tears,79 multiple lesions,83 bipolar lesions,84 or subchondral bone pathology.30,85 Unlike animal models where the entire joint is available for histological analysis, human biopsies are usually collected from the middle of the repair tissue in medial and lateral condylar lesions56,61 and from limited tibial plateau, trochlear, and patellar sites.70,84 In one study, the investigators reported that their trochlear and patellar sites were inaccessible to the biopsy needle by arthroscopy.86 Since some sites may not be amenable to biopsy, this needs to be taken into consideration when designing a clinical trial with histological endpoints. Two clinical studies reported taking 2 biopsies from the defect, one in the middle and one at the edge.62,83 However, for the sake of limiting the amount of tissue removed from the repaired site, and to ensure collection of repair tissue instead of adjacent cartilage, it is recommended that one biopsy be taken from the estimated geometric center of the repaired lesion. Systematic collection of biopsies from the estimated geometric center will minimize selection bias of the “best” or “worst” repair and facilitate comparison between trials.
Animal models of cartilage repair have important differences when compared to the clinical situation. Humans present with existent defects, whereas in most animal models, the defects are treated immediately after being freshly created in healthy tissues. Compared to human lesions, defects created in animals tend to be smaller: 0.3 to 1 cm2 in large animals (sheep, pig, goat, horse)36,37,42,43,48,50,51,87 and 0.01 to 0.2 cm2 in small animals (canine, rabbit), which have a different metabolic rate of repair as well.33,88-90 Critical size defects that are unable to heal spontaneously should be used. Articular cartilage is quite thin in rabbits (0.1-0.3 mm) compared to large animals (0.4-2 mm, from sheep, pig, and goat to horse) and humans (~2-3 mm).15,43,91-93 The average calcified cartilage thickness (0.1-0.2 mm) is similar across species.92 Animal models frequently have full weightbearing immediately postsurgery, and in consequence, many studies use a trochlear location to protect the implanted materials and cells and favor their retention.39,89,94,95 However, selection of a defect site can have additional ramifications as shown in a sheep model, where microfractured trochlear sites regenerated significantly less hyaline tissue than medial condyle sites.43 Such site-specific repair responses are thought to be related to the different loading and weightbearing environments and different opposing articulating surfaces (patella vs. meniscus and tibial plateau).
Timing of Biopsy and Sample Recovery
Human Biopsy Collection
The timing of biopsy collection is important. In many studies, biopsies were collected as they became available (3-36 months postoperatively)62,70,83,84,96,97 (Table 2). The FDA draft recommendation suggested that biopsies could be taken in a subset of subjects at both short-term (e.g., 6 months) and long-term (e.g., 2 years) follow-up.6 It is not realistic or ethically sound to prospectively request multiple biopsies from the same patient, so one endpoint should be chosen. In recent studies of cell-based therapies, later biopsies tended to have a more hyaline character.58,59 However, development of fibrous repair could have been missed at later endpoints (>18 months) if the tissue failed prematurely,56,57,67 leading to the need for further surgery that would eliminate poor-quality tissue from the endpoint analysis and thereby bias late assessments. Given that animal cartilage repair tissues mature33,53 and degenerate with time,32,51 it is recommended that all samples be collected at the same postoperative time point for all subjects. In one RCT, a similar histological profile was obtained in both treatment groups at 24 months, and this was followed by the same failure rate (9 of 40) in both groups at 5 years,56,57 although none of the patients with predominantly hyaline cartilage at 24 months failed at 5 years. In another RCT, significant improvements in the overall tissue quality of one treatment group at 12 months postoperatively were associated with fewer failures at 36 months61,98 (Table 2). Altogether, these results suggest that biopsies obtained at 6 months are still too immature, while biopsies obtained 12 or 24 months could have some prognostic value and serve as interim indicators of potential efficacy of a therapy or technique. To summarize, the optimal time for biopsy collection has not been yet identified, but either 1 or 2 years seems appropriate based on previous studies.
A clinical biopsy is obtained during a second-look arthroscopy. During the procedure, it is essential to document and score the general intra-articular findings. Utilization of a validated scoring system is recommended; a number of studies have used the ICRS macroscopic score.56,61,70,71,74,83,99 The Outerbridge classification has also been used to describe articular cartilage repair; however, the score was originally designed to describe a cartilage injury and therefore is not recommended. The defect size, integration, and repair aspect as well as the biopsy procedure and location and intra-articular findings should be documented using a video-capture system. Documentation of the biopsy site should be sufficiently detailed to permit comparison of histology with other joint parameters (i.e., meniscal status, alignment). Osteochondral biopsies can be taken with a 1.8- to 2.3-mm inner-diameter bone biopsy needle (up to 3-mm nominal outer diameter) such as Jamshidi (11 gauge, Cardinal Health, Dublin, OH),59,71 Manatech (Stoke-on-Trent, England), Trapsystem (11 gauge, MD TECH, Gainesville, FL),72 or Giebel (Karl Storz, Tuttlingen, Germany),83 and video capture can be used to document the biopsy site. The technique may be first practiced ex vivo with animal joints or on human cadaveric specimens. Biopsies should be taken from the estimated geometric center of the initial lesion to minimize the possibility of collecting adjacent (normal or degraded) cartilage and should be as perpendicular as possible to the bone.96 The needle is inserted by very carefully placing the needle and gently tapping with a mallet approximately 1 cm deep in order to obtain several millimeters of subchondral bone and is twisted clockwise and counterclockwise (10 times each way) to core the sample and break the biopsy from the subchondral bone, using a technique similar to the one used for retrieval of osteochondral grafts during a mosaicplasty procedure. The biopsy is extruded backwards through the needle with a fitted wire pressing on the bone core to avoid damaging the soft repair tissue at the opposite end. The biopsy should be photodocumented with a size marker or ruler59 (Fig. 2) and processed immediately (see below, Histoprocessing). All surgical details including problems with biopsy retrieval, time into fixative, and a schematic of the location in the defect should be documented and transferred to the histologist. For some lesion sites, biopsies can only be collected oblique to the cartilage surface, and these may need to go deeper to collect subchondral bone (Figs. 2C and 2D). Also, despite best efforts by the surgeon to extract a biopsy from the middle of the repaired lesion, it is possible that the biopsy is retrieved outside the lesion. It is suggested that the blinded observers flag any biopsies with features suggestive of a nonrepresentative biopsy such as a well-formed tidemark or normal-appearing hyaline cartilage with a lamina splendens. In some repair samples, especially those slightly delaminated, the twisting motion used to extract the biopsy can lead to the bone or surface becoming separated from the cartilage repair in the biopsy specimen. Broken samples have previously been eliminated from some analyses61,68; however, the separate pieces can be processed together to obtain a subset of histological and histomorphometric values, which may reduce bias. Some features can still be scored even if the biopsy is not complete.
Figure 2.
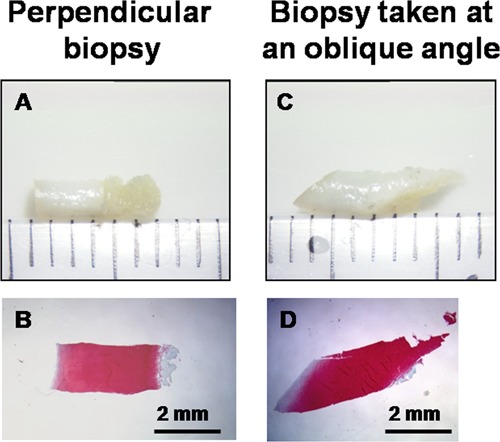
Appearance of human 2-mm-diameter biopsy obtained with a Jamshidi 11-gauge needle (A, C) and corresponding decalcified Safranin O–stained paraffin section (B, D). Samples were obtained ex vivo with an ethics-approved protocol from the same lateral condyle (nonlesional area) obtained after total knee arthroplasty (74-year-old female). (A and B) A biopsy cored perpendicular to the surface and (C and D) a biopsy cored deliberately at an oblique angle to the surface are shown. Both biopsies were initially 6 mm long, but the subchondral bone was missing from the oblique biopsy prior to histoprocessing (C). Part of the subchondral bone in the perpendicular biopsy (B) was lost during histoprocessing.
Animal Sample Collection
In animal studies, a rigorous study design includes histological characterization of the acute defect in a separate group of animals (with and without implanted material, also to assess debridement level) and a repair endpoint relevant to the treatment, for example, 2 to 6 months in rabbits and 6 to 12 months in large animals.6,54 As previously recommended,54 a very short repair period, 1 to 7 days postoperatively, can be used to establish implant residency after weightbearing. Insight into biological mechanisms of action can be gleaned after several weeks’ repair in rabbits33,44,52 and several months in sheep or horses.36,42,49,51 In large animal models, histological analyses can be supplemented with analyses of repair tissue biochemistry.40,43,100 In unilateral cartilage repair models, histology of the contralateral joint can be used as a reference for intact cartilage volume (Fig. 3). Intact age-matched osteochondral specimens also serve as useful controls. Detailed recommendations for animal study design are described in another article in this series.101
Figure 3.
