ABSTRACT
Although many studies have demonstrated intracellular movement of viral proteins or viral replication complexes, little is known about the mechanisms of their motility. In this study, we analyzed the localization and motility of the nucleocapsid protein (NP) of Fig mosaic virus (FMV), a negative-strand RNA virus belonging to the recently established genus Emaravirus. Electron microscopy of FMV-infected cells using immunogold labeling showed that NPs formed cytoplasmic agglomerates that were predominantly enveloped by the endoplasmic reticulum (ER) membrane, while nonenveloped NP agglomerates also localized along the ER. Likewise, transiently expressed NPs formed agglomerates, designated NP bodies (NBs), in close proximity to the ER, as was the case in FMV-infected cells. Subcellular fractionation and electron microscopic analyses of NP-expressing cells revealed that NBs localized in the cytoplasm. Furthermore, we found that NBs moved rapidly with the streaming of the ER in an actomyosin-dependent manner. Brefeldin A treatment at a high concentration to disturb the ER network configuration induced aberrant accumulation of NBs in the perinuclear region, indicating that the ER network configuration is related to NB localization. Dominant negative inhibition of the class XI myosins, XI-1, XI-2, and XI-K, affected both ER streaming and NB movement in a similar pattern. Taken together, these results showed that NBs localize in the cytoplasm but in close proximity to the ER membrane to form enveloped particles and that this causes passive movements of cytoplasmic NBs by ER streaming.
IMPORTANCE Intracellular trafficking is a primary and essential step for the cell-to-cell movement of viruses. To date, many studies have demonstrated the rapid intracellular movement of viral factors but have failed to provide evidence for the mechanism or biological significance of this motility. Here, we observed that agglomerates of nucleocapsid protein (NP) moved rapidly throughout the cell, and we performed live imaging and ultrastructural analysis to identify the mechanism of motility. We provide evidence that cytoplasmic protein agglomerates were passively dragged by actomyosin-mediated streaming of the endoplasmic reticulum (ER) in plant cells. In virus-infected cells, NP agglomerates were surrounded by the ER membranes, indicating that NP agglomerates form the basis of enveloped virus particles in close proximity to the ER. Our work provides a sophisticated model of macromolecular trafficking in plant cells and improves our understanding of the formation of enveloped particles of negative-strand RNA viruses.
INTRODUCTION
The actomyosin system is essential for the intracellular transport of endomembranes and other macromolecular complexes and for the spatial arrangement of organelles (1). Trafficking along actin filaments is primarily mediated by molecular motor myosins. Myosins of Arabidopsis thaliana are divided into the two subfamilies, class VIII and class XI myosins. Class XI myosins provide the motive force for the movement of organelles such as the Golgi apparatus, peroxisomes, and mitochondria (2–6). Of 13 Arabidopsis class XI myosins, the XI-1, XI-2, XI-K, and XI-I myosins are suggested to be involved mainly in the movement of organelles in leaf cells (3, 5, 7). The plant endoplasmic reticulum (ER) is a highly dynamic organelle that streams rapidly in the cortical cytoplasm and in transvacuolar strands. The ER runs along actin filaments and forms an actin/ER network throughout the cytoplasm (8). The streaming of ER is thought to drag the surrounding cytosol, making the ER a strong candidate for the motive force of cytoplasmic streaming (9). ER streaming has been suggested to result from ER remodeling, dynamic changes in the branching points, and movement of ER nodes, with the driving force provided mainly by XI-K myosins (5, 6, 9).
Plant viruses and their components make use of host intracellular transport machineries (10). Several viral proteins relevant to viral cell-to-cell movement hijack the actomyosin cytoskeleton to be targeted to plasmodesmata (11–13). In addition, a number of proteins and protein complexes not directly involved in cell-to-cell movement have been shown to form motile inclusions and to traffic rapidly along the actin/ER network (14–20). One explanation for the motility of viral proteins is that they are anchored to, or in some cases vesiculated by, the endomembrane for the direct use of myosin motors, which provide the motive force (21, 22). However, the mechanisms and biological significance of intracellular movements along the actin/ER network remain to be elucidated.
Negative-sense single-stranded RNA [(–)RNA] viruses induce the modification of intracellular membrane structures to form membrane-enveloped particles (23–26). Glycoprotein (GP), a structural protein encoded by (–)RNA viruses, targets specific organelles and hijacks the membrane to enclose viral ribonucleoprotein complexes (vRNPs), which play key roles in viral replication and translation. Viral genomic RNA molecules that are tightly encapsidated by a large number of nucleocapsid proteins (NPs), and by a comparatively small number of RNA-dependent RNA polymerases (RdRps), are contained in vRNPs (27–29).
Fig mosaic virus (FMV), a (–)RNA plant virus, is a member of the recently established genus Emaravirus (30). FMV contains six genomic RNA segments. The RNA1, RNA2, RNA3, and RNA4 segments encode RdRp, GP, NP, and a movement protein, respectively (30–32). In infected cells, FMV forms enveloped spherical particles, called double-membrane bodies (DMBs), in regions close to ER cisternae and ER tubules (33, 34).
In this study, we analyzed FMV NP localization and motility by using a combination of confocal live imaging and electron microscopy. Our data suggested that ER streaming drags cytoplasmic NP agglomerates, which localize in close proximity to the ER membrane to form enveloped particles. The model presented herein will be broadly applicable to the virus-induced trafficking of macromolecules.
MATERIALS AND METHODS
Construction of binary vectors.
All of the clones generated in this study were constructed using Gateway technology (Invitrogen, Carlsbad, CA) as described previously (35). The NP gene was amplified from an FMV genomic cDNA (AB697843) (31) and cloned into pEarleyGate 101 pEarleyGate 102 (36) and pEarleyGate C3myc, an in-house expression vector built from pEarleyGate 101 to introduce a 3× myc tag at the C terminus. Total RNA was extracted from Nicotiana tabacum cv. Samsun and Arabidopsis thaliana ecotype Columbia by using an RNeasy plant minikit (Qiagen, Venlo, The Netherlands). Reverse transcriptase reactions were carried out using Superscript III reverse transcriptase (Invitrogen) with dT20 oligonucleotide. The Sar1 gene (D87821) was amplified from cDNA derived from N. tabacum cv. Samsun (37) and subcloned into the TOPO vector by using a TOPO TA cloning kit (Invitrogen). An H74L mutation was introduced into Sar1 by using the GeneArt seamless cloning and assembly kit (Invitrogen), and the Sar1(H74L) gene was cloned into pEarleyGate 100. An FABD2 sequence (At4G26700) and all myosin tail sequences (XI-1 [AT1G17580]; XI-2 [AT5G43900]; XI-J [AT3G58160]; XI-K [AT5G20490]) were amplified from cDNA derived from A. thaliana (25, 38) and cloned into pEarleyGate 104 and pEarleyGate NCFP, an in-house expression vector built from pEarleyGate 104 to introduce cyan fluorescent protein (CFP) as an N-terminal fusion. The CFP sequence containing a simian virus 40 (SV40) nuclear localization signal (NLS) sequence was amplified using a primer containing an NLS sequence (39) and cloned into pEarleyGate 100. Expression vectors for fluorescent protein fused to ManI and HDEL (40) were purchased from the Arabidopsis Biological Resource Center (ABRC; http://www.arabidopsis.org/). ABRC stock numbers used were as follows: ER-ck CD3-953 for CFP, ER-yk CD3-957 for yellow fluorescent protein (YFP), ER-rk CD3-959 for mCherry, G-ck CD3-961 for CFP, and G-rk CD3-967 for mCherry. The preparation of an expression vector of β-glucuronidase (GUS) was described previously (32).
