Abstract
Over the past two decades, regenerative therapies using stem cell technologies have been developed for various neurological diseases. Although stem cell therapy is an attractive option to reverse neural tissue damage and to recover neurological deficits, it is still under development so as not to show significant treatment effects in clinical settings. In this review, we discuss the scientific and clinical basics of adult neural stem cells (aNSCs), and their current developmental status as cell therapeutics for neurological disease. Compared with other types of stem cells, aNSCs have clinical advantages, such as limited proliferation, inborn differentiation potential into functional neural cells, and no ethical issues. In spite of the merits of aNSCs, difficulties in the isolation from the normal brain, and in the in vitro expansion, have blocked preclinical and clinical study using aNSCs. However, several groups have recently developed novel techniques to isolate and expand aNSCs from normal adult brains, and showed successful applications of aNSCs to neurological diseases. With new technologies for aNSCs and their clinical strengths, previous hurdles in stem cell therapies for neurological diseases could be overcome, to realize clinically efficacious regenerative stem cell therapeutics.
Keywords: Adult neural stem cell, Neurological diseases, Stem cell therapy, Preclinical trial, Clinical trial
Core tip: In this review, we compare advantages and disadvantages of various types of stem cells for regenerative therapy in neurological disease, and discuss the preclinical and clinical developmental hurdles of stem cell technologies. While at present, adult neural stem cells (aNSCs) have clinical advantages, technical issues in the isolation and expansion of aNSCs prevent active preclinical and clinical applications of aNSCs. However, several papers have recently reported scientific breakthroughs, on the basis of which broad application trials using aNSCs could be performed. In this review, we also summarize the current status of preclinical and clinical applications of aNSCs for various neurological diseases.
STEM CELL THERAPY FOR NEUROLOGICAL DISEASES
Neurological diseases are derived from the loss of functional neurons in the central nervous system (CNS). Although acute localized neurodegeneration could result from a temporal localized injury, such as stroke and trauma, chronic neurodegeneration usually develops over a long period of time, and has unclear multifactorial causes. Functional neurological deficits in chronic neurological diseases originate from either loss of a specific neuronal subtype, or universal brain damage. Alzheimer’s and Huntington’s diseases result in non-specific death of neurons in the brain, whereas Parkinson’s disease is characterized by the specific and localized damage of dopaminergic neurons located in the substantia nigra. In the brain and spinal cord, amyotrophic lateral sclerosis (ALS) and traumatic spinal cord injury induce diffuse motor neuronal loss and localized nonspecific neural tissue damage, respectively. Although these neurodegenerative conditions have unique morphological pathologies, the molecular mechanisms for the neuronal death are complicated and ambiguous, making the development of mechanism-based therapeutic modalities elusive. Since functional loss of neural cells is the common final pathway of various neurological diseases, regardless of specific etiologies, regenerative treatment using stem cells that could repair damaged neural tissue is a viable and non-specific therapeutic option.
TYPES OF STEM CELLS AND THEIR APPLICATIONS TO NEUROLOGICAL DISEASES
Stem cells have two important characteristics: proliferation capacity, and differentiation potential into multiple cellular lineages. According to the source, stem cells can be classified into embryonic, fetal, and adult stem cells (ESCs, FSCs, and ASCs, respectively). Pluripotent ESCs are obtained from the blastocyst of fertilized egg[1]. ESCs proliferate robustly and have multi-potent differentiation potentials into three germ layer cells, which consist of the whole body[2]. However, ethical concerns[3-5], and risks of adverse effects, such as immune rejection and tumor formation[6] prevent their clinical applications. Recently, it was reported that somatic cells could be reprogrammed into pluripotent state, by overexpression of Oct4, Sox2, Klf4, and c-Myc[7,8]. Although these induced pluripotent stem cells (iPSCs) maintain the merits of ESCs, iPSCs still have limitations, such as low efficient generation, and the formation of teratomas or tumors in vivo. These critical limitations provoke hesitation in the use of ESCs and iPSCs as clinical therapies. As other sources of stem cells, fetal organs that contain FSCs have been suggested. In spite of the advantages of FSCs, including proliferation capacity, limited differentiation potential, and lack of teratoma formation[9], ethical problems of using fetal tissues still remain.
ASCs are classically defined as multi-potent cells that originate from various tissues within the adult body, including the bone marrow, skeletal muscle, central nervous system, and adipose tissue[10,11]. The important benefit of ASCs is possible autologous transplantation, in which stem cells can be primarily cultured from and applied to the same patient. This benefit bypasses the ethical problems that ESCs and FSCs harbour. However, in spite of these advantages of ASCs, their limited differentiation and proliferation ability interrupts their widespread use.
Therefore, in the current status, technical and ethical considerations indicate that compared with other stem cells, ASCs are the most clinically applicable.
ADULT NEURAL STEM CELLS FOR NEUROLOGICAL DISEASES
Among various ASCs, mesenchymal stem cells (MSCs) are the most widely used, and furthest progressed in preclinical and clinical trials[12]. The strengths of MSCs are relatively simple isolation, and in vitro expansion techniques. However, there are concerns about the clinical applications of MSCs[13]. First of all, in the culture methods of MSCs, bovine serum should be used. Because the dangers of bovine serum have not been well characterized, potential risks in clinical applications still exist[14]. Although xeno-free culture methods for MSCs have been developed, their quality needs to further study. Moreover, many previous studies suggested that the beneficial effects of MSCs for neurological diseases might originate from their paracrine effects involving immune modulation and/or secretory growth factors, and not from direct neuroregenerative effects producing functional neural cells[15-17].
Compared to MSCs, NSCs are cultivated and expanded in media containing low, or no bovine serum[18-22]. Many preclinical studies using NSCs suggest that NSCs not only have beneficial paracrine effects in the regeneration and repair of neural tissue, but also direct differentiation potential into diverse neuronal lineages, to form networks with surrounding neuronal cells[23-25]. Since the ultimate goal of regenerative treatment for neurodegenerative diseases is the functional repair of damaged neural tissues, NSCs seem to be a more optimal choice for neurological diseases.