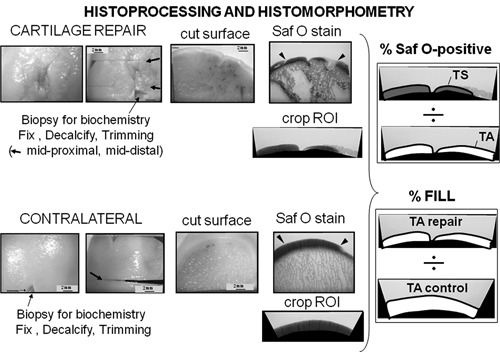
Histoprocessing and histomorphometry of large animal defects. The example is taken from a sheep cartilage repair model (6 months repair43). In this unilateral cartilage repair model, the repaired defect (top panels) was decalcified, trimmed at 2 levels in the defect (midproximal and middistal), and stained with Safranin O/Fast Green. Repair tissue above the projected tidemark was cropped using histomorphometric software, and total area (TA) and total stained repair tissue area (TS) were used to determine percentage of Safranin O–stained repair. The contralateral intact condyle was decalcified, trimmed through the middle, and cropped with matching defect width, and total area was used to determine percentage fill of the defect with repair tissue.
At necropsy, the joint and repair tissue should be photodocumented (preferably with a ruler and label carrying the date and sample identity). The repair tissue should be evaluated for tissue color, homogeneity, surface smoothness, lateral integration, and fill. Macroscopically visible roughening, fibrillation, or degradation of the surfaces articulating with the defect should be noted: for example, patellar surfaces in trochlear defect models, and meniscus and tibial plateaux in condylar defect models. Abnormal reactions such as osteophytes, cysts, adhesions, excess synovial fluid and synovial tissue inflammation, hypertrophy, or pannus should be documented. Any small biopsies for biochemistry and/or biomechanical analyses should be collected (or in situ indentation performed) prior to fixation, outside the repair tissue areas dedicated to histology. The joint should be kept moist with a balanced salt solution until placed in fixative. In general, specimens should be placed in 10 volumes of fixative. For small animal models, the distal femur can be dissected of all soft tissue, cut from the rest of the bone, and placed directly into a 50-mL conical tube filled with fixative. However, in large animal models, the sample block should be further trimmed of excess bone with a hand-held or band saw before placing in fixative to promote good infiltration. Cartilage repair samples should encompass the entire defect width, flanking cartilage to permit observation of lateral integration and enough subchondral bone to permit the observation of subchondral bone reactions as well as the subchondral trabecular bone structure.
Histoprocessing
Once the osteochondral sample is taken, it should be processed immediately. In the majority of animal cartilage repair studies, osteochondral samples are fixed in formalin-based buffer and decalcified in either acid, EDTA, or commercial decalcification solutions.32,33,35-39,42,43,49,50,52,89,102 By comparison, human osteochondral biopsy histoprocessing methods to date have shown a lack of a standardized approach. Clinical biopsies have been fixed in formalin and embedded nondecalcified in paraffin56,60,61,103; frozen nondecalcified, cryosectioned, and stained without the use of fixative58,76,84,96; stored in cell culture media for up to 2 days, trimmed of bone, and the soft repair tissue frozen, sectioned, then fixed70; or fixed and embedded nondecalcified in plastic83,104; or fixed in formalin and EDTA decalcified.62 In several studies, the biopsy histoprocessing methods were not described.59,97,99 It is generally recognized that sectioning of bone-cartilage samples requires “softening” of the bone by a decalcification procedure.105 In order to carry out comprehensive endpoint analyses that include histological scoring for cartilage matrix, osteochondral integration, PLM, subchondral bone structure, and collagen immunostaining, it is recommended that clinical biopsies be fixed in formalin-based buffer, decalcified in acid or EDTA, and either processed in paraffin (5-µm sections) or cryosectioned (8 or 10 µm) according to the preferences of the laboratory. Paraffin embedding presents several advantages over cryoprocessing, such as a more uniform section thickness, better adhesion to slides, and better preservation of the bone structure. Additionally, the thinner paraffin sections lay more flat on the histology slide and can be digitally scanned (40x magnification to observe cell morphology) for histological scoring at a computer, while digitally scanned cryosections tend to have regions out of the focal plane, and therefore, cryosections often need to be scored at a microscope. Antibodies to collagen type I and II work just as well in paraffin as in cryosections for human biopsies.
Samples may also be embedded nondecalcified in plastic; however, it should be noted that collagen immunostaining of thinner plastic sections can be technically challenging compared to paraffin or cryosection. If subchondral bone analysis is not required, or if the goal of the study is to perform analyses on the cartilage repair that are incompatible with fixation and/or decalcification, the biopsies can be trimmed of bone, frozen and sectioned, then analyzed biochemically or fixed for further staining.70 Fresh cartilage vibratome sections may also be frozen, substituted with plastic, and submitted to ultrastructural studies by electron microscopy.106 For structural endpoint analyses, all samples in a given study should be processed identically using standardized histoprocessing methods. Internal control samples (i.e., osteochondral biopsies from cadaveric or arthroplasty specimens, or intact animal osteochondral samples) should be histoprocessed under identical conditions (same fixative, decalcification, embedding media). According to the research objectives, some studies may prefer to separate a group of samples into 2 or more distinct sample sets with different histoprocessing conditions.66,69
Biopsies can be fixed most conveniently in 10% neutral buffered formalin for a minimum of 24 hours (up to 1 week). Fresh 4% paraformaldehyde can also be used, but it is relatively unstable and should be optimally used within 1 to 3 days of preparation. Animal samples are much larger than clinical biopsies and require longer fixation times, as a rule of thumb, a minimum of 3 days (rabbits) to 7 days (large animal blocks) at 4 °C. Long-term storage in fixative should be avoided because overfixation can interfere with immunohistochemical staining for some antigens; samples archived in fixative for a year can show depressed immunostaining for collagen type II or type I and loss of proteoglycan and Safranin O staining. It is recommended that tissues be processed after complete fixation without delay and that the minimal and maximal time limit for fixation be determined for each laboratory.
To analyze cartilage-bone integration and subchondral bone structure, repair biopsies should include bone and should be decalcified intact. Decalcification can be accomplished using acid or EDTA. Acids such 0.5 N HCl43 or 50% formic acid with 68 g/L sodium formate (pH ~3)42,105 are one way to remove calcium. Sufficient decalcification can be obtained in 0.5 N HCl at 4 °C after approximately 30 hours for 2-mm-diameter biopsies, 10 days for rabbit distal femurs, and 4 to 6 weeks for large animal osteochondral samples. Acid decalcification is compatible with immunostaining for both collagen type II and collagen type I in humans and sheep (Fig. 4) as well as rabbits if the samples are cryosectioned.107 However, the low pH can hydrolyze some antigens of interest and destroy their immunoreactivity. For this reason, some laboratories prefer to decalcify samples in EDTA, which chelates calcium at neutral pH, is less damaging to proteins, and preserves enzymatic staining (i.e., alkaline phosphatase in osteoblasts, or tartrate-resistant acid phosphatase in osteoclasts47). The time required for EDTA decalcification will be longer than acid solutions, although this can be somewhat shortened by gently agitating the specimen during the decalcification process and changing solutions frequently. Trace fixative (10% w/v EDTA/0.1% w/v paraformaldehyde, pH 7.2, or 0.5 N HCl/0.1% glutaraldehyde)52 can be included in decalcification solutions, especially during long decalcification procedures. Commercially available decalcification solutions are another alternative, but these tend to be more expensive and utilize the same types of methodologies. As each method has its strengths and drawbacks, it is recommended that the laboratory choose a method of decalcification, optimize it, and then determine how long the tissue can remain in the solution without affecting its immunoreactivity. Decalcification can be monitored and checks carried out to ensure the process is complete by using x-rays (i.e., faxitron of human biopsies62), ammonium oxalate calcium precipitation tests of the decalcification solution (animal specimens only),44,108 or cautiously puncturing into the subchondral bone using a 26-gauge needle.
Figure 4.