Plant material and agroinfiltration.
Transient expression was mediated by infiltration of Agrobacterium tumefaciens cells as described previously (32). Equal volumes of each suspension were mixed before infiltration for coexpression assays. Four-week-old N. benthamiana plants were agroinfiltrated and used for live imaging analysis. Both healthy and infected fig plants (Ficus carica) were maintained in growth chambers at 25°C. Newly emerged leaves were used throughout the study.
Purification of recombinant proteins and electrophoretic mobility shift assay (EMSA).
The NP gene was cloned into the pMal-c5X vector (New England BioLabs, Beverly, MA). The recombinant plasmid and pMal-c5X were transformed and cultured as described previously (41). Maltose binding protein (MBP) and MBP:NP fusion constructs were induced in the transformed Escherichia coli cells with IPTG (isopropyl-β-d-thiogalactopyranoside) at 0.3 mM for 24 h at 16°C and purified according to the manufacturer's instructions.
The 280-nucleotide (nt) 5′-terminal sequence of RNA5 of FMV (31) and the 249-nt tobacco mosaic virus 3′-terminal sequence (42) were transcribed using a MEGAscript T7 kit (Ambion, Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. The transcribed RNA was purified using an RNeasy minikit (Qiagen).
Concentration series of MBP or MBP:NP were mixed with equal volumes of 2× EMSA buffer (20 mM HEPES, 2.5 mM MgCl2, 25 mM KCl, 2 mM dithiothreitol [DTT], 20% [vol/vol] glycerol, and 0.1 mM EDTA). The mixtures were incubated with approximately 200 pg of the 32P-labeled FMV RNA for 15 min on ice. For competitor assays, approximately 1.0 ng of the 32P-labeled FMV RNA and 8.0 μM MBP:NP were mixed with the TMV RNA at the indicated molecular ratios. After the binding reaction, the mixtures were analyzed by electrophoresis on a 2% agarose gel in Tris-acetate-EDTA buffer.
Generation of FMV NP antibodies.
The preparation of recombinant FMV NP and the generation of affinity-purified antibodies were performed as described previously (43). The NP gene was cloned into the pET-30a vector (Merck, Darmstadt, Germany).
Preparation of membrane-rich fractions and sucrose gradient analysis.
N. benthamiana leaves were ground in liquid nitrogen, and the membrane-rich fraction (P30) was collected as described previously (32). Sucrose gradient centrifugation was carried out according to the method of Genovés et al. (44). The P30 fraction, resuspended in 200 μl of lysis buffer with 5 mM MgCl2, was layered onto 2.5 ml of 20-to-60% linear sucrose gradients containing lysis buffer with 5 mM MgCl2. The gradients were centrifuged for 16 h at 100,000 × g in a swing rotor at 4°C, and 18 fractions were collected from the top of the gradient. All fractions were resuspended in aliquots of 2× Laemmli sample buffer (Bio-Rad Laboratories, Hercules, CA).
Coimmunoprecipitation and immunoblot analysis.
N. benthamiana leaves were ground in RIPA buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCI, 1% [vol/vol] Nonidet P-40, 1% [wt/vol] sodium deoxycholate, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], and Complete Mini protease inhibitors [Roche Diagnostics, Basel, Switzerland]) and centrifuged twice at 8,200 × g to remove debris. Myc-tagged proteins were isolated from the supernatant by binding to EZview Red anti-c-Myc agarose (Sigma-Aldrich, St. Louis, MO). Following a wash with RIPA buffer, dissociation from the beads was performed by adding Laemmli buffer and boiling for 5 min.
Immunoblot analysis was carried out essentially as described previously (45). NP was detected using anti-NP antibodies at a dilution of 1:2,000, and the YFP fusion NPs were detected using a mouse monoclonal anti-GFP antibody (Roche Diagnostics) at a dilution of 1:3,000. The membranes were then incubated with peroxidase-conjugated secondary antibodies (GE Healthcare, Chalfont, St. Giles, England) at a dilution of 1:3,000. Can Get signal immunoreaction enhancer solutions (Toyobo, Osaka, Japan) were used as the diluents.
Inhibitor assay.
Latrunculin B (LatB; Sigma-Aldrich) or brefeldin A (BFA; Invitrogen) at the indicated concentrations in 0.5% (vol/vol) dimethyl sulfoxide (DMSO) were infiltrated with N. benthamiana leaves. The leaves at 26 h postinfiltration (hpi) were treated with LatB and observed at 10 h after the treatments. The leaves at 18 hpi were treated with BFA and observed at 6 h after the treatments.
Confocal imaging.
Cells expressing fluorescent protein fusions were imaged using a Leica TCS SP5 laser-scanning confocal microscope (Leica Microsystems, Wetzlar, Germany) with a 63× oil immersion objective. Proteins were monitored as follows: CFP was excited at the 458-nm argon laser line, and the emission was visualized at 460 to 495 nm; the 514-nm argon laser line was used for YFP, and the emission was visualized at 525 to 600 nm; the 543-nm helium/neon laser line was used for mCherry, and the emission was visualized at 585 to 650 nm. CFP and YFP were detected using a 458/514 dichroic mirror. For observing double expression, images were scanned and captured in line-switching mode. For observing triple expression, images were scanned and captured by simultaneous excitation of CFP and YFP in combination with line-switching mode and using a 488/543 dichroic mirror. Images were subsequently processed using the ImageJ (NIH, Bethesda, MD) and Photoshop CS4 (Adobe, San Jose, CA) software.
We counted the number of mobile NBs and immobile NBs from at least 300 NBs by using the ImageJ time-lapse color coder plugin. Ten mobile NBs were selected randomly, and the average velocities were calculated. Experiments were replicated in three individual plants.
The velocities of ER streaming were calculated using the ImageJ KbiFlow plugin (9). Forty frames were captured at 0.32-s intervals. Experiments were replicated at least six times for two individual plants.
Immunogold labeling.
For primary fixation, NP-expressing N. benthamiana leaves at 30 hpi, or symptomatic fig leaves, were chopped into small pieces (1 by 4 mm) and incubated for 2 h in fixative solution (50 mM sodium cacodylate, 4% [wt/vol] paraformaldehyde, 0.1% [vol/vol] glutaraldehyde, 2 mM CaCl2; pH 7.4). Sucrose (60 mM) was added to the fixative solution for fig leaves. Fixed leaves were washed four times in cacodylate buffer (50 mM sodium cacodylate [pH 7.4]), followed by postfixation for 1 h with 4% (wt/vol) osmium tetroxide and 3% (wt/vol) potassium ferricyanide in distilled water. After washing four times in cacodylate buffer with glycine (50 mM sodium cacodylate, 100 mM glycine [pH 7.4]), dehydration was carried out in a series of increasing concentrations of ethanol. LR White embedding resin (Polysciences, Warrington, PA) was infiltrated using mixtures of LR White and ethanol (1:1, 2:1, and 3:1). The samples were eventually transferred to BEEM capsules containing deaerated LR White resin with 1% (vol/vol) benzoyl peroxide (Nisshin EM, Tokyo, Japan). Polymerization was performed by UV irradiation for 5 days at 4°C without accelerator. Ultrathin sectioning (100 nm) was carried out using a Leica EM UC7 ultramicrotome, and sections were mounted on nickel grids. Grids were incubated in blocking solution (2% [wt/vol] polyvinylpyrrolidone [average molecular weight, 40,000; Sigma-Aldrich], 2% [wt/vol] bovine serum albumin, 0.1% [wt/vol] NaN3, and 0.05% [vol/vol] Tween 20 in phosphate-buffered saline [PBS]) for 30 min. All incubations were carried out on droplets of 20 μl. Then, the grids were incubated on anti-NP antiboies diluted 1:100 in PBS for 12 h at 4°C. The grids were washed with PBS, followed by incubation with 15-nm gold-conjugated rabbit antisera diluted 1:100 in PBS for 12 h at 4°C. The grids were washed with PBS and then dried for microscopy analysis.
