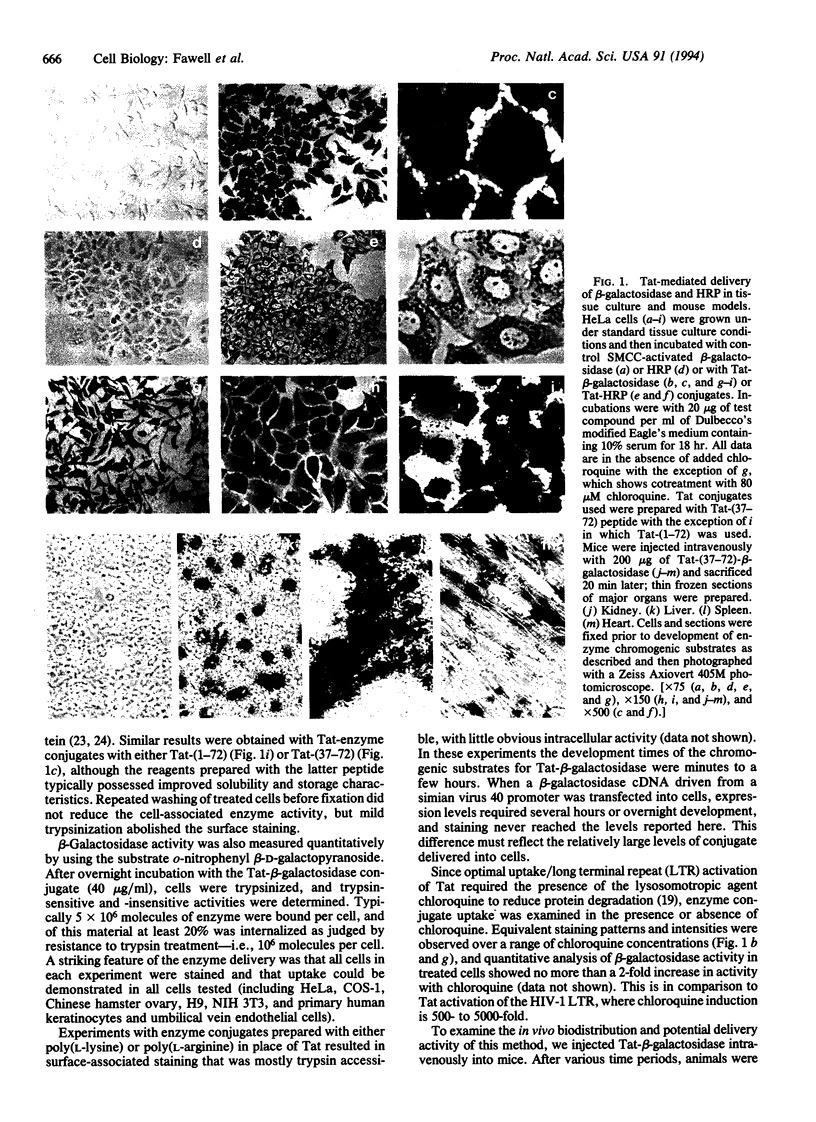
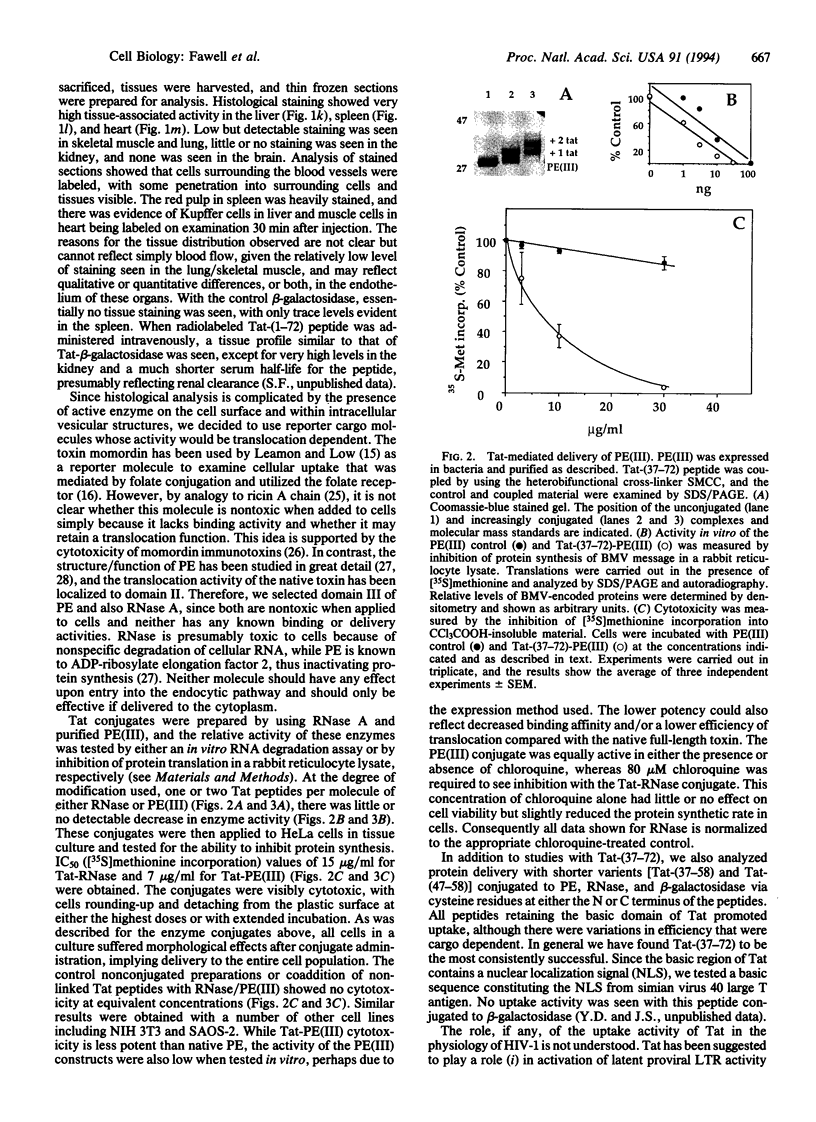
Abstract
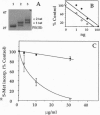
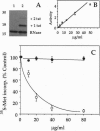
The Tat protein of human immunodeficiency virus 1 (HIV-1) can enter cells efficiently when added exogenously in tissue culture. To assess if Tat can carry other molecules into cells, we chemically cross-linked Tat peptides (residues 1-72 or 37-72) to beta-galactosidase, horseradish peroxidase, RNase A, and domain III of Pseudomonas exotoxin A (PE) and monitored uptake colorimetrically or by cytotoxicity. The Tat chimeras were effective on all cell types tested, with staining showing uptake into all cells in each experiment. In mice, treatment with Tat-beta-galactosidase chimeras resulted in delivery to several tissues, with high levels in heart, liver, and spleen, low-to-moderate levels in lung and skeletal muscle, and little or no activity in kidney and brain. The primary target within these tissues was the cells surrounding the blood vessels, suggesting endothelial cells, Kupffer cells, and/or splenic macrophages. Tat-mediated uptake may allow the therapeutic delivery of macromolecules previously thought to be impermeable to living cells.
Full text
PDF

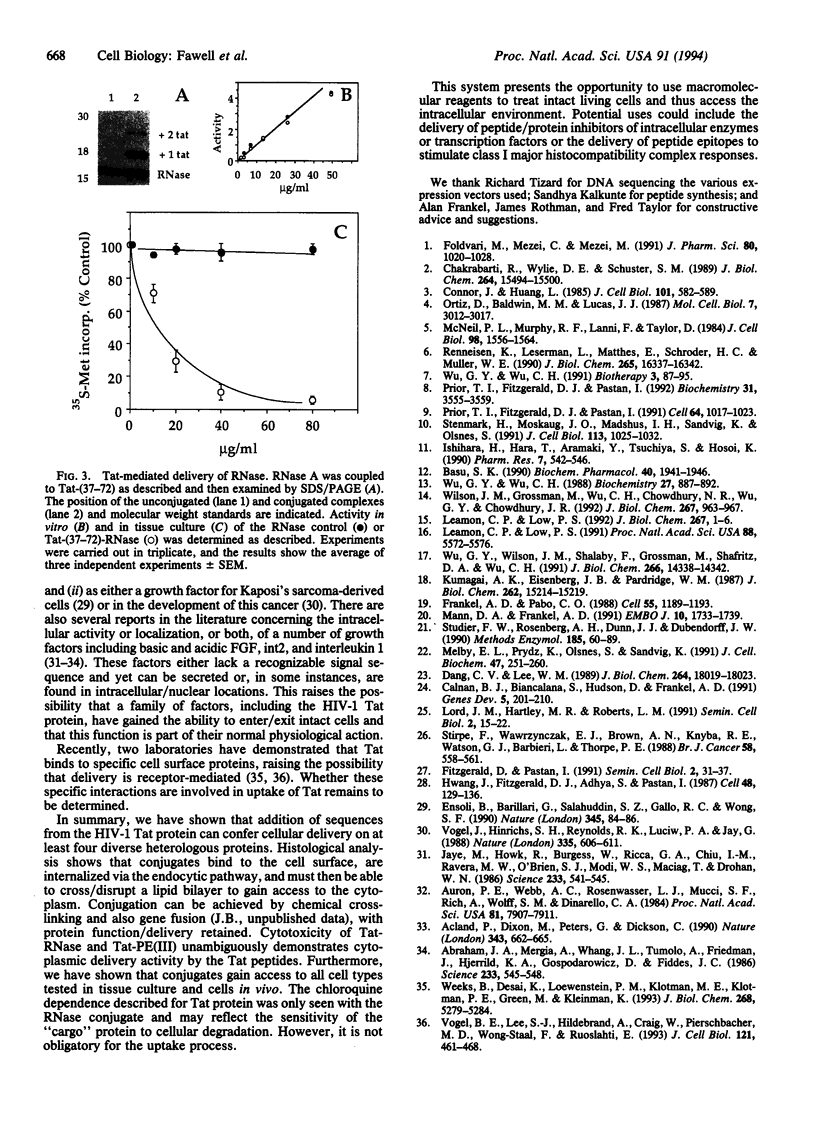
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K. Receptor-mediated endocytosis of macromolecular conjugates in selective drug delivery. Biochem Pharmacol. 1990 Nov 1;40(9):1941–1946. doi: 10.1016/0006-2952(90)90222-7. [DOI] [PubMed] [Google Scholar]
- Calnan B. J., Biancalana S., Hudson D., Frankel A. D. Analysis of arginine-rich peptides from the HIV Tat protein reveals unusual features of RNA-protein recognition. Genes Dev. 1991 Feb;5(2):201–210. doi: 10.1101/gad.5.2.201. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R., Wylie D. E., Schuster S. M. Transfer of monoclonal antibodies into mammalian cells by electroporation. J Biol Chem. 1989 Sep 15;264(26):15494–15500. [PubMed] [Google Scholar]