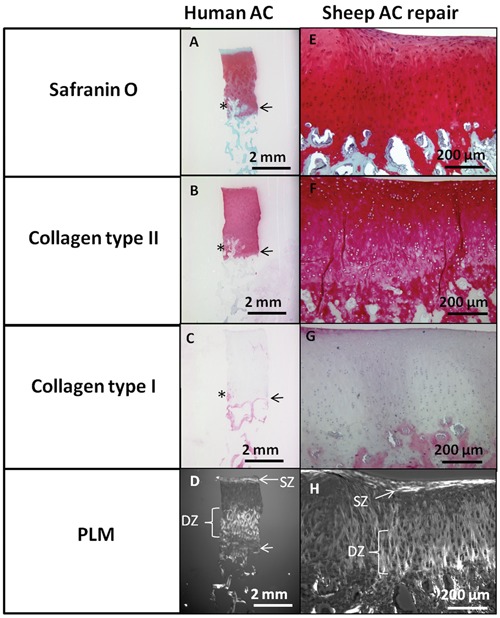
Example of standardized histoprocessing to evaluate a human biopsy (A-D, from cadaveric knee medial femoral condyle) or sheep hyaline repair cartilage 6-month repair after treatment with microfracture and chitosan-GP/blood implant (E-H).43 Sections were stained for Safranin O, immunostained for collagen type II and collagen type I, and observed by polarized light microscopy (PLM). SZ = superficial zone; DZ = deep zone; AC = articular cartilage. Note the abnormal vascular invasion and mineralization (*) in this particular human biopsy above the tidemark (horizontal arrow, A-D), which is frequently observed in osteoarthritis.21
Once decalcified, animal osteochondral sample blocks can be trimmed further, firstly to remove excess bone, which allows adequate infiltration with embedding media, and secondly to generate a cut surface inside the repaired defect from which sections will be collected. Given the typical heterogeneity of cartilage repair tissues, it is recommended to trim the decalcified sample to allow collection of sections from at least 2 distinct levels in the repaired defect. The decalcified sample is trimmed with a flat razor blade, perpendicularly to the surface at 1 or 2 predefined points in the defect (Fig. 3). A clean razor blade should pass readily through the sample during this trimming step; if the tissue is still calcified (“crunchy” or stiff), then the sample should be decalcified further. Fully decalcified and trimmed samples can be postfixed for 24 hours prior to embedding. The trimmed tissue surfaces can be photodocumented for proper sample tracking. Samples from animal specimens should be trimmed to a block that is no more than 0.5 cm thick to ensure adequate infiltration with embedding media. Enough subchondral bone tissue should be retained to permit the analysis of cartilage-bone integration,47,89 bone overgrowth,33,109 fibrosis or callus formation,110 subchondral cyst formation,37,43 and any residual subchondral implanted material.87,111 In animal samples, depending on the type of defect, sections may be collected in the transverse or sagittal plane; transverse sections that retain the outer edges of the joint sample permit observation of osteophytes.
Paraffin embedding uses organic solvents to dehydrate the tissue and can be used to embed and section large sample blocks (2-3 cm3). For plastic embedding, the infiltration times should be increased accordingly for larger samples. If using cryopreservation, small animal femurs should be adequately trimmed (separate condyles and trochlea; maximum, 0.5 cm long) to permit good cryopreservative infiltration and thereby limit sectioning artifacts. Biopsies can be directly embedded in paraffin, cryoprotection agent, or plastic resin. All histology sections in a study should be generated with the same thickness to ensure a uniform and comparable staining intensity. The CryoJane tape-transfer system (Instrumedics, St. Louis, MO) has been used to photo-crosslink frozen cryosections to adhesive-coated histology slides, which improves retention of marrow elements during extensive washing steps.44
Once a sample is embedded, any number of sections can be collected from the specimen. Ideally, sections need to be collected and stained so as to minimize bias in the analysis, a notion that is well defined in the stereology literature as “systematic random sampling.”112 The systematic aspect means that one is covering as completely and homogeneously as possible the sample to be analyzed. Some studies analyze 2 distinct levels per defect; some studies analyze serial sections through the entire defect. The random aspect indicates that the location is not biased by operator choice, meaning the trim plane should be determined prior to sample recovery. Another systematic aspect could involve a preplanned order of distinct stains to perform on a series of sections that are collected from each level in the specimen. Each laboratory should determine the best approach for the sectioning and staining plan that generates unbiased sections for analysis.
Staining, Immunostaining, and Routine Light Microscopy
The selection of stains utilized to visualize the tissue will depend on what parameter is being examined. Similar stains are used in the evaluation of human biopsies and animal repair cartilage. Routine staining of 2 sections in the same level (on the same slide, or on separate slides) can save time because if one section is folded (or lost) during staining, this will allow for selection of the better section for analysis. Duplicate sections can also be used to confirm whether minor artifacts (tears, missing tissue) are consistently present in distinct sections and that the staining techniques are reproducible.
Routine Histostaining and Polarized Light Microscopy
Hematoxylin and eosin (H&E) staining of the tissue section will allow evaluation of the overall tissue, cell morphology, presence of abnormal calcification, and the bone-cartilage interface (Fig. 1). The tidemark is well visualized using H&E staining (Fig. 1A, 1E, 1G). Hematoxylin will stain cell nuclei blue, while eosin will stain protein in bone, cartilage, and fibrous tissues pink. When the staining method is properly done, H&E is helpful for differentiating mineralized collagen (deep pink hue23) from nonmineralized fibrous tissues (light pink), which otherwise stain uniformly green with Safranin O-fast green stain (Fig. 1C and 1D).
Safranin O and toluidine blue are most frequently utilized to stain proteoglycans and demonstrate their presence and location within the tissue. Safranin O/fast green/iron hematoxylin stain provides the best contrast between red- or pink-stained sulfated glycosaminoglycan and green-stained subchondral bone with iron hematoxylin counterstain to color cell nuclei dark blue (Fig. 1B).113 Prolonged incubation in the ethanol baths during the dehydration steps for permanent mounting can leach Safranin O from the section and give a false-negative result. Toluidine blue is a metachromatic stain that stains collagenous matrix blue and sulfated glycosaminoglycan purple. The similar hue of toluidine blue–stained fibrous and fibrocartilage tissue104 can make it difficult to evaluate percentage area of glycosaminoglycan positivity with this stain. Alcian blue is another metachromatic stain but is more difficult to perform reliably as it must be done at the right pH (pH ≤1). Thionin is a nonmetachromatic purple stain that is simple to perform114 but, like toluidine blue, generates a similar hue for hyaline and fibrous tissue. To control for batch-to-batch consistency in proteoglycan staining intensity, a control section from the same reference sample (i.e., osteochondral cadaveric or animal specimen) should be included in each staining batch.
PLM is used to determine the presence of organized collagen fibrils in the matrix. PLM can be carried out on either unstained sections mounted with permanent mounting media22 or H&E-stained sections.59 Collagen organization can also be evaluated at selected sites in the repair tissue with hydrated and unmounted sections by scanning environmental electron microscopy in low vacuum mode.22
Immunohistochemical Staining
Adult hyaline articular cartilage is comprised of a wide variety of matrix molecules organized in a particular manner; to date, no single marker specific for hyaline cartilage has been identified. Hyaline articular cartilage contains type II collagen, fibrocartilage contains a mixture of type I and type II collagen, and fibrous tissue contains type I collagen and no type II collagen.41,60,69 Thus, the extent of collagen type II staining could be considered a marker of the degree of differentiation towards hyaline cartilage61,96; however, collagen type II alone cannot be used to determine the extent of hyaline cartilage formation because type II collagen is also found in fibrocartilage. Therefore, the extent of hyaline cartilage formation can only be truly demonstrated by the presence of collagen type II, low or absent collagen type I staining, and an organized collagen matrix when viewed under polarized light43,67,69,96,107 (Tables 1 and 2). Appropriate zonal organization is a feature that distinguishes articular hyaline cartilage from other types of hyaline cartilage (e.g., nasal). For collagen immunostaining, the monoclonal antibody I-8H5 (MP Biomedicals, Solon, OH) recognizes collagen type I matrix in human, rabbit, and sheep tissues.43,69,107 The presence of decalcified bone in the section serves as a good internal positive control for collagen type I (Fig. 4). Monoclonal antibodies II-II6B3, CIIC1 (Developmental Studies Hybridoma Bank, Iowa City, IA), and II-4C11 (MP Biomedicals) have been used to detect collagen type II in humans69,115 and monoclonal II-II6B3 to detect collagen type II in rabbit, sheep, and horse tissues.43,52,89,92,107 Positive controls using tissues taken from other orthopedic procedures and processed identically should be included in all immunostaining batches as controls to ensure reagents are working and to demonstrate reproducibility of the technique.