Electron microscopy.
Spurr low-viscosity embedding medium (Sigma-Aldrich) was used for electron microscopy. NP-expressing N. benthamiana leaves or symptomatic fig leaves were chopped and fixed as described above, except that 1% (vol/vol) glutaraldehyde was added to the fixative. Fixed leaves were washed with cacodylate buffer as described above, followed by postfixation for 2 h with 2% (wt/vol) osmium tetroxide in distilled water. Dehydration was carried out as described above, and Spurr resin was infiltrated using mixtures of Spurr resin and acetone (1:3, 1:1, and 3:1). The samples were eventually transferred to deaerated Spurr mixture, and polymerization was performed at 60°C for 3 days. Ultrathin sections (80 nm) were examined under a JEM 1400 Plus transmission electron microscope (JEOL, Tokyo, Japan).
RESULTS
NP formed agglomerates that were enveloped by the ER membrane in FMV-infected fig leaves.
A cytological study of FMV-infected fig leaves was performed using electron microscopy. Observation of the FMV-infected mesophyll cells revealed that DMBs, enveloped particles, were located adjacent to the ER (Fig. 1A). The two membrane layers were clearly distinguishable in some DMBs (Fig. 1A, inset). DMBs frequently protruded from the ER membrane, and DMB budding appeared to be taking place (Fig. 1B). DMBs appeared as electron-opaque bodies, possibly because they had a double membrane and were tightly packed with vRNPs. DMBs occasionally accompanied the so-called “matrix,” which is a large filamentous inclusion body-like structure observed in previous studies of FMV-infected leaves (Fig. 1C) (33, 46). Matrices appeared to be composed predominantly of the modified ER, in that they contained electron-opaque ribosome-like particles.
FIG 1.
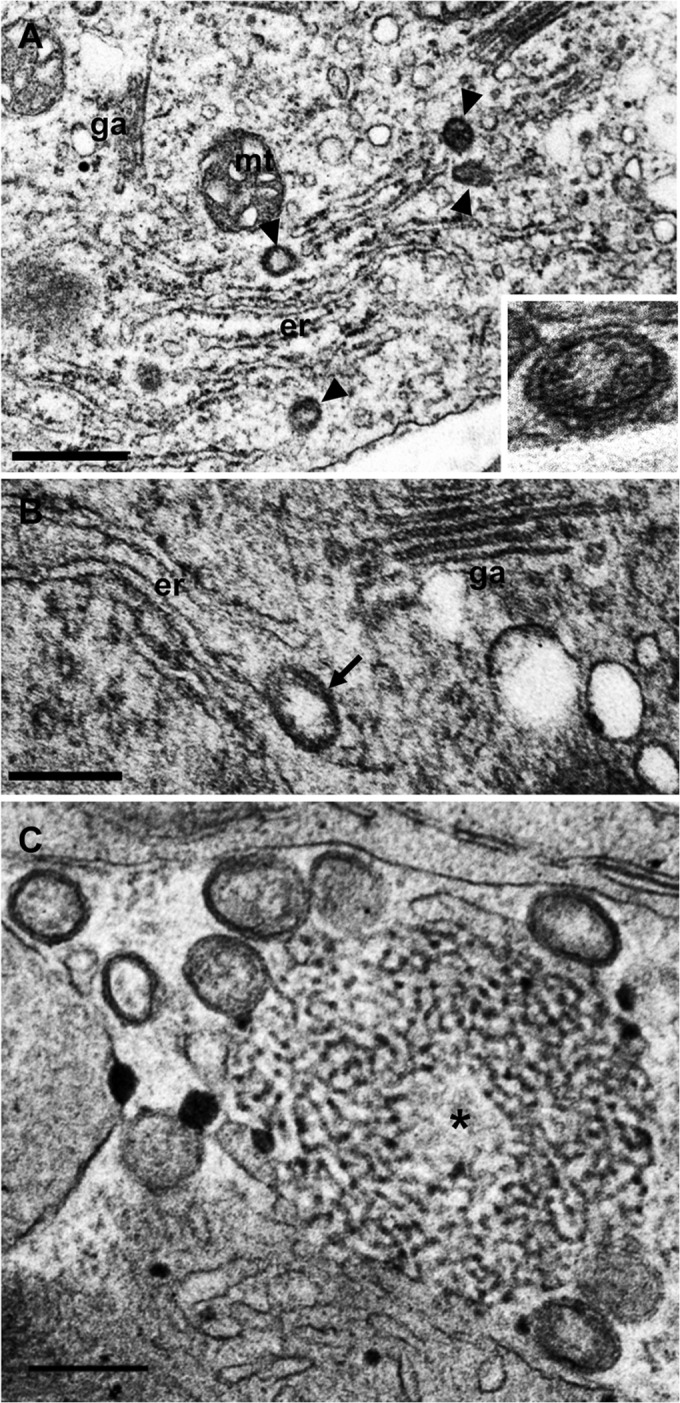
Overview of membranous structures induced by FMV infection in fig leaves. (A) Electron-opaque DMBs were observed adjacent to the ER (arrowheads). DMBs have two layers (inset). (B) A DMB appearing to bud from ER cisterna (arrow). (C) Matrix (asterisk) associated with DMBs. er, endoplasmic reticulum; ga, Golgi apparatus; mt, mitochondria. Bars: 500 nm (A); 200 nm (B and C).
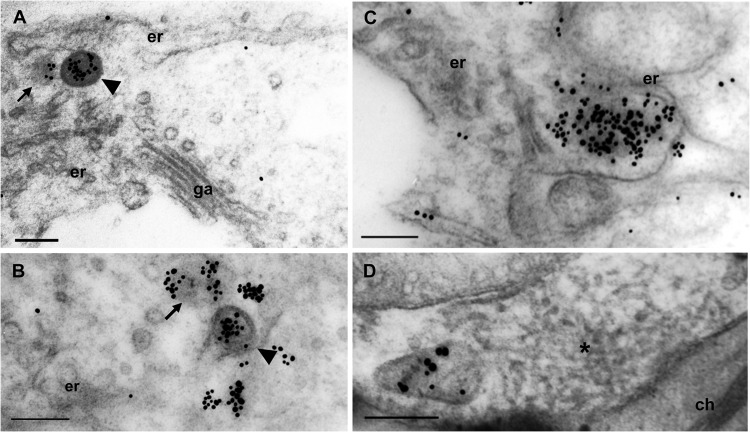
To analyze NP localization using immunogold labeling, we generated anti-NP antibodies. We confirmed that anti-NP antibodies showed little or no nonspecific background labeling on healthy fig leaves (data not shown). Anti-NP antibodies recognized the interiors of DMBs in FMV-infected fig leaves, indicating dense packing of NPs in virus particles (Fig. 2A and B, arrowheads). Intriguingly, some NPs formed agglomerates which were not surrounded by the membrane despite the close association with the ER (Fig. 2A and B, arrows). Membrane-free agglomerates occasionally increased in size, which was accompanied by a distorted ER (Fig. 2C). No labeling of anti-NP antibodies was observed in the matrix despite the close association with a DMB (Fig. 2D), suggesting that the matrices did not contain NPs. Neither cytological alteration nor specific labeling was found in the other organelles. Taken together, these findings suggest that NP agglomerates are predominantly enveloped by the ER membrane; however, nonenveloped NP agglomerates were also observed in close proximity to the ER in FMV-infected cells. These observations suggested that the nonenveloped NP agglomerates localize in close proximately to the ER to exploit the membrane for particle formation.