Adult NSCs are tissue-resident multi-potent neural progenitor cells that have self-renewal capacity, so long as they can be maintained undifferentiated. NSCs have the potential, under appropriate culture conditions, to differentiate into multiple neural cells, such as neurons, astrocytes, and oligodendrocytes. NSCs are observed in the developmental stage and mature CNS of mammalian species[26-29], specifically in the subventricular (SVZ) and subgranular zones (SGZ)[30-32]. The neurogenic niche surrounding SVZ and SGZ represents a unique microenvironment that regulates the survival and differentiation of NSCs[28,33].
TECHNICAL HURDLES AND RECENT BREAKTHROUGHS IN THE USE OF ADULT NEURAL STEM CELLS FOR NEUROLOGICAL DISEASES
Depending on the types of neurological diseases, undifferentiated NSCs themselves, or differentiated neural cells, have been applied to verify their efficacy in preclinical animal models. However, in vitro expansion of differentiated neural cells to acquire the necessary amount of cells for transplantation is very difficult, because differentiated cells cannot proliferate well. Therefore, regardless of transplantation cell types, aNSCs first need to be properly isolated, and effectively expanded in vitro. Compared with other stem cells, such as ESCs, fetal NSCs, and MSCs, aNSCs reside in restricted areas of the adult CNS[31,32], and have limited capacity to proliferate[34,35]. Therefore, difficulties in the primary isolation and stable in vitro expansion of aNSCs are major technical obstacles to be resolved, for the utilization of aNSCs.
Up to now, several research teams have addressed these difficulties, using various scientific and technical approaches. Surgical samples from the adult CNS are usually very small (1-2 mL). As the number of resident aNSCs within the tissue is also very small, isolation techniques have been optimized to increase the success rate of the primary isolation of aNSCs. To acquire aNSCs, CNS tissues are physically minced, and enzymatically digested into single cells. Among them, the enzymatic digestion is a critical step, because it directly affects the survival of aNSCs. The compositions of dissociating enzymes and incubation times are various among investigators. Papain, trypsin, and collagenase have usually been used, and in some reports, papain dissociation was suggested to be most optimal for the primary isolation of aNSCs[36,37].
After the mechanical and enzymatic dissociation of CNS tissues, the resulting single cells have been cultured by two alternative methods: the neurosphere, and adherent culture methods. Conventionally, the neurosphere culture method has been used for in vitro culture of NSCs[38-47]. This method was first used in the primary isolation of NSCs from murine brains. The neurosphere culture method was also applied to maintain aNSCs from human brains. However, difficulties in the stable in vitro expansion of aNSCs using suspension culture methods resulted in the need for another culture method to be developed. Moreover, a single neurosphere may not be derived from a single NSC[48]. The possible heterogenic origin of neurospheres could not guarantee the homogeneity of in vitro expanded aNSCs in the suspension culture conditions[49-51].
To overcome the weak points of the neurosphere culture method, others, as well as ourselves, developed alternative adherent culture methods for NSCs[18-21,44,52-54]. Each group used their own coating plates to attach NSCs to the plates, and various culture medium compositions. Laminin and poly-L-ornithine (PLO) have frequently been used to coat plates, which increase the adherent efficiency of NSCs. To maintain stemness and proliferation of NSCs, the amount of EGF and basic FGF have been optimized[55]. For example, we expanded aNSCs from temporal lobectomy samples of epilepsy patients without any neoplasmic diseases, on PLO-coated plates in a DMEM/F12 media supplemented with 1% B27, 1% penicillin/streptomycin, EGF (50 ng/mL), bFGF (50 ng/mL), and 0.5% fetal bovine serum (Table 1)[18]. Using the adherent culture method, aNSCs were expanded in vitro from 104 to 1012 cells within 8 subcultures for 2 mo. Moreover, the expression of Nestin and Sox2 as NSC markers was stably maintained[18]. If the number of aNSCs required for transplantation is 107 per patient, at least one hundred thousand patients could be treated with a primary culture of aNSCs.
Table 1.
Isolation and in vitro culture methods for adult neural stem cells
| Culture methods | Cell source | Dissociating method | Media composition | Plate coating | Maximal in vitro culture | Ref. |
| Adherent culture method | Temporal lobe | Physical Mincing and enzymatic digestion with papain | DMEM/F12 supplemented with 10 ng/mL bFGF, 20 ng/mL TGFα, 2.5 μg/mL heparin, 2% B27 (without retinoic acid), 10 mmol/L hepes, and 1% FBS | [54] | ||
| Temporal lobe | Mechanical trituration and enzymatic dissociation using papain and DNase I | DMEM/F12 supplemented with 1% B27, 50 ng/mL EGF, 50 ng/mL bFGF, and 0.5% FBS | Poly-L-ornithine | 18 passages | [18] | |
| Neurosphere culture method | Hippocampal and lateral ventricle wall tissue | Mechanical dissociation and enzyme digestion using hyaluronic acid, kynurenic acid, and trypsin | DMEM/F12 supplemented with 10 ng/mL EGF, 20 ng/mL EGF, B27, and 2 mmol/L glutamine | [39] | ||
| Temporal lobe | Enzymic digestion with trypsin | N2 medium supplemented with 5% FBS | Poly-2-hydroxyethyl methacrylate | [40] | ||
| Hippocampus Amygdala Frontal cortex Temporal cortex | Enzymic digestion with hyaluronidase, kynurenic acid, and trypsin | DMEM/F12 supplemented with 0.6% glucose, 2 mmol/L glutamine, 3 mmol/L sodium bicarbonate, 5 mmol/L HEPES buffer, 25 mg/mL insulin, 10 mg/mL heparan sulfate, 100 mg/mL transferrin, 20 nmol/L progesterone, 60 mmol/L putrescine, 30 nmol/L selenium chloride, 20 ng/mL EGF, and 20 ng/mL bFGF-2 | [41] | |||
| Temporal lobe from 11-wk-old postnatal male Hippocampus, ventricular zone, motor cortex and corpus callosum from and a 27-year-old male | Enzymic digestion with papain, DNase I, and neutral protease | Initially, DMEM/F-12 supplemented with glutamine and 10% FBS After 24 h, DMEM/F12 supplemented with BIT-9500 (bovine serum albumin, transferrin, insulin, 20 ng/mL bFGF, 20 ng/mL EGF, and 20 ng/mL PDGF-AB) and 25% conditioned medium from rat stem cells that produces secretory bFGF and glycosylated form of cystatin C | Fibronectin | More than 70 population doublings in the 11-wk-old postnatal male More than 30 population doublings in the 27-year-old male | [42] | |
| Temporal lobe | Mechanical dissociation and enzymic digestion with DNase I and trypsin | DMEM/F12 supplemented with 1 mol/L HEPES, 2% B27, 0.1% EGF, and 0.1% bFGF | 11 mo | [43] | ||
| Temporal lobe | Enzymic digestion with Papain and DNase I | DMEM/F12 supplemented with bFGF and EGF | 3–6 wk | [44,114] | ||
| Lateral ventricular roof | Mechanical dissociation and enzyme digestion with DNase I and trypsin | DMEM/F12 supplemented with bFGF, EGF, and B27 | 3-7 wk | [45] | ||
| Hippocampus containing hilus, temporal cortex, and subventricular zone including anterior horn and segmented lateral ventricle | Physical mincing and enzyme digestion with trypsin | DMEM/F12 supplemented with N2, 35 μg/mL bovine pituitary extract, 5% fetal calf serum, 40 ng/mL EGF, and 20 ng/mL bFGF | > 60 population doublings | [46] | ||
| Biopsies from filum terminale below conus medullaris | Physical mincing and enzyme digestion with trypsin | DMEM/F12 supplemented with B27, 10 ng/mL LIF, 10 ng/mL bFGF, and 20 ng/mL EGF | Ultra-low attachment dish | [47] |
TGFα: Transforming growth factor alpha; DMEM/F12: Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12; FBS: Fetal bovine serum; bFGF: Basic fibroblast growth factor; EGF: Epidermal growth factor.