For research purposes, additional markers have been used to analyze repair cartilage, including collagen type IIa,69 a propeptide domain isoform of collagen type II expressed in fetal cartilage and perichondrium,116 metalloproteinases and aggrecanases that indicate ongoing remodeling or cartilage matrix degradation, proteoglycan isotypes, and collagen type X as a secondary marker of calcified cartilage69,96,117 and bone overgrowth in the defect.67 The FDA guidance additionally recommended histological evaluation of aggrecan (size, composition), dermatan sulfate, fibronectin, and tenacin.6 As the above markers are not necessarily indicators of articular cartilage but supportive findings, we would suggest that they not be required markers in animal or clinical studies.
To summarize, cartilage structural characterization minimally includes immunostaining for collagen type II and collagen type I, in combination with staining with H&E to assess osteochondral tissue features and Safranin O or toluidine blue to assess glycosaminoglycan deposition. Additional unstained sections can be generated to analyze collagen fibril organization by PLM, and unmounted sections can be analyzed for collagen structure by electron microscopy.
Evaluation
Stained sections should be evaluated to determine how closely the structure and organization of the repair tissue resembles normal adult articular cartilage. Histological evaluation can be qualitative and somewhat subjective. We suggest particular parameters be assessed in an attempt to standardize the evaluation. Firstly, an initial appraisal of the quality of the sampling and processing should be undertaken. For example, has a full-depth biopsy been obtained, encompassing the articulating surface and at least some of the subchondral bone? The quality of sections should be confirmed, that is, that it is cut in the correct orientation and whether there are artifactual tears or “holes,” knife score marks, or folds within the section that make certain assessments impossible or unreliable. A torn cartilage-bone interface or missing bone would invalidate, for example, the scoring of cartilage-bone integration. Biopsy samples that have no surface or bone attached can only be evaluated for a limited set of features. Sections that are poorly stained, folded, or torn should be replaced by newly cut and stained sections prior to histological and histomorphometry analysis. A complete set of interpretable sections should be generated, and then the sections should be blinded by a third party prior to evaluation.
Once the satisfactory quality of the sample and sections is established, the level and type of evaluation (i.e., qualitative/quantitative) will depend on the particular study or purpose of the investigation and the questions being asked. The only parameter that may be of interest to the investigator(s) might be the tissue morphology, for example, whether the majority of the tissue is hyaline, fibrocartilaginous, fibrous, or none of these (Table 2). Such an analysis facilitates comparison of new data with the literature. However, it is in the interest of the investigator to analyze and quantify a variety of histological parameters to provide a more complete assessment of the effects of treatment, which may be positive or negative. Note that hyaline repair can still fail if mechanical irritation persists, for example, due to poor defect fill,56,57,74,118 nonintegration (perichondral flaps66), bone overgrowth (periosteal graft67), or coexistent unstable joint conditions such as meniscal tears or malalignment. Hyaline repair could also have limited durability if the surface is disintegrated, a feature of OA cartilage also linked to weakened compressive strength.119 It should be emphasized here that many characteristics are just as important as the extracellular matrix composition in determining repair cartilage structural quality, namely, viable cells with zonal-specific morphology at an appropriate density, cell organization, collagen fibril organization and orientation, cartilage-bone integration, tissue stratification, and surface integrity, for example (Table 1). A comprehensive and quantitative analysis of tissue character and anatomy provides a more complete and unbiased assessment of the effects of treatment.
Establishing the Region of Interest
Objective scoring and good interreader agreement depend on the ability to score the same region of interest in a blinded sample. In normal osteochondral samples, this is not an issue; however, in repair tissue biopsies, the reader’s perception of the soft repair tissue zone can be complicated by an irregular bone-cartilage interface. This type of artifact can generate interreader variations in biopsy analyses simply due to reader-specific scoring of different regions of interest within the same section.115 When the biopsy contains several regions of bone mixed with soft repair, the cartilage-bone interface could be defined by scanning from the surface downwards and marking a line across the first encountered area where bone covers more than 50% of the biopsy width (Fig. 1C and 1D, black arrows). The use of blinded sections that contain both a test and control group will help prevent selection bias (when analyzing inhomogeneous tissues). Standardized methods to establish the cartilage-bone interface will increase interreader agreement for parameters such as repair tissue thickness and abnormal calcification.
In animal cartilage repair studies, the acute defect may or may not include subchondral bone damage, and during repair, extensive subchondral bone resorption can sometimes occur.38,42,43,120 When there is subchondral bone resorption, and all soft tissue present in the section is evaluated, this will lead to artifactual overestimates of the cartilage repair tissue volume. Likewise, bone overgrowth leads to thinning of the articular layer and the presence of bone in the cartilage defect area.109 To specifically analyze chondral repair tissue versus subchondral repair tissue, a curved “projected tidemark” should be drawn across the defect, using the tidemark of the flanking cartilage, to separate the cartilage repair from the subchondral repair tissue (Fig. 5).
Figure 5.
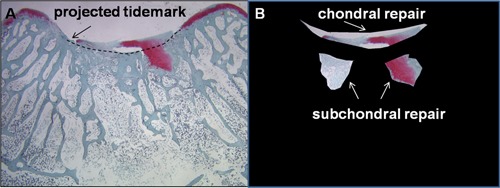
Histomorphometry of chondral versus subchondral soft repair tissues. The example is from a 2-month repair of a trochlear full-thickness rabbit knee defect with two 0.9-mm microdrill holes.44 (A) Safranin O–stained trochlear repair tissue, with the “projected tidemark” drawn through the defect area. (B) The chondral repair is cropped separately from the subchondral soft tissue repair for further histomorphometric analysis.
Histological Scoring
Histological scoring provides an important outcome measure of preclinical and clinical repair cartilage. Before undertaking histological assessments of cartilage repair, it is essential that the readers are trained to recognize morphological and structural features of normal and repair osteochondral tissues. In most histological scoring systems, readers judge the predominant repair tissue feature on an ordinal scale of 0 to 3 or 4, which gives a semiquantitative and relatively crude approximation (i.e., 0, +, ++, +++). The data are then analyzed by nonparametric statistical tests (Mann Whitney U, Kruskal-Wallis). Semiquantitative scoring systems are notorious for having reader-specific interpretation, and more recently, it was shown that among 3 independent readers, interreader and intrareader agreement is actually quite low.55,60 Therefore, to aim for the highest objectivity in a study, it is advisable that histological evaluations are performed by 2 or 3 independent blinded readers. Any wide discrepancies between the readers, that is, differences greater than 30% on a 100-point scale, or differences of 2 ordinal numbers on a 0- to 3-point scale, should be agreed upon in a consensus meeting, while the samples are still blinded. The final data set for statistical analyses can either be averaged for all readers (where consensus scores are not required) or expressed as a consensus score.121 Interreader and intrareader reproducibility can be calculated using intraclass correlation coefficients122 that are superior to Pearson correlations for this purpose. Although outside the scope of this article, a well-defined preplanned statistical analysis accounting for multiple outcome measures is essential to ensure proper control of type I and type II errors and the effects of multiplicity and missing values. A good review of these issues can be found in the companion article on patient-reported outcomes for cartilage repair.123
In animal models for which whole joints are available for study, a variety of cartilage histological grading systems for cartilage repair tissue have been developed (recently reviewed by Rutgers et al.55). A frequently employed assessment tool is the O’Driscoll score,41 while some studies have used the more simple Pineda score.102 The Pineda system evaluates 4 histological features: filling of defect, reconstitution of the osteochondral junction, matrix staining, and cell morphology102; a score of 0 represents normal articular cartilage. The O’Driscoll score additionally evaluates surface regularity, structural integrity, tissue thickness, lateral integration (bonding), cellularity, chondrocyte clustering, and adjacent cartilage degeneration,41 and a score of 24 represents normal articular cartilage. Modified O’Driscoll scores (MODS) have also been reported, where matrix staining (0-4) was replaced by a continuum of percentage staining (0%-100%),124 or additional categories such as inflammation and subchondral bone health were added.47,125
To specifically evaluate the quality of repair tissue in patient biopsies, the Histological Endpoint Committee of the International Cartilage Repair Society (ICRS) developed a Visual Assessment Histological Scale in 2003.68 This histological scoring system, termed ICRS-I, assesses 6 components of repair, including surface architecture, matrix, cell distribution, cell population viability, subchondral bone, and abnormal cartilage mineralization. Like the O’Driscoll score, the ICRS-I system uses discrete integer scores (0-3) to describe a continuum of tissue features in heterogeneous tissues and has shown low interreader agreement.60 Therefore, a new histological scoring system was developed (ICRS-II), using a continuous visual analog scale (VAS) and 14 criteria to assess parameters related to chondrocyte phenotype and tissue structure.60 In the VAS system, the reader generates a pen mark along a 100-mm line according to worst (0) to ideal cartilage features (100), which is then measured with a ruler and converted to a numerical score from 0 to 100. Better interreader consistency was found using the ICRS-II VAS scoring system compared to MODS or ICRS-I, which use discrete integers.60 In the ICRS-II, the “overall assessment” and “matrix staining” scores showed the highest correlation coefficients for interreader and intrareader variability (r > 0.80).60 The ICRS-II represents an improvement over previous histological cartilage repair grading systems in terms of reader reproducibility, but its ability to predict long-term repair durability will need to be determined.