FIG 2.
NP forming cytoplasmic agglomerates in close proximity to the ER and NP agglomerates predominantly enveloped by the ER membrane in FMV-infected fig leaves. Immunogold labeling was performed using an anti-NP antibody and 15-nm gold particle-conjugated anti-rabbit antibody. (A and B) A DMB (arrowhead) and an agglomerate that is not enveloped (arrowhead). (C) Larger agglomerates curving the ER cisternae. (D) NP was not detected in the matrix (asterisk). er, endoplasmic reticulum; ch, chlorophyll. Bars, 200 nm.
Intracellular distribution of NP expressed transiently in Nicotiana benthamiana leaves.
Based on electron microscopy in FMV-infected cells, NP agglomerates may play central roles as core structures of DMBs in particle formation. To further investigate NP function, a C-terminal fusion with yellow fluorescent protein (NP:YFP) was transiently expressed in N. benthamiana leaves by using Agrobacterium tumefaciens. Observation of epidermal cells at 36 hpi revealed that NP:YFP formed motile and small punctate structures throughout the cytoplasm (Fig. 3A; see also Video S1 in the supplemental material). The punctate structures composed of NPs are referred to here as NP bodies (NBs). Some NBs were almost stationary, whereas others moved rapidly (Fig. 3B). Although the velocities of NB motion were highly variable, the average velocity was estimated to be approximately 0.5 μm/s.
FIG 3.
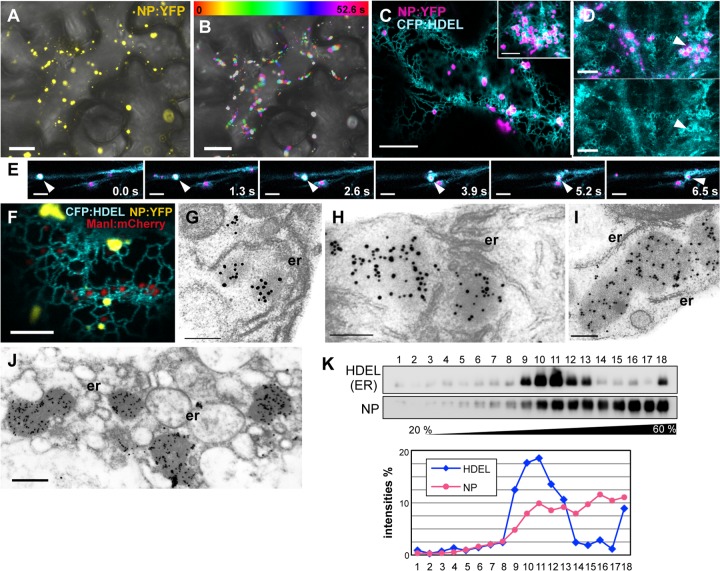
Transiently expressed NP forming NBs that move rapidly along with the ER. (A and B) Intracellular distribution and motility of NBs. NP:YFP was expressed transiently in N. benthamiana epidermal cells by using agroinfiltration. Fluorescence of the infiltrated cells was observed at 36 hpi. Bright-field images were merged. (A) NP:YFP forming punctate agglomerates (NBs) throughout the cell. (B) Integration of time-lapse images of the experiment shown in panel A. Twenty frames were captured at 2.6-s intervals. (C to E) Cells coexpressing NP:YFP (pseudocolored magenta) and CFP:HDEL as observed at 36 hpi (C and E) or 48 hpi (D). (C) NBs localized within the ER network. (Inset) A magnified image of the association of NBs with the distorted ER. (D) Three-dimensional projection of entire z-stacks. The ER was severely distorted by expression of NP:YFP. A merged image of NP:YFP and CFP:HDEL (upper panel) and an image of CFP:HDEL only (lower panel) are shown. (E) NBs moved together with transvacuolar ER streaming. Arrowheads point to an NB and the ER. (F) ManI:mCherry was coexpressed with NP:YFP and CFP:HDEL. (G to J) Immunogold labeling with thin sections of NP:YFP-expressing N. benthamiana leaves by using an anti-NP antibody and a 15-nm gold particle-conjugated anti-rabbit antibody. The leaves were fixed at 30 hpi (G to I) or 40 hpi (J). (G) NBs in close proximity to ER cisterna; (H) NBs surrounded by ER tubules; (I) NBs clearly separated from the ER; (J) NBs localized near to the drastically modified ER. er, endoplasmic reticulum. (K) Subcellular fractionation analysis of NP. The membrane-enriched fraction (P30) extracted from leaves coexpressing NP:3myc and CFP:HDEL was subjected to sucrose density gradient centrifugation. An immunoblot analysis using anti-GFP and anti-myc antibodies was subsequently performed. The fractions were numbered from top to bottom (1 to 18). The intensities of NP:3myc and CFP:HDEL signals were quantitated and are presented in the graph. Bars: 25 μm (A and B); 10 μm (C and D); 5 μm (C [inset], E, and F); 200 nm (G to I); 500 nm (J).
Since NP agglomerates were associated with the ER in FMV-infected cells, a protein fusion of CFP with an ER retention signal (CFP:HDEL) was coexpressed with NP:YFP (40). At 36 hpi, NBs that localized within the ER network caused abnormal ER tubule shrinkage and partial distortion of the mesh-like pattern (Fig. 3C). Such modification of the ER structure was not observed in cells expressing CFP:HDEL alone (data not shown). At 48 hpi, more severe disruption of the ER network was observed in NP:YFP-expressing cells (Fig. 3D). NBs were surrounded by the distorted ER, and the mesh-like pattern of the ER network was barely visible.
Closer observation of cells coexpressing NP:YFP and CFP:HDEL revealed that NBs and the ER moved together. The synchronous movement of NBs and the ER was difficult to recognize, because the ER network and its motility were highly intricate; however, it was comparatively easy to recognize in transvacuolar strands due to their straight and unidirectional streaming (Fig. 3E; see also Video S2 in the supplemental material).
Because the morphology and motion of NBs resembled those of Golgi complexes, the Golgi apparatus was visualized using an mCherry fusion with ManI (ManI:mCherry) (40). When coexpressed with NP:YFP and CFP:HDEL, the fluorescent signal of NP:YFP was clearly distinguished from that of ManI:mCherry (Fig. 3F). NBs filled small polygonal mesh regions of the ER, whereas the Golgi apparatuses were located in larger polygons slightly apart from the ER tubules. Time-lapse imaging showed that NBs and Golgi bodies moved similarly along with the ER (see Video S3 in the supplemental material).
To examine NB localization more closely, NP:YFP-expressing cells were observed by electron microscopy using immunogold labeling. At 30 hpi, a large number of electron-opaque agglomerates localized in close proximity to the ER membrane (Fig. 3G). Anti-NP antibodies identified these agglomerates as NBs. NBs were frequently amorphous, with estimated diameters of 100 to 500 nm, and were similar to the NP agglomerates observed in FMV-infected cells in terms of size, morphology, and localization. Some NBs localized within the intricate ER network (Fig. 3H). NBs were close to the ER but clearly separated from the ER membrane, and they appeared to localize in the cytoplasm (Fig. 3I). At 40 hpi, NBs became larger than those at 30 hpi, and the ER was transformed into ring-like structures (Fig. 3J). These structures were similar to those observed by confocal microscopy (Fig. 2D).