Table 1 summarizes various primary culture and in vitro expansion techniques for aNSCs. As indicated in Table 1, major obstacles in the primary isolation and stable in vitro expansion of aNSCs have been, or are being resolved, which would increase their clinical applicability.
CURRENT THERAPEUTIC STATUS OF ADULT NEURAL STEM CELLS FOR NEUROLOGICAL DISEASES
Since technical breakthroughs for the preclinical and clinical utilization of aNSCs were introduced relatively recently, scientific results showing treatment effects of aNSCs against neurological diseases are, at present, limited[18,56-59]. However, many previous reports have indicated that NSCs, compared with other stem cell types, are optimal for neurological diseases, since neural functional recovery requires direct neural cell supplementation, besides indirect paracrine effects. With brief presentation of the current developmental status of stem cell therapeutics for individual neurological disease, Tables 2 and 3 summarize preclinical and clinical results, respectively, of aNSCs against various neurological diseases.
Table 2.
Preclinical results of adult neural stem cells against neurodegenerative diseases
| Targeted disease animal model | Cell source | Injection method | Result | Animal species | Ref. |
| Demyelinated spinal cord injury | Frontal cortex, temporal cortex, hippocampus, and subventricular/subependymal zone of frontal lobe | The midline of the dorsal columns of the spinal cord at three longitudinal sites | The cells elicited extensive remyelination with a peripheral myelin pattern similar to Schwanncell myelination The remyelinated axons conducted impulses at near normal conduction velocities | Rat | [56] |
| Multiple sclerosis (lysolecithin-demyelinated brain) | Temporal lobe | Local injection to demyelinated brain regions | Transplanted cells migrated to lesions without extending into normal white matter. Implanted progenitors matured as oligodendrocytes, and developed myelin-associated antigens | Rat | [57] |
| Global brain ischemia | Temporal lobe | The posterior periventricular region above the hippocampus | Adult human NPCs survived, migrated into ischemic regions, and differentiated into functional neural cells No information about therapeutic effects | Rat | [58] |
| Global brain ischemia | Temporal lobe | Left hippocampus After in vitro differentiation | Injected cells migrated preferentially into an ischemic lesion, which was mediated by SDF-1α and CXCR4 signaling pathways No information about therapeutic effects | Rat | [59] |
| Focal ischemic stroke | Temporal lobe | Contralateral lateral ventricle | Transplanted cells reduced infarction volumes and enhanced motor activity | Rat | [18] |
Table 3.
Current clinical trials of neural stem cells against neurodegenerative diseases
| Disease | NSC source | Brief title | Trial ID | Condition | Location | Period | Cell source | Route | Phase |
| ALS | Fetal NSCs | Human neural stem cell transplantation in amyotrophic lateral sclerosis | NCT01640067 | Recruiting | Italy | 2011-12 -2016-09 | Fetal neural stem cells | Spinal cord | I |
| Dose escalation and safety study of human spinal cord derived neural stem cell transplantation for the treatment of amyotrophic lateral sclerosis | NCT01730716 | Ongoing | United States | 2013-05 -2014-09 | Spinal cord of a single fetus 8 wk of gestation | Spinal cord after laminectomy | II | ||
| Human spinal cord derived neural stem cell transplantation for the treatment of amyotrophic lateral sclerosis | NCT01348451 | Ongoing | United States | 2009-01 -2015-12 | Spinal cord of a single fetus 8 wk of gestation | Spinal cord after laminectomy | I | ||
| Stoke | Adult NSCs | Pilot investigation of stem cells in stroke | NCT01151124 | Ongoing | United Kingdom | 2010-06 -2015-03 | CTX0E03 DP allogeneic neural stem cells | Putamen region of the brain | I |
| Pilot Investigation of stem cells in stroke phase II efficacy | NCT02117635 | Recruiting | United Kingdom | 2014-06 -2015-12 | CTX0E03 DP allogeneic neural stem cells | Intracranially via stereotaxic | II | ||
| SCI | Adult NSCs | Safety study of human spinal cord-derived neural stem cell transplantation for the treatment of chronic SCI | NCT01772810 | Recruiting | United States | 2014-08 -2016-02 | Human spinal cord-derived neural stem cell | Direct injections into spinal parenchyma | I |
| Study of human central nervous system stem cells in patients with thoracic spinal cord Injury | NCT01321333 | Ongoing | Canada, Switzerland | 2011-03 -2015-05 | Human central nervous system stem cells | Thoracic spinal cord | I, II | ||
| Study of human central nervous system stem cell transplantation in cervical spinal cord injury | NCT02163876 | Recruiting | United States | 2014-10 -2017-05 | Human central nervous system stem cells | Cervical spine | II |
NSCs: Neural stem cells; ALS: Amyotrophic lateral sclerosis; iPSCs: Induced pluripotent stem cells.