- Connor J., Huang L. Efficient cytoplasmic delivery of a fluorescent dye by pH-sensitive immunoliposomes. J Cell Biol. 1985 Aug;101(2):582–589. doi: 10.1083/jcb.101.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, HSP70, and HIV tat proteins. J Biol Chem. 1989 Oct 25;264(30):18019–18023. [PubMed] [Google Scholar]
- Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., Wong-Staal F. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature. 1990 May 3;345(6270):84–86. doi: 10.1038/345084a0. [DOI] [PubMed] [Google Scholar]
- FitzGerald D., Pastan I. Redirecting Pseudomonas exotoxin. Semin Cell Biol. 1991 Feb;2(1):31–37. [PubMed] [Google Scholar]
- Foldvari M., Mezei C., Mezei M. Intracellular delivery of drugs by liposomes containing P0 glycoprotein from peripheral nerve myelin into human M21 melanoma cells. J Pharm Sci. 1991 Nov;80(11):1020–1028. doi: 10.1002/jps.2600801105. [DOI] [PubMed] [Google Scholar]
- Frankel A. D., Pabo C. O. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988 Dec 23;55(6):1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Hara T., Aramaki Y., Tsuchiya S., Hosoi K. Preparation of asialofetuin-labeled liposomes with encapsulated human interferon-gamma and their uptake by isolated rat hepatocytes. Pharm Res. 1990 May;7(5):542–546. doi: 10.1023/a:1015833220179. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Khorana H. G. Rhodopsin, photoreceptor of the rod cell. An emerging pattern for structure and function. J Biol Chem. 1992 Jan 5;267(1):1–4. [PubMed] [Google Scholar]
- Kumagai A. K., Eisenberg J. B., Pardridge W. M. Absorptive-mediated endocytosis of cationized albumin and a beta-endorphin-cationized albumin chimeric peptide by isolated brain capillaries. Model system of blood-brain barrier transport. J Biol Chem. 1987 Nov 5;262(31):15214–15219. [PubMed] [Google Scholar]