To gain further insights into the subcellular distribution of NPs, we performed sucrose density gradient centrifugation. We prepared an expression vector encoding an NP carrying a 3-myc tag at its C terminus (NP:3myc). Total membrane proteins extracted from leaves coexpressing CFP:HDEL and NP:3myc were subjected to sucrose gradient centrifugation. Immunoblot analysis of gradient fractions showed that NP:3myc did not comigrate with CFP:HDEL (Fig. 3K). An increasing amount of NP:3myc was detected from the top to bottom fractions, whereas the amount of CFP:HDEL peaked in the middle fractions. Taking the results of microscopic analysis into consideration, NBs localized in the cytoplasm but were enriched in the total membrane fraction due to their high density.
Intracellular movement of NBs was actomyosin dependent.
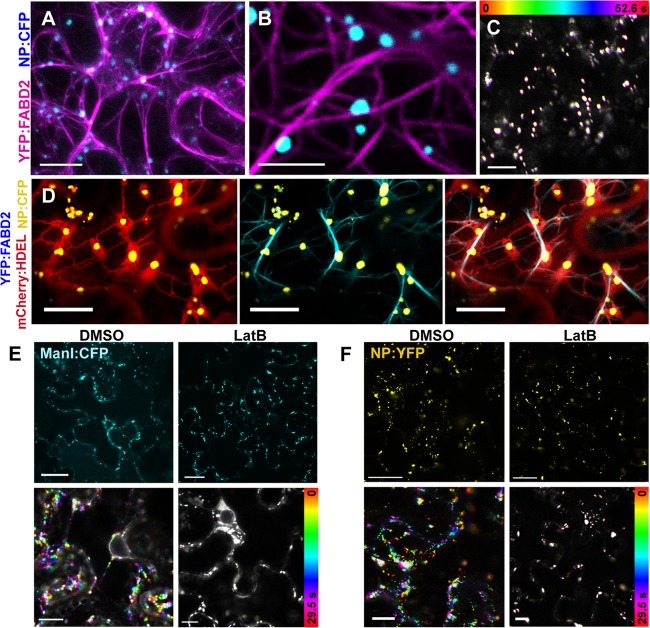
Since the rapid movement along the ER was reminiscent of the actomyosin system, an actin filament marker was coexpressed with NP:CFP. Actin filaments were visualized with an N-terminal fusion of YFP to the C-terminal half of A. thaliana fimbrin1 (YFP:FABD2), which contains an actin-binding domain (38). NBs localized along YFP:FABD2-labeled actin filaments in epidermal cells (Fig. 4A and B). Unlike the ER, actin filaments were not affected by NP:CFP expression, but YFP:FABD2 expression unexpectedly halted the movement of NBs (Fig. 4C), probably due to the actin-binding activity of FABD2. Similarly, previous studies showed that transient overexpression of FABD2 inhibited the actomyosin system and disrupted the trafficking of viral replication complexes or organelles along actin filaments (47, 48). To obtain further information on NB localization, NP:CFP, YFP:FABD2, and an mCherry fusion were coexpressed with an ER retention signal (mCherry:HDEL). We found that the ER ran parallel with actin filaments, whereas NBs were surrounded by the ER (Fig. 4D).
FIG 4.
The actomyosin system is involved in NB motility. (A to C) Confocal imaging of NBs and actin filaments. NP:CFP and YFP:FABD2 (pseudocolored magenta) were coexpressed in N. benthamiana leaves and observed at 36 hpi. (A) Three-dimensional projection of entire z-stacks. NP:CFP localized along actin filaments. (B) A magnified image of the experiment shown in panel A. (C) Integration of time-lapse images of NP:CFP. (D) YFP:FABD2 (pseudocolored cyan), NP:CFP (pseudocolored yellow), and mCherry:HDEL were coexpressed and observed at 36 hpi. (E and F) Effects of inhibition of actin filaments on the motilities of the Golgi apparatus and NBs. Leaves expressing ManI:CFP (E) or NP:YFP (F) at 26 hpi were treated with 0.5% DMSO (left column) or 10 μM LatB (right column) and observed at 36 hpi. Upper panels show localization of the fluorescent proteins. Lower panels are integrated time-lapse images. Bars: 10 μm (A and D); 5 μm (B); 25 μm (C, E, and F).
To verify NB movement by the actomyosin system, an inhibition assay using LatB, which induces the disassembly of actin filaments, was performed. To confirm the intended effect of LatB on actomyosin-based trafficking, the effect on Golgi apparatus motility was assessed, because the Golgi bodies are known to undergo rapid trafficking along the actin/ER network (8, 49). The Golgi apparatus was visualized using ManI:CFP (41). As expected, LatB treatment abolished the movement of the Golgi apparatus (Fig. 4E). The solvent, 0.5% (vol/vol) DMSO, had no effect on movement of the Golgi apparatus. LatB treatment almost abolished NB movement and did not change its localization (Fig. 4F). These results indicate that the intracellular movement of NBs is actomyosin dependent.
NB localization was dependent on the ER network configuration.
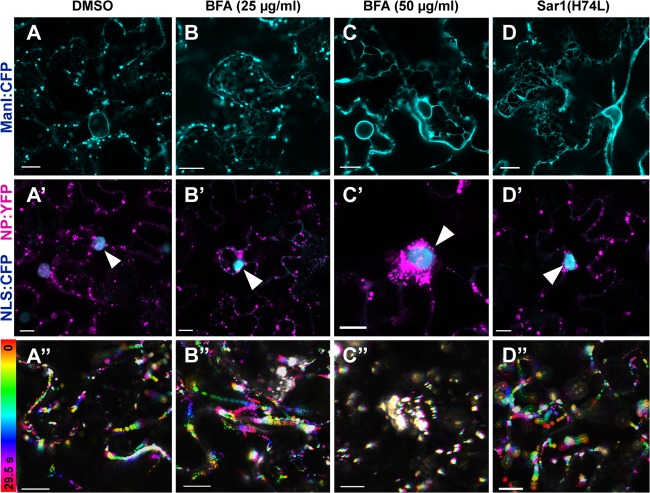
Involvement of the configuration of the ER network in NB localization and motility was assessed using BFA, which is an inhibitor that disables transport from the ER to the Golgi apparatus but also disrupts the ER network when used at concentrations of at least 50 μg/ml (50). To examine the effect of BFA on the Golgi apparatus and the ER network, ManI:CFP-expressing leaves were treated with BFA at various concentrations. At 25 μg/ml, although ManI:CFP was still distributed in the Golgi apparatus, a greater amount of ManI:CFP was retained in the ER than in solvent-treated cells (Fig. 5A and B). At 50 μg/ml, transport of ManI:CFP from the ER to the Golgi apparatus was almost completely blocked, and the abnormal ER network configuration was observed (Fig. 5C). When NP:YFP-expressing cells were treated with BFA at 25 μg/ml, no abnormality in NB localization or motility was found (Fig. 5B′ and B″). In contrast, at 50 μg/ml, excessive accumulation of NBs was observed surrounding the nuclei (Fig. 5C′), which were visualized using a CFP fusion of the nuclear localization signal of SV40 (NLS:CFP) (39). In these cells, the movements of NBs were abolished (Fig. 5C″). Such aberrant localization and motility of NBs were not observed in solvent-treated cells (Fig. 5A′ and A″).
FIG 5.