Ischemic stroke
Various human stem cells and their derivatives can differentiate into neurons restoring functional losses in the rodent stroke model[60,61]. In particular, human ESC-derived NSCs, injected into the ischemic penumbra region in rat brains with ischemic stroke, have been reported to move to the lesions, and improve motor performances[62]. Moreover, human adult temporal lobe-derived NSCs, grafted into the contralateral ventricle of the rat brains with focal cerebral stroke, significantly reduced the infarction area, and showed recovery of motor function[18]. When human fetal NSCs were transplanted into ischemic lesions of rodent brains, they migrated toward the injured regions and differentiated into neurons[63,64].
Initial clinical trials with stem cells have been completed in stroke[61]. Unfortunately, no significant clinical outcomes were observed when autologous MSCs were injected intravenously into ischemic patients[65]. Although other clinical studies adopting intravenous or intra-arterial administration of autologous bone marrow-derived stem cells in stroke patients are in progress or planning[23], NSC-based regenerative treatment with both paracrine and neuronal supplementation effects would be more effective. Recently, a clinical trial for stroke with immortalized NSCs generated from human fetal cortex was planned in the United Kingdom[23], which would yield scientific data that might possibly demonstrate the superior regenerative and treatment activities of NSCs.
Although there are scientific data demonstrating the therapeutic effects of aNSCs on ischemic stroke, aNSCs have not applied to clinical trials for ischemic stroke yet. In contrast, clinical trials using MSCs for ischemic stroke are continuously planned and performed world widely. Compared with MSC clinical trials, the most different feature of NSC trials is the injection route; while MSCs are usually injected intravenously, NSCs are stereotactically transplanted in the brain. Since the penetration of MSCs across brain-blood barrier is still controversial, direct implantation of NSCs into the brain would potentiate the therapeutic effects against ischemic stroke.
Spinal cord injury
Human NSCs transplanted into a mouse model of spinal cord injury were observed to differentiate into neurons and oligodendrocytes to lead the recovery of locomotion[66]. Treatment mechanism study indicated that neurons derived from transplanted stem cells integrated into the host neuronal circuitry and mediated functional recovery[19,67]. On the other hand, the functional recovery after NSC transplantation into spinal cord injury models was proportional to the number of transplanted stem cell-derived oligodendrocytes and the amount of regenerated myelin[68]. Those preclinical results indicate that the supplementation of mature neural cells by implanted stem cells would also be important in clinical settings, for the functional recovery of spinal cord injury patients.
Highly refined oligodendrocyte progenitor cells (OPCs) generated in vitro from human ESCs differentiated into oligodendrocytes, and induced remyelination of the demyelinated spinal cord of mouse[69]. Based on these observations, a first phase I clinical trial using human ESC-derived OPCs is under planning by the United States company, Geron[23]. This first clinical trial has raised worries about the risk for tumorigenicity, which is difficult to determine in preclinical situations[70]. Since the results from animal models could not be directly translated into human, the possible risks need to be further validated. Moreover, utilization of aNSCs, instead of fetal origin stem cells, would reduce the possible tumorigenicity, due to their limited proliferation potential.
Parkinson’s disease
Human embryonic mesencephalic tissue which contains many post-mitotic dopaminergic neuroblasts was tried clinically, which have showed proof of concept that regenerative approach could have therapeutic effects in Parkinson’s disease (PD) patients[71]. Dopaminergic neuroblasts for preclinical animal models have been cultured from various different stem cell sources, including ESCs[72-79], fetal NSCs and precursors of embryonic ventral mesencephalon[80-83], adult NSCs from the SVZ[84], bone marrow stem cells[85], and fibroblast-derived iPSC cells[86].
Although a small portion of dopaminergic neurons derived from transplanted cells contain disease-specific Lewy bodies 11 to 16 years after transplantation[87,88], implanted cells remained viable[23,89]. However, definitive successful clinical trials have not yet been reported in the case of human stem cell-derived dopaminergic neurons. In contrast, a group of patients who had embryonic mesencephalic graft showed dyskinesia[90-92]. Those reports have provoked major concern about the possible side effects of transplanted ESC cell-derived dopaminergic neuroblasts[77], and the need for safer stem cell sources, such as aNSCs.
Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a lethal neuro-degenerative disease with premature degeneration of motor neurons in the CNS[23,93]. For the regeneration and/or supplementation of motor neurons, motor neurons were generated in vitro from ESCs[94-97], fetal NSCs[98-100], and iPSCs[101,102].
Recently, transplantation of fetal spinal cord- or iPSC-derived NSCs was reported to be effective in slowing down the disease progression of ALS animal models[103,104]. Based on the preclinical results, a phase I clinical trial of intra-spinal cord injection of fetal NSCs into ALS patients was attempted in the United States. Clinical outcomes from 6 to 18 mo after the transplantation showed that the intervention did not accelerate disease progress[105]. In contrast, their efficacy could not be determined, although higher dose of injection showed better results in some evaluating factors. On-going and/or planned phase II and III clinical trials would further determine the optimal therapeutic dose, and their therapeutic efficacy against ALS[106].
Until now, there have been few preclinical and clinical trials using aNSCs for ALS. However, ALS could be a good treatment target of aNSCs, having regard to its fatality, and lack of proven therapeutic options for it.
Alzheimer’s disease
Alzheimer’s disease (AD) is the most frequent neurological disease, which is characterized by the increased amyloid plaques and neurofibrillary tangles in the brain[107]. Amyloid plaques are extracellular aggregations consisting of amyloid-peptides. Neurofibrillary tangles are intracellular aggregations of hyperphosphorylated tau, a microtubule-associated protein within neuron[108]. The causative relationship between amyloid plaques/neurofibrillary tangles and AD is still under investigation[109]. Widespread non-specific neuronal death in the AD brain makes stem cell-based regeneration challenging. For effective cell therapy for AD, NSCs need to migrate to multiple regions of the brain and then differentiate into numerous multiple subtype neural cells[110]. Moreover, the effect of amyloid plaques on the survival, migration, and differentiation of injected stem cells should be taken into consideration[111].