Histomorphometric Evaluation of Cartilage
Quantitative histomorphometry can be performed on human biopsies and animal repair tissue, using histomorphometric software. Negative controls using omission of primary antibody, isotype antibodies or antigen-preabsorbed antibody solutions should always be included to assess background staining, and positive controls using intact cartilage should also be included. The use of scanned digital images can ensure that all digital section images have the same light intensity. For samples that include bone, repair tissue thickness can be evaluated using a line tool.43,91,92,96 If the tissue is irregular, an average thickness can be generated from several line thickness measurements. In animal repair samples, defect width, percentage coverage of the defect bone base with integrated repair tissue, and percentage coverage of the defect bone base with detached repair tissue can be further analyzed using line tools.89
Repair tissue area can be measured using volume tools and thresholding software (for example, ImageJ, NIH, Bethesda, MD and Northern Eclipse, Mississauga, ON, Canada). Manual or automatic thresholding software algorithms can be used to further determine percentage staining for collagen type I, collagen type II, and Safranin O for glycosaminoglycan69,115 (Fig. 3). First, a calibrated digital image of the osteochondral specimen is acquired, and using the cropping tool, a new image file is created that contains only the soft cartilage repair tissue (the region of interest [ROI]). Next, in the cropped image, the value for total tissue area (TA) is determined by training the threshold tool to recognize the whole soft cartilage repair tissue area. Finally, in the cropped image, the threshold tool is trained to recognize total stained tissue (TS) (immunopositive or histostained). The percentage of stained repair tissue is then determined by the following equation: % stained = TS/TA*100.
A cautionary note in using percentage staining as the main structural outcome should be made. Strong staining for collagen type II and Safranin O can be obtained in structurally disintegrated samples,126 indicating the need for histological scores in addition to histomorphometric findings, to properly evaluate the biopsy.
Evaluation of Subchondral Bone
The importance of subchondral bone health in cartilage repair is being increasingly recognized.68,89,102,125,127 In animal cartilage repair models, subchondral cysts are abnormal and can be identified as visible fibrous white tissue or tissue voids in the subchondral tissue. To evaluate cyst severity, 2 sections from a distinct level in the defect are given a cyst score according to the area percentage of the bone section occupied by the cyst (0 = no cyst, 1 = 0%-25%, 2 = 25%-50%, 3 = 50%-75%, and 4 = more than 75% of the bone area is occupied by a cyst). The average score is used as the final cyst score for a particular defect.43
Subchondral bone is histologically evaluated in the ICRS-I and ICRS-II scales, but the current scoring systems are limited in that different subchondral repair tissues can yield a similar score. For example, subchondral callus and subchondral bone remodeling could receive similar scores of 2 on a 0- to 3-point scale68 or a score of 60 on a 0-to-100 VAS scale.60 Alternatively, quantitative structural data of subchondral bone parameters can be generated using stereology and histomorphometry. The bone volume fraction of a specific region of interest, thickness of the subchondral plate, and subchondral bone porosity (surface density) can be evaluated using bone histomorphometric software. Bone surface volume (Sv) and volume fraction (Vv) can be analyzed in a particular region of interest by stereology.89 The significance of these bone parameters has yet to be fully understood, although more complete bone repair44,47 and a more porous subchondral bone repair43,89 of marrow-stimulated defects were previously correlated with a more hyaline repair cartilage in animal models.
Collagen Structural Organization
Collagen organization in adult hyaline articular cartilage is important for maintenance of tissue load-bearing capacity.128 To observe zonal collagen organization, PLM can be performed on H&E-stained sections or unstained sections that are dehydrated and mounted in permanent mounting media. In normal articular cartilage, the radial deep zone contains fibers oriented perpendicularly and anchored into the tidemark, and the superficial zone contains horizontal collagen bundles (Table 1). The intermediate zone contains anisotropic fibers that do not change light polarization and thus do not generate birefringence. Cartilage exposed to collagenases that degrade the collagen architecture will diminish PLM birefringence, while exposure to aggrecanases can enhance PLM birefringence.128 PLM can demonstrate horizontal surface and deep zone perpendicular collagen fibril orientation (Figs. 4D and 4H). A semiquantitative PLM scoring system was recently developed to grade collagen architecture and zonal stratification.22 Blinded sections are graded on a scale of 0 to 5, where 0 = no organization, 1 = vertical deep zone apparent, 2 = deep zone well developed, 3 = 3 zones present, 4 = zonal proportions of normal articular cartilage, and 5 = hyaline cartilage organization. The average score of 3 readers is taken to represent the degree of tissue stratification and zonal organization of collagen fibers.
Conclusions
Preclinical studies and RCTs for new cartilage repair strategies have successfully applied histological structural endpoints to evaluate the effects of treatment; in such studies, a control group must be included and assessments carried out with a trained team of blinded readers. Structural endpoints can be useful to interpret and validate noninvasive imaging and may be able to predict functional outcomes. Cartilage repair tissue and resurfacing is frequently heterogeneous, so it is paramount for the investigator to use methods that minimize bias insofar as it is possible by preplanned analyses with unbiased and representative sampling methods and uniform validated staining methodologies. Standardization of histological sample collection, processing, staining, and quantitative evaluation will further our collective efforts to develop better analytical and possibly prognostic tools to evaluate the outcome of cartilage repair treatments, thus leading to their improvement and/or new algorithms for treating symptomatic chondral and osteochondral lesions. Currently, there is no suitable substitute for histological analyses when tissue quality and cartilaginous character are to be assessed.
Footnotes
Acknowledgments and Funding: The authors thank Drs. Alberto Restrepo and Matthew Shive for advice on biopsy retrieval methods; Dr. William Stanish and Piramal Healthcare for providing selected human osteochondral samples; Gaoping Chen, Viorica Lascau, and Adele Changoor for assistance in generating the figures; and Dr. Gerjo van Osch for critical reading of the article. Salary support is acknowledged for C.D.H. (Fonds de Recherche en Santé du Québec), S.R. (MRC grant number G0800248, UK), L.C. (Dutch Arthritis Association), A.P.H. (Endowed Chair from Arthritis Research UK), and M.D.B. (Canada Research Chair) as well as operating grants from Canadian Institutes of Health Research (C.D.H., M.D.B.) and the Natural Sciences and Engineering Research Council of Canada (C.D.H., M.D.B.).
Declaration of Conflicting Interests: S. Méthot is an employee of Piramal Healthcare. None of the other authors has any conflicts or apparent conflicts of interest to declare in relation to this article.