Effects of higher concentrations of BFA on NB localization and the Golgi apparatus. All images show leaves at 24 hpi. Leaves expressing ManI:CFP (A to C) or NP:YFP (pseudocolored magenta) and NLS:CFP (A′ to C′) at 18 hpi were treated with 0.5% DMSO (A and A′), BFA at 25 μg/ml (B and B′), BFA at 50 μg/ml (C and C′). Sar1(H74L) was coexpressed with ManI:CFP (D) or NP:YFP (D′). Arrowheads indicate nuclei. (A″ to D″) Integrated time-lapse images of experimental results shown in panels (A′ to D′). Bars, 10 μm.
To rule out the possibility that the inhibition of transport from the ER caused the aberrant NB localization, we specifically blocked transport from the ER to the Golgi apparatus by using Sar1(H74L) (51). Expression of Sar1(H74L) with ManI:CFP caused the disappearance of the Golgi apparatus, but the ER network configuration did not appear to be affected (Fig. 5D). Expression of Sar1(H74L) with NP:YFP did not affect NB localization or motility (Fig. 5D′ and D″). These observations indicated that disruption of the ER network configuration rather than inhibition of transport from the ER causes the aberrant localization. Taken together, these results indicate that NB localization is dependent on the ER network configuration.
Expression of the tail domains of XI-1, XI-2, and XI-K class XI myosins interfered with NB movement.
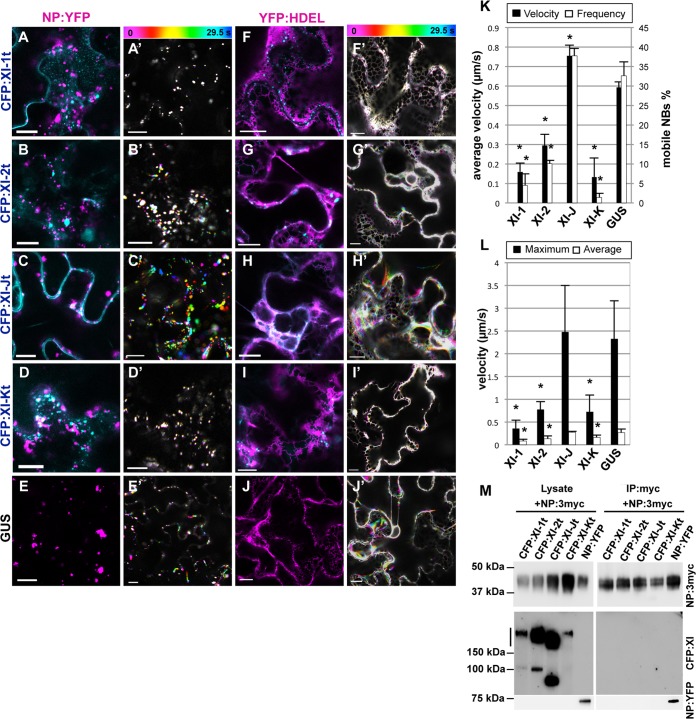
To elucidate the mechanisms of NB movement, we investigated the involvement of myosin motors, which are responsible for trafficking along actin filaments. N-terminal CFP fusions with a tail domain of class XI myosins of A. thaliana, XI-1, XI-2, XI-J, and XI-K, were coexpressed with NP:YFP. These headless myosins, which contain the cargo-binding domain and lack the motor domain, act as a dominant negative form of myosins to inhibit the cargo-binding function (2, 3, 5). GUS and NP:YFP were also coexpressed as controls. Initially, we investigated the subcellular localization of the myosin tails. CFP fusions with the myosin tails of XI-1, XI-2, and XI-K (CFP:XI-1t, CFP:XI-2t, and CFP:XI-Kt, respectively) labeled as punctate structures (Fig. 6A, B, and D), whereas a CFP fusion with the XI-J tail (CFP:XI-Jt) exhibited cytosolic localization (Fig. 6C). These observations were in accordance with the results of a previous study (6). NB localization was not apparently affected in cells expressing the myosin tails, compared to a GUS control (Fig. 6A to E). These myosin tails occasionally localized close to, but did not colocalize with, NBs. In cells expressing CFP:XI-1t, CFP:XI-2t, or CFP:XI-Kt, NB movement was strongly disrupted (Fig. 6A′, B′, and D′). To calculate the velocity, NBs that showed wobbling motion indistinguishable from the drift of the specimen were selectively eliminated. After counting the number of mobile NBs, the percentage and the average velocity of mobile NBs were calculated. Quantification of NB movement velocities revealed that the average velocities in cells expressing CFP:XI-1t, CFP:XI-2t, or CFP:XI-Kt were 0.16, 0.29, and 0.13 μm/s, respectively (Fig. 6K); the percentages of mobile NBs were 4.4%, 10.0%, and 1.3%, respectively, which correlated with the average velocities. In contrast, NBs in CFP:XI-Jt-expressing cells demonstrated more rapid movement than NBs in GUS-expressing cells, with average velocities of 0.75 μm/s and 0.59 μm/s and percentages of mobile NBs of 37.7% and 32.6%, respectively (Fig. 6C′, E′, and K).
FIG 6.
NB trafficking and ER streaming were affected by dominant negative inhibition of class XI myosins in a similar pattern. NP:YFP (pseudocolored magenta) (A to E) or YFP:HDEL (pseudocolored magenta) (F to J) was coexpressed with CFP:XI-1t (A and F), CFP:XI-2t (B and G), CFP:XI-Jt (C and H), CFP:XI-Kt (D and I), and GUS (E and J). (A′ to J′) Integrated time-lapse images of experiments shown in panels (A to J). (K and L) Quantification of the velocities of NB motility and ER streaming. (K) Percentages of mobile NBs and their average velocities in the presence of the respective myosin tails or GUS. Three movies were analyzed, and the average values with standard deviations are presented. (L) The average and maximum velocities of ER streaming in the presence of myosin tails or GUS. Six movies were analyzed, and the average values and standard deviations are presented. Asterisks indicate significant differences from a GUS control (P < 0.01). (M) A coimmunoprecipitation experiment with NP and class XI myosins. The four CFP fusions with myosin tails or NP:YFP were coexpressed with NP:3myc. The inputs and precipitants were analyzed by immunoblotting using anti-myc and anti-GFP antibodies. Bars, 10 μm.
To ascertain whether the myosin tails were bound to NBs, a coimmunoprecipitation assay was carried out. NP:3myc was coexpressed with CFP:XI-1t, CFP:XI-2t, CFP:XI-Jt, CFP:XI-Kt, or NP:YFP. Immunoblot analysis of input extracts confirmed the expression of NP:3myc, NP:YFP, and the CFP-tagged myosin tails (Fig. 6M). The myosin tails were detected predominantly at twice the size of the monomer (approximately 170 to 200 kDa), which indicated homodimerization of the myosin tails and was in accordance with a previous report (52). NP:YFP coprecipitated with NP:3myc, consistent with agglomerate formation of NPs in vivo. However, none of the myosin tails precipitated with NP:3myc. These data suggested that the NP molecules interacted with each other, but not with the myosin tails.
Expression of myosin XI-1, XI-2, and XI-K tails also interfered with ER streaming.