Human NSCs transplanted into the brains of AD animal models showed little neurogenesis, but unwanted gliosis around the plaque-like structures[112]. Therefore, stem cell-based regenerative therapies need to be further developed preclinically, before clinical applications to AD. The disappointing preclinical data have resulted in few clinical trials using NSCs against AD. However, MSC is in relative advanced clinical trial stages. For example, human umbilical cord blood-derived MSCs are currently in a phase I clinical trial. Most trials using MSCs hire one-time direct injection of MSCs into the patient’s brain. As AD is a progressive disease, long term investigations are necessary, to examine the lasting effects, as well as the safety of transplanted stem cells[113].
PERSPECTIVES
Based on few scientifically proven treatment modalities for neurological diseases, and the regenerative potentials of stem cells, cell therapies using various stem cells have been preclinically and clinically applied to neurological diseases. There are many controversies about the therapeutic effects of stem cell treatments and their treatment mechanisms. The controversies could be derived from the diverse types of stem cells, and from their unique pros and cons. Compared with other stem cell sources, aNSCs have several advantages, such as differentiation potential into functional neural cells, limited proliferation capacity, and few ethical problems (Figure 1). Although difficulties in the isolation and in vitro expansion of aNSCs prevent the active applications of aNSCs to neurological diseases, the technical obstacles have been continuously resolved. Therefore, at the current status, aNSCs can be attractive stem cell sources, to be introduced into the preclinical and clinical trials targeting various neurological diseases.
Figure 1.
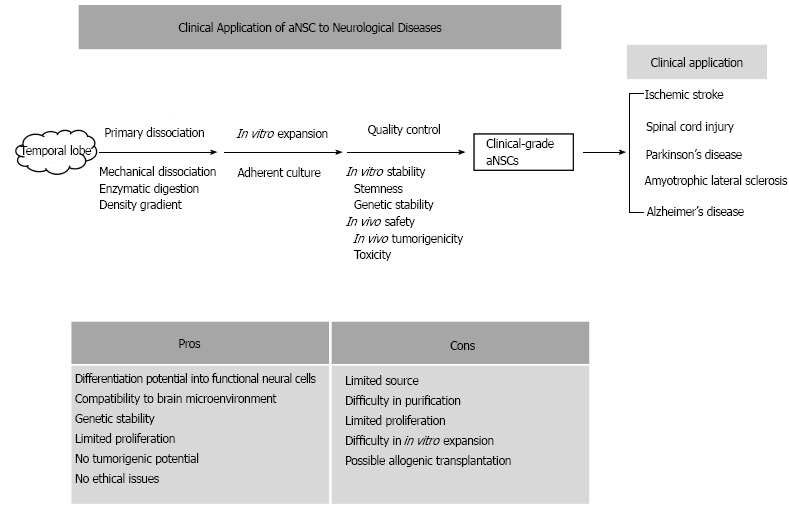
A diagrammatic summary of the approaches/strategies of adult neural stem cells and their pros/cons. aNSCs: Adult neural stem cells.
Footnotes
P- Reviewer: Dawe GS, Leanza G, Li JX S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Supported by The Korea Ministry of Food and Drug Safety in 2014, No. 10172KFDA993
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 29, 2014
First decision: August 14, 2014
Article in press: December 16, 2014
References
- 1.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Mimeault M, Hauke R, Batra SK. Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin Pharmacol Ther. 2007;82:252–264. doi: 10.1038/sj.clpt.6100301. [DOI] [PubMed] [Google Scholar]
- 3.Juengst E, Fossel M. The ethics of embryonic stem cells--now and forever, cells without end. JAMA. 2000;284:3180–3184. doi: 10.1001/jama.284.24.3180. [DOI] [PubMed] [Google Scholar]
- 4.McLaren A. Ethical and social considerations of stem cell research. Nature. 2001;414:129–131. doi: 10.1038/35102194. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin T. Morality and human embryo research. Introduction to the Talking Point on morality and human embryo research. EMBO Rep. 2009;10:299–300. doi: 10.1038/embor.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JY, Christophersen NS, Hall V, Soulet D, Brundin P. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–153. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Mimeault M, Batra SK. Concise review: recent advances on the significance of stem cells in tissue regeneration and cancer therapies. Stem Cells. 2006;24:2319–2345. doi: 10.1634/stemcells.2006-0066. [DOI] [PubMed] [Google Scholar]
- 10.Barker N, Bartfeld S, Clevers H. Tissue-resident adult stem cell populations of rapidly self-renewing organs. Cell Stem Cell. 2010;7:656–670. doi: 10.1016/j.stem.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Ehnert S, Glanemann M, Schmitt A, Vogt S, Shanny N, Nussler NC, Stöckle U, Nussler A. The possible use of stem cells in regenerative medicine: dream or reality? Langenbecks Arch Surg. 2009;394:985–997. doi: 10.1007/s00423-009-0546-0. [DOI] [PubMed] [Google Scholar]
- 12.Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–1437. doi: 10.1111/trf.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alagesan S, Griffin MD. Autologous and allogeneic mesenchymal stem cells in organ transplantation: what do we know about their safety and efficacy? Curr Opin Organ Transplant. 2014;19:65–72. doi: 10.1097/MOT.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 14.Asher DM. Bovine sera used in the manufacture of biologicals: current concerns and policies of the U.S. Food and Drug Administration regarding the transmissible spongiform encephalopathies. Dev Biol Stand. 1999;99:41–44. [PubMed] [Google Scholar]
- 15.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 16.Salem HK, Thiemermann C. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 2010;28:585–596. doi: 10.1002/stem.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joo KM, Kang BG, Yeon JY, Cho YJ, An JY, Song HS, Won JH, Kim SJ, Hong SC, Nam DH. Experimental and clinical factors influencing long-term stable in vitro expansion of multipotent neural cells from human adult temporal lobes. Exp Neurol. 2013;240:168–177. doi: 10.1016/j.expneurol.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Yan J, Xu L, Welsh AM, Hatfield G, Hazel T, Johe K, Koliatsos VE. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Gorp S, Leerink M, Kakinohana O, Platoshyn O, Santucci C, Galik J, Joosten EA, Hruska-Plochan M, Goldberg D, Marsala S, et al. Amelioration of motor/sensory dysfunction and spasticity in a rat model of acute lumbar spinal cord injury by human neural stem cell transplantation. Stem Cell Res Ther. 2013;4:57. doi: 10.1186/scrt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hefferan MP, Johe K, Hazel T, Feldman EL, Lunn JS, Marsala M. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell Transplant. 2011;20:1153–1161. doi: 10.3727/096368910X564553. [DOI] [PubMed] [Google Scholar]