- Leamon C. P., Low P. S. Delivery of macromolecules into living cells: a method that exploits folate receptor endocytosis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5572–5576. doi: 10.1073/pnas.88.13.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Hartley M. R., Roberts L. M. Ribosome inactivating proteins of plants. Semin Cell Biol. 1991 Feb;2(1):15–22. [PubMed] [Google Scholar]
- Mann D. A., Frankel A. D. Endocytosis and targeting of exogenous HIV-1 Tat protein. EMBO J. 1991 Jul;10(7):1733–1739. doi: 10.1002/j.1460-2075.1991.tb07697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melby E. L., Prydz K., Olsnes S., Sandvig K. Effect of monensin on ricin and fluid phase transport in polarized MDCK cells. J Cell Biochem. 1991 Nov;47(3):251–260. doi: 10.1002/jcb.240470311. [DOI] [PubMed] [Google Scholar]
- Ortiz D., Baldwin M. M., Lucas J. J. Transient correction of genetic defects in cultured animal cells by introduction of functional proteins. Mol Cell Biol. 1987 Aug;7(8):3012–3017. doi: 10.1128/mcb.7.8.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior T. I., FitzGerald D. J., Pastan I. Barnase toxin: a new chimeric toxin composed of pseudomonas exotoxin A and barnase. Cell. 1991 Mar 8;64(5):1017–1023. doi: 10.1016/0092-8674(91)90325-s. [DOI] [PubMed] [Google Scholar]
- Prior T. I., FitzGerald D. J., Pastan I. Translocation mediated by domain II of Pseudomonas exotoxin A: transport of barnase into the cytosol. Biochemistry. 1992 Apr 14;31(14):3555–3559. doi: 10.1021/bi00129a001. [DOI] [PubMed] [Google Scholar]
- Renneisen K., Leserman L., Matthes E., Schröder H. C., Müller W. E. Inhibition of expression of human immunodeficiency virus-1 in vitro by antibody-targeted liposomes containing antisense RNA to the env region. J Biol Chem. 1990 Sep 25;265(27):16337–16342. [PubMed] [Google Scholar]
- Stenmark H., Moskaug J. O., Madshus I. H., Sandvig K., Olsnes S. Peptides fused to the amino-terminal end of diphtheria toxin are translocated to the cytosol. J Cell Biol. 1991 Jun;113(5):1025–1032. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Wawrzynczak E. J., Brown A. N., Knyba R. E., Watson G. J., Barbieri L., Thorpe P. E. Selective cytotoxic activity of immunotoxins composed of a monoclonal anti-Thy 1.1 antibody and the ribosome-inactivating proteins bryodin and momordin. Br J Cancer. 1988 Nov;58(5):558–561. doi: 10.1038/bjc.1988.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Vogel B. E., Lee S. J., Hildebrand A., Craig W., Pierschbacher M. D., Wong-Staal F., Ruoslahti E. A novel integrin specificity exemplified by binding of the alpha v beta 5 integrin to the basic domain of the HIV Tat protein and vitronectin. J Cell Biol. 1993 Apr;121(2):461–468. doi: 10.1083/jcb.121.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J., Hinrichs S. H., Reynolds R. K., Luciw P. A., Jay G. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature. 1988 Oct 13;335(6191):606–611. doi: 10.1038/335606a0. [DOI] [PubMed] [Google Scholar]
- Weeks B. S., Desai K., Loewenstein P. M., Klotman M. E., Klotman P. E., Green M., Kleinman H. K. Identification of a novel cell attachment domain in the HIV-1 Tat protein and its 90-kDa cell surface binding protein. J Biol Chem. 1993 Mar 5;268(7):5279–5284. [PubMed] [Google Scholar]
- Wilson J. M., Grossman M., Wu C. H., Chowdhury N. R., Wu G. Y., Chowdhury J. R. Hepatocyte-directed gene transfer in vivo leads to transient improvement of hypercholesterolemia in low density lipoprotein receptor-deficient rabbits. J Biol Chem. 1992 Jan 15;267(2):963–967. [PubMed] [Google Scholar]
- Wu G. Y., Wilson J. M., Shalaby F., Grossman M., Shafritz D. A., Wu C. H. Receptor-mediated gene delivery in vivo. Partial correction of genetic analbuminemia in Nagase rats. J Biol Chem. 1991 Aug 5;266(22):14338–14342. [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Delivery systems for gene therapy. Biotherapy. 1991;3(1):87–95. doi: 10.1007/BF02175102. [DOI] [PubMed] [Google Scholar]
- Wu G. Y., Wu C. H. Evidence for targeted gene delivery to Hep G2 hepatoma cells in vitro. Biochemistry. 1988 Feb 9;27(3):887–892. doi: 10.1021/bi00403a008. [DOI] [PubMed] [Google Scholar]