Because NB localization and motility were closely related to the ER, we assumed that ER streaming caused NB movement. To test this hypothesis, we investigated a dominant negative effect of class XI myosins on ER streaming in the same way as for NBs. CFP:XI-1t, CFP:XI-2t, CFP:XI-Jt, CFP:XI-Kt, and GUS were coexpressed with YFP:HDEL. In GUS-expressing cells, transvacuolar ER strands were visible (Fig. 6J). However, in CFP:XI-1t-expressing cells, transvacuolar ER strands disappeared, and only a slightly distorted mesh-like pattern was observed (Fig. 6F). CFP:XI-Kt severely affected the structure of the ER, and distortion and collapse of the ER network were observed frequently (Fig. 6I). CFP:XI-2t and CFP:XI-Jt did not appear to modify the ER structure (Fig. 6G and H). Color-coded visualization of time-lapse images revealed minimal ER remodeling in the CFP:XI-1t-, CFP:XI-2t-, or CFP:XI-Kt-expressing cells (Fig. 6F′, G′, and I′). In contrast, expression of CFP:XI-Jt and GUS had negligible effects on ER remodeling (Fig. 6H′ and J′). Calculation of the maximum and average velocities of ER streaming showed that velocities in CFP:XI-1t-, CFP:XI-2t-, or CFP:XI-Kt-expressing cells were significantly lower than those in CFP:XI-J- or GUS-expressing cells (Fig. 6L). This pattern was similar to that of NBs (Fig. 6K). We note that the average velocities of ER streaming were uniformly lower than that of NB movement because we used different approaches to measure these velocities. To measure ER streaming, we employed the ImageJ plugin software KbiFlow (9), which facilitates detection of even slight movements of fluorescent points of interest in images. Taken together, these data suggest that the patterns of the dominant negative effect on NB movement and ER streaming were similar, suggesting that the motive forces underlying NB movement and ER streaming are produced by the same mechanism.
RNA-binding activity of FMV NP.
Although NPs are characterized by their general RNA-binding activities (25, 29), NPs of emaraviruses, including FMV, had not been assessed for RNA-binding activity. RNA-binding activity was verified in an EMSA using a recombinant fusion of NP with maltose-binding protein (MBP:NP). A 32P-labeled minus-stranded RNA encompassing the 5′ terminal region of FMV RNA5 was used as a probe. As expected, MBP:NP at 1.0 μM retarded probe mobility. The probe that bound to MBP:NP did not migrate into the gel, indicating that MBP:NP formed a large NP-RNA complex (Fig. 7A). In contrast, a comparative amount of the full-length MBP did not retard the probe, excluding the possibility that MBP contributes to RNA-binding activity.
FIG 7.
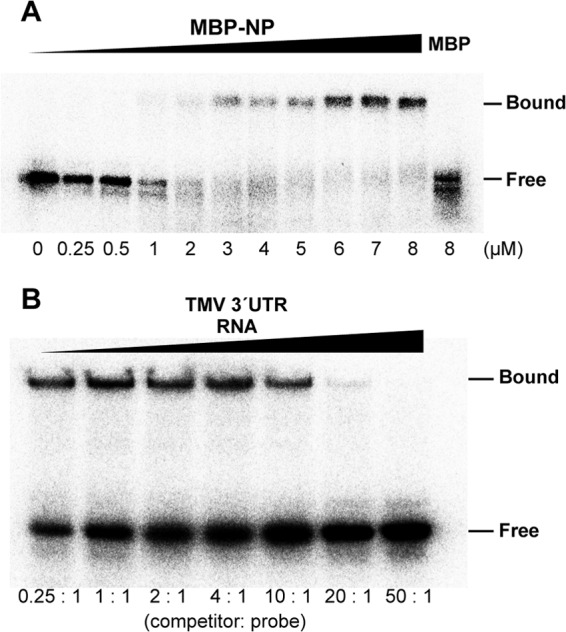
Analysis of NP RNA-binding activity. (A) Electron mobility shift assay. The indicated concentrations of MBP:NP and MBP were incubated with the 32P-labeled 5′-terminal region of RNA5 from FMV as a probe. Positions of free and bound RNAs are indicated. (B) Competitor assay. MBP:NP and the FMV probe were incubated with the unlabeled 3′-terminal region of TMV genomic RNA at the indicated molar ratios.
To examine whether MBP:NP bound preferentially to FMV genomic RNA, we performed a competition assay using the unlabeled 3′-terminal region of the TMV genome. The 3′-TMV RNA began to compete with the probe at an FMV:TMV ratio of 1:20 (mol:mol) (Fig. 7B). Given that viral proteins bind to a favorable RNA sequence in the presence of competitors in 100-fold excess (53, 54), these results showed that MBP:NP has little or no preference for FMV genomic RNA. The NPs of (–)RNA viruses themselves are known to have little or no preference for their own genomic RNAs, but they exhibit specificity for their own genomic RNAs in the presence of other viral factors, such as RdRps (29).
DISCUSSION
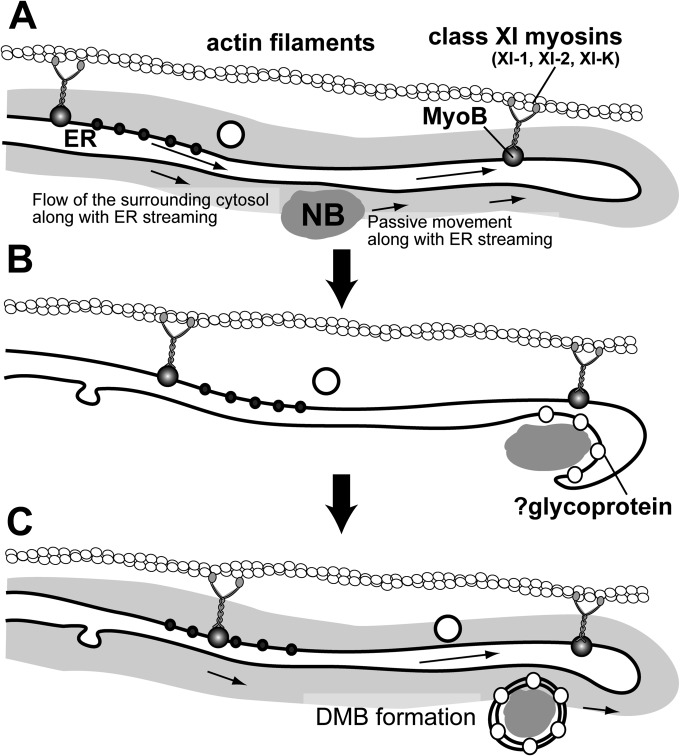
Based on the results of this study, we concluded that NBs localize in the cytoplasm in close proximity to the ER membrane, which enables NBs to move passively throughout the cells by ER streaming, with a motive force provided by class XI myosins and actin filaments running along the ER (Fig. 8A). Four lines of evidence obtained in this study support this model of movement.
FIG 8.
Schematic representation of an NB trafficking model in FMV-infected cells. (A) NPs form NBs, which localize in close proximity to the ER membrane. An NB is passively moved by ER streaming. The cytoplasmic flow generated by ER streaming may be responsible for NB motility. ER streaming is caused by the class XI myosins, XI-1, XI-2, XI-K, and MyoB receptors. ER streaming leads an NB to a site of viral budding. (B) At the budding site, the NB was enveloped by the ER membrane to form a DMB. To establish the envelopment, other viral components, such as glycoprotein, are required. (C) The DMB also moves along with the ER to further the infection process.
First, NPs formed motile agglomerates whose motility was dependent on the actomyosin system. Agglomerate formation of NPs was most evident in the electron microscopic analysis using immunogold labeling (Fig. 2 and 3G to J). Such NBs rapidly moved along the actin/ER network throughout the cells (Fig. 3A and B and 4A, B, and D). LatB treatment confirmed that NB movements were dependent on the actomyosin system (Fig. 4E and F).
Second, the motility of NBs was not caused by interactions with actin filaments. Although expression of class XI myosin tails abolished NB movement, myosin tails and NBs showed neither colocalization nor interaction with each other (Fig. 6A to D and M). Furthermore, NB localization was hampered by a higher concentration of BFA, which did not inhibit the actomyosin system (8) (Fig. 5C′). Given that myosins require specific receptor proteins that are anchored to the acceptor membrane (55, 56), NBs were probably dragged by the movement of membrane-bound organelles, which are directly propelled by myosin motors.