References
- 1. Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33(3):443-60. [DOI] [PubMed] [Google Scholar]
- 2. Mithoefer K, McAdams TR, Scopp JM, Mandelbaum BR. Emerging options for treatment of articular cartilage injury in the athlete. Clin Sports Med. 2009;28(1):25-40. [DOI] [PubMed] [Google Scholar]
- 3. Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91A(7):1778-90. [PubMed] [Google Scholar]
- 4. Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92A(4):994-1009. [DOI] [PubMed] [Google Scholar]
- 5. van Osch G, Brittberg M, Dennis JE, Bastiaansen-Jenniskens YM, Erben RG, Konttinen YT, Luyten FP. Cartilage repair: past and future. Lessons for regenerative medicine. J Cell Mol Med. 2009;13(5):792-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McFarland R, Kaiser A. Guidance for industry: preparation of IDEs and INDs for products intended to repair or replace knee cartilage. Rockville, MD: U.S. Department of Health and Human Services; 2007. Available from: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm072952.htm [Google Scholar]
- 7. FDA. 38th meeting, topic I: Cellular, Tissue, and Gene Therapies Advisory Committee. Rockville, MD: U.S. Food and Drug Administration; 2005. Available from: http://www.fda.gov/ohrms/dockets/ac/05/transcripts/2005-4093T1.htm [Google Scholar]
- 8. Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest. 1995;96(6):2859-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mankin HJ. The reaction of articular cartilage to injury and osteoarthritis (first of two parts). N Engl J Med. 1974;291(24):1285-92. [DOI] [PubMed] [Google Scholar]
- 10. Sabatini M, Lesur C, Thomas M, Chomel A, Anract P, de Nanteuil G, Pastoureau P. Effect of inhibition of matrix metalloproteinases on cartilage loss in vitro and in a guinea pig model of osteoarthritis. Arthritis Rheum. 2005;52(1):171-80. [DOI] [PubMed] [Google Scholar]
- 11. Pastoureau P, Hunziker E, Pelletier JP. Cartilage bone and synovial histomorphometry in animal models of osteoarthritis. Osteoarthritis Cartilage. 2010;18:S106-12. [DOI] [PubMed] [Google Scholar]
- 12. Kraus VB, Nevitt M, Sandell LJ. Summary of the OA biomarkers workshop 2009 biochemical biomarkers: biology, validation, and clinical studies. Osteoarthritis Cartilage. 2010;18(6):742-5. [DOI] [PubMed] [Google Scholar]
- 13. Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II: correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523-37. [PubMed] [Google Scholar]
- 14. Hoemann CD. Molecular and biochemical assays of cartilage components. In: De Ceuninck F, Sabatini M, Pastoureau P, editors. Cartilage and osteoarthritis. Totowa, NJ: Humana Press; 2004:127-156. [DOI] [PubMed] [Google Scholar]
- 15. Hunziker EB, Quinn TM, Hauselmann HJ. Quantitative structural organization of normal adult human articular cartilage. Osteoarthritis Cartilage. 2002;10(7):564-72. [DOI] [PubMed] [Google Scholar]
- 16. Li LP, Buschmann MD, Shirazi-Adl A. A fibril reinforced nonhomogeneous poroelastic model for articular cartilage: inhomogeneous response in unconfined compression. J Biomech. 2000;33(12):1533-41. [DOI] [PubMed] [Google Scholar]
- 17. Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64(1):88-94. [PubMed] [Google Scholar]
- 18. Treppo S, Koepp H, Quan EC, Cole AA, Kuettner KE, Grodzinsky AJ. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18(5):739-48. [DOI] [PubMed] [Google Scholar]
- 19. Laasanen MS, Toyras J, Korhonen RK, Rieppo J, Saarakkala S, Nieminen MT, et al. Biomechanical properties of knee articular cartilage. Biorheology. 2003;40(1-3):133-40. [PubMed] [Google Scholar]
- 20. Legare A, Garon M, Guardo R, Savard P, Poole AR, Buschmann MD. Detection and analysis of cartilage degeneration by spatially resolved streaming potentials. J Orthop Res. 2002;20(4):819-26. [DOI] [PubMed] [Google Scholar]
- 21. Rieppo J, Hyttinen MM, Halmesmaki E, Ruotsalainen H, Vasara A, Kiviranta I, et al. Changes in spatial collagen content and collagen network architecture in porcine articular cartilage during growth and maturation. Osteoarthritis Cartilage. 2009;17(4):448-55. [DOI] [PubMed] [Google Scholar]
- 22. Changoor A, Tran-Khanh N, Methot S, Garon M, Hurtig M, Shive MS, Buschmann MB. A polarized light microscopy method for accurate and reliable grading of collagen organization in cartilage repair. Osteoarthritis Cartilage. 2011;19(1):126-35. [DOI] [PubMed] [Google Scholar]
- 23. Gilmore RS, Palfrey AJ. A histological study of human femoral condylar articular cartilage. J Anat. 1987;155:77-85. [PMC free article] [PubMed] [Google Scholar]
- 24. Wang FY, Ying Z, Duan XJ, Tan HB, Yang B, Guo L, et al. Histomorphometric analysis of adult articular calcified cartilage zone. J Struct Biol. 2009;168(3):359-65. [DOI] [PubMed] [Google Scholar]
- 25. Gannon JM, Walker G, Fischer M, Carpenter R, Thompson RC, Oegema TR. Localization of Type-X collagen in canine growth plate and adult canine articular-cartilage. J Orthop Res. 1991;9(4):485-94. [DOI] [PubMed] [Google Scholar]
- 26. Oegema TR, Carpenter RJ, Hofmeister F, Thompson RC. The interaction of the zone of calcified cartilage and subchondral bone in osteoarthritis. Microsc Res Tech. 1997;37(4):324-32. [DOI] [PubMed] [Google Scholar]
- 27. Gomoll A, Madry H, Knutsen G, van Dijk N, Seil R, Brittberg M, Kon E. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madry H. The subchondral bone: a new frontier in articular cartilage repair. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):417-8. [DOI] [PubMed] [Google Scholar]
- 29. Madry H, van Dijk C, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):419-33. [DOI] [PubMed] [Google Scholar]
- 30. Johnson DL, Urban WP, Caborn DNM, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409-14. [DOI] [PubMed] [Google Scholar]
- 31. Burr DB, Schaffler MB. The involvement of subchondral mineralized tissues in osteoarthrosis: quantitative microscopic evidence. Microsc Res Tech. 1997;37(4):343-57. [DOI] [PubMed] [Google Scholar]
- 32. Wei XC, Messner K. Maturation-dependent durability of spontaneous cartilage repair in rabbit knee joint. J Biomed Mater Res. 1999;46(4):539-48. [DOI] [PubMed] [Google Scholar]
- 33. Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75A(4):532-53. [DOI] [PubMed] [Google Scholar]
- 34. Shamis LD, Bramlage LR, Gabel AA, Weisbrode S. Effect of subchondral drilling on repair of partial-thickness cartilage defects of third carpal bones in horses. Am J Vet Res. 1989;50(2):290-5. [PubMed] [Google Scholar]
- 35. Breinan HA, Martin SD, Hsu HP, Spector M. Healing of canine articular cartilage defects treated with microfracture, a type-II collagen matrix, or cultured autologous chondrocytes. J Orthop Res. 2000;18(5):781-9. [DOI] [PubMed] [Google Scholar]
- 36. Frisbie DD, Trotter GW, Powers BE, Rodkey WG, Steadman JR, Howard RD, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242-55. [DOI] [PubMed] [Google Scholar]
- 37. Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model: a preliminary study. J Bone Joint Surg Am. 2001;83A(1):53-64. [DOI] [PubMed] [Google Scholar]
- 38. Gill TJ, McCulloch PC, Glasson SS, Blanchet T, Morris EA. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005;33(5):680-5. [DOI] [PubMed] [Google Scholar]
- 39. Fortier LA, Potter HG, Rickey EJ, Schnabel LV, Foo LF, Chong LR, et al. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J Bone Joint Surg Am. 2010;92(10):1927-37. [DOI] [PubMed] [Google Scholar]
- 40. Vachon AM, McIlwraith CW, Keeley FW. Biochemical-study of repair of induced osteochondral defects of the distal portion of the radial carpal bone in horses by use of periosteal autografts. Am J Vet Res. 1991;52(2):328-32. [PubMed] [Google Scholar]
- 41. O’Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free autogenous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion: a follow-up report at one year. J Bone Joint Surg Am. 1988;70(4):595-606. [PubMed] [Google Scholar]
- 42. Hurtig MB, Fretz PB, Doige CE, Schnurr DL. Effects of lesion size and location on equine articular cartilage repair. Can J Vet Res. 1988;52(1):137-46. [PMC free article] [PubMed] [Google Scholar]
- 43. Hoemann CD, Hurtig M, Rossomacha E, Sun J, Chevrier A, Shive MS, Buschmann MD. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87A(12):2671-86. [DOI] [PubMed] [Google Scholar]
- 44. Hoemann CD, Chen G, Marchand C, Sun J, Tran-Khanh N, Chevrier A, et al. Scaffold-guided subchondral bone repair: implication of neutrophils and alternatively activated arginase-1+ macrophages. Am J Sports Med. 2010;38(9):1845-56. [DOI] [PubMed] [Google Scholar]