- 22.Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- 23.Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29–40. doi: 10.1172/JCI40543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunn JS, Sakowski SA, Hur J, Feldman EL. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70:353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gincberg G, Arien-Zakay H, Lazarovici P, Lelkes PI. Neural stem cells: therapeutic potential for neurodegenerative diseases. Br Med Bull. 2012;104:7–19. doi: 10.1093/bmb/lds024. [DOI] [PubMed] [Google Scholar]
- 26.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nunes MC, Roy NS, Keyoung HM, Goodman RR, McKhann G, Jiang L, Kang J, Nedergaard M, Goldman SA. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 28.Reekmans K, Praet J, Daans J, Reumers V, Pauwels P, Van der Linden A, Berneman ZN, Ponsaerts P. Current challenges for the advancement of neural stem cell biology and transplantation research. Stem Cell Rev. 2012;8:262–278. doi: 10.1007/s12015-011-9266-2. [DOI] [PubMed] [Google Scholar]
- 29.Yoneyama M, Shiba T, Hasebe S, Ogita K. Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J Pharmacol Sci. 2011;115:425–432. doi: 10.1254/jphs.11r02cp. [DOI] [PubMed] [Google Scholar]
- 30.Madhavan L, Collier TJ. A synergistic approach for neural repair: cell transplantation and induction of endogenous precursor cell activity. Neuropharmacology. 2010;58:835–844. doi: 10.1016/j.neuropharm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Mathieu P, Battista D, Depino A, Roca V, Graciarena M, Pitossi F. The more you have, the less you get: the functional role of inflammation on neuronal differentiation of endogenous and transplanted neural stem cells in the adult brain. J Neurochem. 2010;112:1368–1385. doi: 10.1111/j.1471-4159.2009.06548.x. [DOI] [PubMed] [Google Scholar]
- 34.Signer RA, Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell. 2013;12:152–165. doi: 10.1016/j.stem.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 36.Maric D, Maric I, Barker JL. Buoyant density gradient fractionation and flow cytometric analysis of embryonic rat cortical neurons and progenitor cells. Methods. 1998;16:247–259. doi: 10.1006/meth.1998.0682. [DOI] [PubMed] [Google Scholar]
- 37.Panchision DM, Chen HL, Pistollato F, Papini D, Ni HT, Hawley TS. Optimized flow cytometric analysis of central nervous system tissue reveals novel functional relationships among cells expressing CD133, CD15, and CD24. Stem Cells. 2007;25:1560–1570. doi: 10.1634/stemcells.2006-0260. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 39.Johansson CB, Svensson M, Wallstedt L, Janson AM, Frisén J. Neural stem cells in the adult human brain. Exp Cell Res. 1999;253:733–736. doi: 10.1006/excr.1999.4678. [DOI] [PubMed] [Google Scholar]
- 40.Kukekov VG, Laywell ED, Suslov O, Davies K, Scheffler B, Thomas LB, O’Brien TF, Kusakabe M, Steindler DA. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999;156:333–344. doi: 10.1006/exnr.1999.7028. [DOI] [PubMed] [Google Scholar]
- 41.Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Déglon N, Kostic C, Zurn A, Aebischer P. Isolation of multipotent neural precursors residing in the cortex of the adult human brain. Exp Neurol. 2001;170:48–62. doi: 10.1006/exnr.2001.7691. [DOI] [PubMed] [Google Scholar]
- 42.Palmer TD, Schwartz PH, Taupin P, Kaspar B, Stein SA, Gage FH. Cell culture. Progenitor cells from human brain after death. Nature. 2001;411:42–43. doi: 10.1038/35075141. [DOI] [PubMed] [Google Scholar]
- 43.Westerlund U, Moe MC, Varghese M, Berg-Johnsen J, Ohlsson M, Langmoen IA, Svensson M. Stem cells from the adult human brain develop into functional neurons in culture. Exp Cell Res. 2003;289:378–383. doi: 10.1016/s0014-4827(03)00291-x. [DOI] [PubMed] [Google Scholar]
- 44.Moe MC, Varghese M, Danilov AI, Westerlund U, Ramm-Pettersen J, Brundin L, Svensson M, Berg-Johnsen J, Langmoen IA. Multipotent progenitor cells from the adult human brain: neurophysiological differentiation to mature neurons. Brain. 2005;128:2189–2199. doi: 10.1093/brain/awh574. [DOI] [PubMed] [Google Scholar]
- 45.Westerlund U, Svensson M, Moe MC, Varghese M, Gustavsson B, Wallstedt L, Berg-Johnsen J, Langmoen IA. Endoscopically harvested stem cells: a putative method in future autotransplantation. Neurosurgery. 2005;57:779–784; discussion 779-784. [PubMed] [Google Scholar]
- 46.Walton NM, Sutter BM, Chen HX, Chang LJ, Roper SN, Scheffler B, Steindler DA. Derivation and large-scale expansion of multipotent astroglial neural progenitors from adult human brain. Development. 2006;133:3671–3681. doi: 10.1242/dev.02541. [DOI] [PubMed] [Google Scholar]
- 47.Varghese M, Olstorn H, Berg-Johnsen J, Moe MC, Murrell W, Langmoen IA. Isolation of human multipotent neural progenitors from adult filum terminale. Stem Cells Dev. 2009;18:603–613. doi: 10.1089/scd.2008.0144. [DOI] [PubMed] [Google Scholar]
- 48.Rietze RL, Reynolds BA. Neural stem cell isolation and characterization. Methods Enzymol. 2006;419:3–23. doi: 10.1016/S0076-6879(06)19001-1. [DOI] [PubMed] [Google Scholar]