- 45. Driesang IM, Hunziker EB. Delamination rates of tissue flaps used in articular cartilage repair. J Orthop Res. 2000;18(6):909-11. [DOI] [PubMed] [Google Scholar]
- 46. Dell’Accio F, Vanlauwe J, Bellemans J, Neys J, De Bari C, Luyten FP. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J Orthop Res. 2003;21(1):123-31. [DOI] [PubMed] [Google Scholar]
- 47. Chen G, Sun J, Lascau-Coman V, Chevrier A, Marchand C, Hoemann CD. Acute osteoclast activity following subchondral drilling is promoted by chitosan and associated with improved cartilage tissue integration. Cartilage. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mainil-Varlet P, Rieser F, Grogan S, Mueller W, Saager C, Jakob RP. Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study. Osteoarthritis Cartilage. 2001;9:S6-15. [DOI] [PubMed] [Google Scholar]
- 49. Erggelet C, Neumann K, Endres M, Haberstroh K, Sittinger M, Kaps C. Regeneration of ovine articular cartilage defects by cell-free polymer-based implants. Biomaterials. 2007;28(36):5570-80. [DOI] [PubMed] [Google Scholar]
- 50. Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26(17):3617-29. [DOI] [PubMed] [Google Scholar]
- 51. Convery FR, Akeson WH, Keown GH. The repair of large osteochondral defects: an experimental study in horses. Clin Orthop Relat Res. 1972;82:253-62. [PubMed] [Google Scholar]
- 52. Chevrier A, Hoemann CD, Sun J, Buschmann MD. Chitosan-glycerol phosphate/blood implants increase cell recruitment, transient vascularization and subchondral bone remodeling in drilled cartilage defects. Osteoarthritis Cartilage. 2007;15(3):316-27. [DOI] [PubMed] [Google Scholar]
- 53. Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215-27. [DOI] [PubMed] [Google Scholar]
- 54. Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432-63. [DOI] [PubMed] [Google Scholar]
- 55. Rutgers M, van Pelt MJP, Dhert WJA, Creemers LB, Saris DBF. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. In press. [DOI] [PubMed] [Google Scholar]
- 56. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: a randomized trial. J Bone Joint Surg Am. 2004;86A(3):455-64. [DOI] [PubMed] [Google Scholar]
- 57. Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. J Bone Joint Surg Am. 2007;89A(10):2105-12. [DOI] [PubMed] [Google Scholar]
- 58. Brun P, Dickinson S, Zavan B, Cortivo R, Hollander A, Abatangelo G. Characteristics of repair tissue in second-look and third-look biopsies from patients treated with engineered cartilage: relationship to symptomatology and time after implantation. Arthritis Res Ther. 2008;10(6):R132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gikas PD, Morris T, Carrington R, Skinner J, Bentley G, Briggs T. A correlation between the timing of biopsy after autologous chondrocyte implantation and the histological appearance. J Bone Joint Surg Br. 2009;91B(9):1172-7. [DOI] [PubMed] [Google Scholar]
- 60. Mainil-Varlet P, van Damme B, Nesic D, Knutsen G, Kandel R, Roberts S. A new histology scoring system for the assessment of the quality of human cartilage repair: ICRS II. Am J Sports Med. 2010;38(5):880-90. [DOI] [PubMed] [Google Scholar]
- 61. Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235-46. [DOI] [PubMed] [Google Scholar]
- 62. Briggs TWR, Mahroof S, David LA, Flannelly DJ, Pringle J, Bayliss M. Histological evaluation of chondral defects after autologous chondrocyte implantation of the knee. J Bone Joint Surg Br. 2003;85B(7):1077-83. [DOI] [PubMed] [Google Scholar]
- 63. Frisbie DD, Morisset S, Ho CP, Rodkey WG, Steadman JR, McIlwraith CW. Effects of calcified cartilage on healing of chondral defects treated with microfracture in horses. Am J Sports Med. 2006;34(11):1824-31. [DOI] [PubMed] [Google Scholar]
- 64. Insall JN. Intra-articular surgery for degenerative arthritis of the knee: a report of the work of the late K. H. Pridie. J Bone Joint Surg Br. 1967;49(2):211-28. [PubMed] [Google Scholar]
- 65. Ficat RP, Ficat C, Gedeon P, Toussaint JB. Spongialization: a new treatment for diseased patellae. Clin Orthop Relat Res. 1979;144:74-83. [PubMed] [Google Scholar]
- 66. Bouwmeester P, Kuijer R, Terwindt-Rouwenhorst E, van der Linden T, Bulstra K. Histological and biochemical evaluation of perichondrial transplants in human articular cartilage defects. J Orthop Res. 1999;17(6):843-9. [DOI] [PubMed] [Google Scholar]
- 67. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop. 1999;365:149-62. [DOI] [PubMed] [Google Scholar]
- 68. Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS). J Bone Joint Surg Am. 2003;85A:45-57. [PubMed] [Google Scholar]
- 69. Roberts S, Menage J, Sandell LJ, Evans EH, Richardson JB. Immunohistochemical study of collagen types I and II and procollagen IIA in human cartilage repair tissue following autologous chondrocyte implantation. Knee. 2009;16(5):398-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hollander AP, Dickinson SC, Sims TJ, Brun P, Cortivo R, Kon E, et al. Maturation of tissue engineered cartilage implanted in injured and osteoarthritic human knees. Tissue Eng. 2006;12(7):1787-98. [DOI] [PubMed] [Google Scholar]
- 71. ClinicalTrials. A Randomized, Comparative Multicenter Clinical Trial Evaluating BST-CarGel™ and Microfracture in Repair of Focal Articular Cartilage Lesions on the Femoral Condyle. Montreal, Canada: NCT00314236 CgI; 2007. Available from: http://clinicaltrials.gov/ct2/show/NCT00314236. [Google Scholar]
- 72. Moriya T, Wada Y, Watanabe A, Sasho T, Nakagawa K, Mainil-Varlet P, Moriya H. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12(3):265-73. [DOI] [PubMed] [Google Scholar]
- 73. Peterfy CG, Guermazi A, Zaim S, Tirman P.FJ, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-90. [DOI] [PubMed] [Google Scholar]
- 74. Mithoefer K, Williams RJ, 3rd, Warren RF, Potter HG, Spock CR, Jones EC, et al. The microfracture technique for the treatment of articular cartilage lesions in the knee: a prospective cohort study. J Bone Joint Surg Am. 2005;87(9):1911-20. [DOI] [PubMed] [Google Scholar]
- 75. Watanabe A, Wada Y, Obata T, Ueda T, Tamura M, Ikehira H, Moriya H. Delayed gadolinium-enhanced MR to determine glycosaminoglycan concentration in reparative cartilage after autologous chondrocyte implantation: preliminary results. Radiology. 2006;239(1):201-8. [DOI] [PubMed] [Google Scholar]
- 76. Tins BJ, McCall IW, Takahashi T, Cassar-Pullicino V, Roberts S, Ashton B, Richardson J. Autologous chondrocyte implantation in knee joint: MR imaging and histologic features at 1-year follow-up. Radiology. 2005;234(2):501-8. [DOI] [PubMed] [Google Scholar]
- 77. Mithoefer K, Saris DBF, Farr J, Kon E, Zaslav K, Cole BJ, et al. Guidelines for the design and conduct of clinical studies in knee articular cartilage repair: international cartilage repair society recommendations based on current scientific evidence and standards of clinical care. Cartilage 2(2). DOI: 10.1177/1947603510392913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Trattnig S, Winalski CS, Marlovits S, Jurvelin JS, Welsch GH, Potter HG. Magnetic resonance imaging of cartilage repair: a review. Cartilage 2011;2:5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 80. Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7(4):300-4. [Google Scholar]
- 81. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 82. Shive MS, Hoemann CD, Restrepo A, Hurtig MB, Duval N, Ranger P, et al. BST-CarGel: in situ chondroinduction for cartilage repair. Oper Tech Orthop. 2006;16(4):271-8. [Google Scholar]
- 83. Henderson I, Francisco R, Oakes B, Cameron J. Autologous chondrocyte implantation for treatment of focal chondral defects of the knee: a clinical, arthroscopic, MRI and histologic evaluation at 2 years. Knee. 2005;12(3):209-16. [DOI] [PubMed] [Google Scholar]
- 84. Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, et al. Articular cartilage engineering with Hyalograft (R) C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96-105. [DOI] [PubMed] [Google Scholar]
- 85. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am. 2003;85(Suppl 2):17. [DOI] [PubMed] [Google Scholar]
- 86. Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253-61. [DOI] [PubMed] [Google Scholar]
- 87. Vasara AI, Hyttinen MM, Lammi MJ, Lammi PE, Langsjo TK, Lindahl A, et al. Subchondral bone reaction associated with chondral defect and attempted cartilage repair in goats. Calcif Tissue Int. 2004;74(1):107-14. [DOI] [PubMed] [Google Scholar]
- 88. Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of.large, full-thickness defects of articular-cartilage. J Bone Joint Surg Am. 1994;76A(4):579-92. [DOI] [PubMed] [Google Scholar]