- 49.Bez A, Corsini E, Curti D, Biggiogera M, Colombo A, Nicosia RF, Pagano SF, Parati EA. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993:18–29. doi: 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 50.Suslov ON, Kukekov VG, Ignatova TN, Steindler DA. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA. 2002;99:14506–14511. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reynolds BA, Rietze RL. Neural stem cells and neurospheres--re-evaluating the relationship. Nat Methods. 2005;2:333–336. doi: 10.1038/nmeth758. [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Ryugo DK, Pongstaporn T, Johe K, Koliatsos VE. Human neural stem cell grafts in the spinal cord of SOD1 transgenic rats: differentiation and structural integration into the segmental motor circuitry. J Comp Neurol. 2009;514:297–309. doi: 10.1002/cne.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 54.Murrell W, Palmero E, Bianco J, Stangeland B, Joel M, Paulson L, Thiede B, Grieg Z, Ramsnes I, Skjellegrind HK, et al. Expansion of multipotent stem cells from the adult human brain. PLoS One. 2013;8:e71334. doi: 10.1371/journal.pone.0071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 56.Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- 57.Windrem MS, Roy NS, Wang J, Nunes M, Benraiss A, Goodman R, McKhann GM, Goldman SA. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69:966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 58.Olstorn H, Moe MC, Røste GK, Bueters T, Langmoen IA. Transplantation of stem cells from the adult human brain to the adult rat brain. Neurosurgery. 2007;60:1089–1098; discussion 1089-1098. doi: 10.1227/01.NEU.0000255461.91892.0D. [DOI] [PubMed] [Google Scholar]
- 59.Olstorn H, Varghese M, Murrell W, Moe MC, Langmoen IA. Predifferentiated brain-derived adult human progenitor cells migrate toward ischemia after transplantation to the adult rat brain. Neurosurgery. 2011;68:213–222; discussion 222. doi: 10.1227/NEU.0b013e3181fd2c11. [DOI] [PubMed] [Google Scholar]
- 60.Bacigaluppi M, Pluchino S, Martino G, Kilic E, Hermann DM. Neural stem/precursor cells for the treatment of ischemic stroke. J Neurol Sci. 2008;265:73–77. doi: 10.1016/j.jns.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Locatelli F, Bersano A, Ballabio E, Lanfranconi S, Papadimitriou D, Strazzer S, Bresolin N, Comi GP, Corti S. Stem cell therapy in stroke. Cell Mol Life Sci. 2009;66:757–772. doi: 10.1007/s00018-008-8346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daadi MM, Maag AL, Steinberg GK. Adherent self-renewable human embryonic stem cell-derived neural stem cell line: functional engraftment in experimental stroke model. PLoS One. 2008;3:e1644. doi: 10.1371/journal.pone.0001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kelly S, Bliss TM, Shah AK, Sun GH, Ma M, Foo WC, Masel J, Yenari MA, Weissman IL, Uchida N, et al. Transplanted human fetal neural stem cells survive, migrate, and differentiate in ischemic rat cerebral cortex. Proc Natl Acad Sci USA. 2004;101:11839–11844. doi: 10.1073/pnas.0404474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kallur T, Darsalia V, Lindvall O, Kokaia Z. Human fetal cortical and striatal neural stem cells generate region-specific neurons in vitro and differentiate extensively to neurons after intrastriatal transplantation in neonatal rats. J Neurosci Res. 2006;84:1630–1644. doi: 10.1002/jnr.21066. [DOI] [PubMed] [Google Scholar]
- 65.Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- 66.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hooshmand MJ, Sontag CJ, Uchida N, Tamaki S, Anderson AJ, Cummings BJ. Analysis of host-mediated repair mechanisms after human CNS-stem cell transplantation for spinal cord injury: correlation of engraftment with recovery. PLoS One. 2009;4:e5871. doi: 10.1371/journal.pone.0005871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hofstetter CP, Holmström NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisén J, Olson L. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005;8:346–353. doi: 10.1038/nn1405. [DOI] [PubMed] [Google Scholar]
- 69.Nistor GI, Totoiu MO, Haque N, Carpenter MK, Keirstead HS. Human embryonic stem cells differentiate into oligodendrocytes in high purity and myelinate after spinal cord transplantation. Glia. 2005;49:385–396. doi: 10.1002/glia.20127. [DOI] [PubMed] [Google Scholar]
- 70.Erdö F, Bührle C, Blunk J, Hoehn M, Xia Y, Fleischmann B, Föcking M, Küstermann E, Kolossov E, Hescheler J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 71.Lindvall O, Björklund A. Cell therapy in Parkinson’s disease. NeuroRx. 2004;1:382–393. doi: 10.1602/neurorx.1.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bjorklund LM, Sánchez-Pernaute R, Chung S, Andersson T, Chen IY, McNaught KS, Brownell AL, Jenkins BG, Wahlestedt C, Kim KS, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349. doi: 10.1073/pnas.022438099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho MS, Lee YE, Kim JY, Chung S, Cho YH, Kim DS, Kang SM, Lee H, Kim MH, Kim JH, et al. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, Nishikawa SI, Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- 75.Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez-Gómez JA, Lu JQ, Velasco I, Rivera S, Zoghbi SS, Liow JS, Musachio JL, Chin FT, Toyama H, Seidel J, et al. Persistent dopamine functions of neurons derived from embryonic stem cells in a rodent model of Parkinson disease. Stem Cells. 2007;25:918–928. doi: 10.1634/stemcells.2006-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat Med. 2006;12:1259–1268. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez-Pernaute R, Lee H, Patterson M, Reske-Nielsen C, Yoshizaki T, Sonntag KC, Studer L, Isacson O. Parthenogenetic dopamine neurons from primate embryonic stem cells restore function in experimental Parkinson’s disease. Brain. 2008;131:2127–2139. doi: 10.1093/brain/awn144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takagi Y, Takahashi J, Saiki H, Morizane A, Hayashi T, Kishi Y, Fukuda H, Okamoto Y, Koyanagi M, Ideguchi M, et al. Dopaminergic neurons generated from monkey embryonic stem cells function in a Parkinson primate model. J Clin Invest. 2005;115:102–109. doi: 10.1172/JCI21137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O'Keeffe FE, Scott SA, Tyers P, O’Keeffe GW, Dalley JW, Zufferey R, Caldwell MA. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson’s disease. Brain. 2008;131:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- 81.Parish CL, Castelo-Branco G, Rawal N, Tonnesen J, Sorensen AT, Salto C, Kokaia M, Lindvall O, Arenas E. Wnt5a-treated midbrain neural stem cells improve dopamine cell replacement therapy in parkinsonian mice. J Clin Invest. 2008;118:149–160. doi: 10.1172/JCI32273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sánchez-Pernaute R, Studer L, Bankiewicz KS, Major EO, McKay RD. In vitro generation and transplantation of precursor-derived human dopamine neurons. J Neurosci Res. 2001;65:284–288. doi: 10.1002/jnr.1152. [DOI] [PubMed] [Google Scholar]
- 83.Studer L, Tabar V, McKay RD. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- 84.Shim JW, Park CH, Bae YC, Bae JY, Chung S, Chang MY, Koh HC, Lee HS, Hwang SJ, Lee KH, et al. Generation of functional dopamine neurons from neural precursor cells isolated from the subventricular zone and white matter of the adult rat brain using Nurr1 overexpression. Stem Cells. 2007;25:1252–1262. doi: 10.1634/stemcells.2006-0274. [DOI] [PubMed] [Google Scholar]
- 85.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, Tajima N, Yamada H, Sawada H, Ishikawa H, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 88.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 89.Piccini P, Brooks DJ, Björklund A, Gunn RN, Grasby PM, Rimoldi O, Brundin P, Hagell P, Rehncrona S, Widner H, et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson’s patient. Nat Neurosci. 1999;2:1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 90.Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med. 2001;344:710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 91.Olanow CW, Goetz CG, Kordower JH, Stoessl AJ, Sossi V, Brin MF, Shannon KM, Nauert GM, Perl DP, Godbold J, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 92.Hagell P, Piccini P, Björklund A, Brundin P, Rehncrona S, Widner H, Crabb L, Pavese N, Oertel WH, Quinn N, et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 93.Lagier-Tourenne C, Cleveland DW. Rethinking ALS: the FUS about TDP-43. Cell. 2009;136:1001–1004. doi: 10.1016/j.cell.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Singh Roy N, Nakano T, Xuing L, Kang J, Nedergaard M, Goldman SA. Enhancer-specified GFP-based FACS purification of human spinal motor neurons from embryonic stem cells. Exp Neurol. 2005;196:224–234. doi: 10.1016/j.expneurol.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 95.Lee H, Shamy GA, Elkabetz Y, Schofield CM, Harrsion NL, Panagiotakos G, Socci ND, Tabar V, Studer L. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells. 2007;25:1931–1939. doi: 10.1634/stemcells.2007-0097. [DOI] [PubMed] [Google Scholar]
- 96.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23:215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 97.Li XJ, Hu BY, Jones SA, Zhang YS, Lavaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jordan PM, Ojeda LD, Thonhoff JR, Gao J, Boehning D, Yu Y, Wu P. Generation of spinal motor neurons from human fetal brain-derived neural stem cells: role of basic fibroblast growth factor. J Neurosci Res. 2009;87:318–332. doi: 10.1002/jnr.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wu P, Tarasenko YI, Gu Y, Huang LY, Coggeshall RE, Yu Y. Region-specific generation of cholinergic neurons from fetal human neural stem cells grafted in adult rat. Nat Neurosci. 2002;5:1271–1278. doi: 10.1038/nn974. [DOI] [PubMed] [Google Scholar]
- 100.Gao J, Coggeshall RE, Tarasenko YI, Wu P. Human neural stem cell-derived cholinergic neurons innervate muscle in motoneuron deficient adult rats. Neuroscience. 2005;131:257–262. doi: 10.1016/j.neuroscience.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 101.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 102.Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, et al. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806–811. doi: 10.1002/stem.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu L, Yan J, Chen D, Welsh AM, Hazel T, Johe K, Hatfield G, Koliatsos VE. Human neural stem cell grafts ameliorate motor neuron disease in SOD-1 transgenic rats. Transplantation. 2006;82:865–875. doi: 10.1097/01.tp.0000235532.00920.7a. [DOI] [PubMed] [Google Scholar]
- 104.Popescu IR, Nicaise C, Liu S, Bisch G, Knippenberg S, Daubie V, Bohl D, Pochet R. Neural progenitors derived from human induced pluripotent stem cells survive and differentiate upon transplantation into a rat model of amyotrophic lateral sclerosis. Stem Cells Transl Med. 2013;2:167–174. doi: 10.5966/sctm.2012-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- 106.Feldman EL, Boulis NM, Hur J, Johe K, Rutkove SB, Federici T, Polak M, Bordeau J, Sakowski SA, Glass JD. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75:363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 108.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 109.Israel MA, Yuan SH, Bardy C, Reyna SM, Mu Y, Herrera C, Hefferan MP, Van Gorp S, Nazor KL, Boscolo FS, et al. Probing sporadic and familial Alzheimer’s disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen WW, Blurton-Jones M. Concise review: Can stem cells be used to treat or model Alzheimer’s disease? Stem Cells. 2012;30:2612–2618. doi: 10.1002/stem.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H. Stem-cell challenges in the treatment of Alzheimer’s disease: a long way from bench to bedside. Med Res Rev. 2014;34:957–978. doi: 10.1002/med.21309. [DOI] [PubMed] [Google Scholar]
- 112.Sugaya K, Kwak YD, Ohmitsu O, Marutle A, Greig NH, Choumrina E. Practical issues in stem cell therapy for Alzheimer’s disease. Curr Alzheimer Res. 2007;4:370–377. doi: 10.2174/156720507781788936. [DOI] [PubMed] [Google Scholar]
- 113.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Moe MC, Westerlund U, Varghese M, Berg-Johnsen J, Svensson M, Langmoen IA. Development of neuronal networks from single stem cells harvested from the adult human brain. Neurosurgery. 2005;56:1182–1188; discussion 1182-1188. doi: 10.1227/01.neu.0000159881.09663.6d. [DOI] [PubMed] [Google Scholar]