- 89. Hoemann CD, Sun J, McKee MD, Chevrier A, Rossomacha E, Rivard GE, et al. Chitosan-glycerol phosphate/blood implants elicit hyaline cartilage repair integrated with porous subchondral bone in microdrilled rabbit defects. Osteoarthritis Cartilage. 2007;15(1):78-89. [DOI] [PubMed] [Google Scholar]
- 90. Brittberg M, Nilsson A, Lindahl A, Ohlsson C, Peterson L. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;326:270-83. [DOI] [PubMed] [Google Scholar]
- 91. Ahern BJ, Parvizi J, Boston R, Schaer TP. Preclinical animal models in single site cartilage defect testing: a systematic review. Osteoarthritis Cartilage. 2009;17(6):705-13. [DOI] [PubMed] [Google Scholar]
- 92. Frisbie DD, Cross MW, McIlwraith CW.A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19(3):142-6. [PubMed] [Google Scholar]
- 93. Temple MM, Bae WC, Chen MQ, Lotz M, Amiel D, Coufts RD, Sah RL. Age- and site-associated biomechanical weakening of human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2007;15(9):1042-52. [DOI] [PubMed] [Google Scholar]
- 94. Nixon AJ, Fortier LA, Williams J, Mohammed H. Enhanced repair of extensive articular defects by insulin-like growth factor-I-laden fibrin composites. J Orthop Res. 1999;17(4):475-87. [DOI] [PubMed] [Google Scholar]
- 95. Breinan HA, Minas T, Hsu HP, Nehrer S, Sledge CB, Spector M. Effect of cultured autologous chondrocytes on repair of chondral defects in a canine model. J Bone Joint Surg Am. 1997;79(10):1439-51. [DOI] [PubMed] [Google Scholar]
- 96. Roberts S, McCall I, Darby A, Menage J, Evans H, Harrison P, Richardson J. Autologous chondrocyte implantation for cartilage repair: monitoring its success by magnetic resonance imaging and histology. Arthritis Res Ther. 2003;5(1):R60-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. J Bone Joint Surg Am. 2003;85A(2):185-92. [DOI] [PubMed] [Google Scholar]
- 98. Saris DBF, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, Luyten FP. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37:10S-19S. [DOI] [PubMed] [Google Scholar]
- 99. Bartlett W, Skinner JA, Gooding CR, Carrington RWJ, Flanagan AM, Briggs TWR, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee. J Bone Joint Surg Br. 2005;87B(5):640-5. [DOI] [PubMed] [Google Scholar]
- 100. Hendrickson DA, Nixon AJ, Grande DA, Todhunter RJ, Minor RM, Erb H, Lust G. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular-cartilage defects. J Orthop Res. 1994;12(4):485-97. [DOI] [PubMed] [Google Scholar]
- 101. Hurtig M, Buschmann MD, Fortier L, Hoemann CD, Hunziker EB, Jurvelin JS, et al. Pre-Clinical Studies for Cartilage Repair: Recommendations from the International Cartilage Repair Society. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pineda S, Pollack A, Stevenson S, Goldberg V, Caplan A. A semiquantitative scale for histologic grading of articular-cartilage repair. Acta Anatomica. 1992;143(4):335-40. [DOI] [PubMed] [Google Scholar]
- 103. Selmi TAS, Verdonk P, Chambat P, Dubrana F, Potel JF, Barnouin L, Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90-B(5):597-604. [DOI] [PubMed] [Google Scholar]
- 104. Henderson IJP, Tuy B, Connell D, Oakes B, Hettwer WH. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85B(7):1060-6. [DOI] [PubMed] [Google Scholar]
- 105. Kristensen HK. An improved method of decalcification. Stain Technol. 1948;23(3):151-4. [DOI] [PubMed] [Google Scholar]
- 106. Hunziker EB, Michel M, Studer D. Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech. 1997;37(4):271-84. [DOI] [PubMed] [Google Scholar]
- 107. Chevrier A, Rossomacha E, Buschmann MD, Hoemann CD. Optimization of histoprocessing methods to detect glycosaminoglycan, collagen type II, and collagen type I in decalcified rabbit osteochondral sections. J Histotechnol. 2005;28(3):165-75. [Google Scholar]
- 108. Rosen AD. End-point determination in EDTA decalcification using ammonium oxalate. Stain Technol. 1981;56(1):48-9. [DOI] [PubMed] [Google Scholar]
- 109. Qiu YS, Shahgaldi BF, Revell WJ, Heatley FW. Observations of subchondral plate advancement during osteochondral repair: a histomorphometric and mechanical study in the rabbit femoral condyle. Osteoarthritis Cartilage. 2003;11(11):810-20. [DOI] [PubMed] [Google Scholar]
- 110. Hoemann CD, Sun J, Legare A, McKee MD, Buschmann MD. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthritis Cartilage. 2005;13(4):318-29. [DOI] [PubMed] [Google Scholar]
- 111. Niederauer GG, Slivka MA, Leatherbury NC, Korvick DL, Harroff HH, Ehler WC, et al. Evaluation of multiphase implants for repair of focal osteochondral defects in goats. Biomaterials. 2000;21(24):2561-74. [DOI] [PubMed] [Google Scholar]
- 112. Griffiths G. Quantitative aspects of immunocytochemistry: Estimation of volume density and surface density in practice. In: Griffiths G, editor. Fine structure immunocytochemistry. 377-383 Berlin: Springer-Verlag; 1993. [Google Scholar]
- 113. Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69-82. [PubMed] [Google Scholar]
- 114. Bulstra SK, Drukker J, Kuijer R, Buurman WA, Vanderlinden AJ. Thionin staining of paraffin and plastic embedded sections of cartilage. Biotech Histochem. 1993;68(1):20-8. [DOI] [PubMed] [Google Scholar]
- 115. Hoemann CD, Tran-Khanh N, Lascau-Coman V, Garon M, Chen H, Jarry C, et al. Quantitative histomorphometry of collagen types I & II and safranin-O in human osteochondral biopsies. Miami: 2009 Conference Proceedings of the International Cartilage Repair Society. [Google Scholar]
- 116. Zhu Y, Oganesian A, Keene DR, Sandell LJ. Type IIA procollagen containing the cysteine-rich amino propeptide is deposited in the extracellular matrix of prechondrogenic tissue and binds to TGF-beta 1 and BMP-2. J Cell Biol. 1999;144(5):1069-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Roberts S, Hollander AP, Caterson B, Menage J, Richardson JB. Matrix turnover in human cartilage repair tissue in autologous chondrocyte implantation. Arthritis Rheum. 2001;44(11):2586-98. [DOI] [PubMed] [Google Scholar]
- 118. Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, et al. Microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180-6. [DOI] [PubMed] [Google Scholar]
- 119. Temple-Wong MM, Bae WC, Chen MQ, Bugbee WD, Amiel D, Coutts RD, et al. Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage. 2009;17(11):1469-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. von Rechenberg B, Akens MK, Nadler D, Bittmann P, Zlinszky K, Kutter A, et al. Changes in subchondral bone in cartilage resurfacing: an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11(4):265-77. [DOI] [PubMed] [Google Scholar]
- 121. Méthot S, Hoemann CD, Rossomacha E, Restrepo A, Stanish WD, MacDonald P, et al. ICRS histology scores of biopsies from an interim analysis of a randomized controlled clinical trial show significant improvement in tissue quality at 13 months for BST-CarGel versus microfracture. Barcelona: 2010 Conference Proceedings of the International Cartilage Repair Society. [Google Scholar]
- 122. Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420-8. [DOI] [PubMed] [Google Scholar]
- 123. Roos EM, Engelhart L, Ranstam J, Anderson AF, Irrgang JJ, Marx RG, Tegner Y, Davis AM. ICRS recommendation document: patient-reported outcome instruments for use in patients with articular cartilage defects. Cartilage 1947603510391084, first published on January 25, 2011 as 10.1177/1947603510391084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. O’Driscoll SW, Marx RG, Beaton DE, Miura Y, Gallay SH, Fitzsimmons JS. Validation of a simple histological- histochemical cartilage scoring system. Tissue Eng. 2001;7(3):313-20. [DOI] [PubMed] [Google Scholar]
- 125. Igarashi T, Iwasaki N, Kasahara Y, Minami A. A cellular implantation system using an injectable ultra-purified alginate gel for repair of osteochondral defects in a rabbit model. J Biomed Mater Res A. 2010;94A(3):844-55. [DOI] [PubMed] [Google Scholar]
- 126. Hoemann CD, Tran-Khanh N, Méthot S, Chen G, Marchand C, Lascau-Coman V, et al. Correlation of tissue histomorphometry with ICRS histology scores in biopsies obtained from a randomized controlled clinical trial comparing BST-CarGel versus microfracture. Barcelona: 2010 Conference Proceedings of the International Cartilage Repair Society. [Google Scholar]
- 127. Minas T, Gomoll AH, Rosenberger R, Royce RO, Bryant T. Increased failure rate of autologous chondrocyte implantation after previous treatment with marrow stimulation techniques. Am J Sports Med. 2009;37:902-8. [DOI] [PubMed] [Google Scholar]
- 128. Rieppo J, Toyras J, Nieminen MT, Kovanen V, Hyttinen MM, Korhonen RK, et al. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs. 2003;175(3):121-32. [DOI] [PubMed] [Google Scholar]