Third, NBs localized in the cytoplasm in close proximity to the ER membrane. Confocal and electron microscopic analyses showed that NBs were located very close to the ER (Fig. 2 and 3C to J); however, electron microscopy also revealed that NBs were clearly separated from the ER membrane in both FMV-infected cells and NP-expressing cells (Fig. 2A and 3G to J). Furthermore, NP did not comigrate with the ER marker in the sucrose gradient analysis (Fig. 3K). These results suggested that NBs are not anchored to the ER membrane and that they localize in the cytoplasm.
Finally, NBs exhibited behavior remarkably similar to ER streaming. We observed the movements of NBs along with ER streaming (Fig. 3E; see also Video S2 in the supplemental material), and the dominant negative effects by class XI myosin tails on NB movement and ER streaming occurred in a similar pattern (Fig. 6K and L). Therefore, ER streaming likely provides the motive force for NB movement.
Taken together, these results indicate that ER streaming hauls FMV NBs in close proximity to the ER, which is arranged in parallel with actin filaments (Fig. 8A). A previous study suggested that the cytosol surrounding the ER flows concomitant with ER streaming (9). Therefore, the flow of the cytosol surrounding the ER is considered to provide the force for NB movement (Fig. 8A).
The model for NB movement presented here is in good agreement with the model for vesicle transport in plant cells. Recent studies on class XI myosin motors have suggested that attachment of class XI myosins (XI-1, XI-K, and XI-I) to endomembranes is mediated by MyoB receptors (56). Intriguingly, although dominant negative inhibition of XI-K myosins abolished the movements of almost all organelles and endomembranes (2, 3), XI-K and MyoB did not colocalize with these organelles or endomembranes, the motilities of which were inhibited by the knockout of XI-K or expression of XI-K tail (56). Therefore, a model was proposed where XI-K and MyoB directly provide the motive force for specific organelles; other organelles, to which XI-K and MyoB do not localize, move passively. Importantly, XI-K and MyoB cofractionated predominantly with the ER, and hence the ER was most likely to be directly driven by XI-K and MyoB complexes (9, 56) (Fig. 8). These findings are highly compatible with our results, which indicated passive movements of NBs by ER streaming. In this context, our data not only corroborate the model for vesicle transport but also extend the potential targets for passive movements to viral protein complexes in this model.
Many viral factors have been reported to display rapid intracellular movement (10). Some of these proteins and protein complexes have been postulated to be anchored to, or vesiculated by, the endomembrane, which allows these proteins to make use of the actomyosin cytoskeleton to move rapidly throughout the cell (10, 21, 22). However, the model presented in this study suggests that the viral protein trafficking can be a passive movement caused by ER streaming. We suggest that the model presented herein is widely applicable to the intracellular movements of viral proteins or protein complexes.
Although rapid movement of protein bodies induced by plant viruses has generally been linked to viral cell-to-cell movement (10, 57, 58), our results provide another explanation for rapid intracellular movement: the proximity of viral protein bodies to the ER causes passive movements with ER streaming. In other words, rapid movements with ER streaming can reflect spatial and functional relationships between these protein bodies and the ER. Regarding FMV NP, given that NBs were enveloped by the ER membrane in the infected cells, NBs localized in close proximity to the ER to form enveloped particles (Fig. 8B).
The results of this study raised a further question: do DMBs move rapidly in FMV-infected cells, as is the case for NBs formed by expression of NP alone? In (–)RNA plant viruses, complete infectious cDNA clones have not yet been established. Therefore, the difficulty of a reverse genetics approach prevents live imaging of these viruses, and the dynamics of vRNPs or enveloped particles in infected cells remains enigmatic. We demonstrated that DMBs as well as NBs localized adjacent to the ER in FMV-infected cells (Fig. 1, 2, and 3G to J). These observations allowed us to speculate that DMBs were also dragged passively by ER streaming (Fig. 8C). While DMBs were readily observed in FMV-infected cells, membrane-enveloped NBs were not observed in FMV NP-expressing cells (Fig. 3G to J). This suggested that other viral components, presumably GP, a transmembrane spike protein, are required to form enveloped particles (Fig. 8B) (59).
NPs of Tomato spotted wilt virus (TSWV), another (–)RNA plant virus, have been reported to form cytoplasmic agglomerates (60, 61) and to move along the actin/ER network (16). Since these features are quite similar to those of FMV NP, we considered the possibility that ER streaming also mediates TSWV NP movement. However, even though a headless XI-K myosin abolished TSWV NP movement, a headless XI-2 myosin had a negligible effect (16), which is inconsistent with the results of our study. Naturally, we cannot dismiss the possibility that NPs of the two viruses move in different systems, but there is another explanation for this inconsistency. Myosins used in the previous study originated from N. benthamiana. Unlike A. thaliana, the role of N. benthamiana myosins in ER streaming has not been assessed, and we suspect that myosin orthologs from N. benthamiana have a different effect on the dominant negative inhibition.
The dominant negative inhibition by the four class XI myosins, XI-1, XI-2, XI-J, and XI-K, provided important insight into the involvement of these myosins in the structure and dynamics of the ER. Expression of the XI-1 tail resulted in the disappearance of transvacuolar ER strands. In contrast, transvacuolar ER strands were observed in XI-2 or XI-K tail-expressing cells, despite the disruption of ER streaming (data not shown). Actin polymerization is involved in the composition of transvacuolar strands, and several inhibitors of actin filaments have been reported to induce the disappearance of transvacuolar strands (62–65). The XI-1 tail may cause structural perturbation of actin filaments. In the case of the XI-2 tail, no inhibitory effect was found except for the abolition of ER streaming (Fig. 6G, G′, and L), implying that the XI-2 tail specifically inhibited ER streaming. The expression of the XI-K tail had the most severe effect on the ER structure (Fig. 6I), although the decline in ER streaming velocity was the same as in XI-2 tail-expressing cells (Fig. 6L). These different structural modifications in XI-2- and XI-K-expressing cells were also reported in a recent study (66). Therefore, and taken together, these results indicate that XI-1, XI-2, and XI-K contribute to ER streaming in different ways.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kenichi Ikeda and Kanako Inoue (Kobe University) for valuable advice on the immunogold labeling technique. We are grateful to Yu Inoue and Kyoko Watanabe (Tamagawa University) for their technical assistance in electron microscopy.
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02527-14.
REFERENCES
- 1.Vale RD. 2003. The molecular motor toolbox for intracellular transport. Cell 112:467–480. doi: 10.1016/S0092-8674(03)00111-9. [DOI] [PubMed] [Google Scholar]
- 2.Avisar D, Prokhnevsky AI, Makarova KS, Koonin EV, Dolja VV. 2008. Myosin XI-K is required for rapid trafficking of Golgi stacks, peroxisomes, and mitochondria in leaf cells of Nicotiana benthamiana. Plant Physiol 146:1098–1108. doi: 10.1104/pp.107.113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avisar D, Abu-Abied M, Belausov E, Sadot E, Hawes C, Sparkes IA. 2009. A comparative study of the involvement of 17 Arabidopsis myosin family members on the motility of Golgi and other organelles. Plant Physiol 150:700–709. doi: 10.1104/pp.109.136853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparkes IA, Teanby NA, Hawes C. 2008. Truncated myosin XI tail fusions inhibit peroxisome, Golgi, and mitochondrial movement in tobacco leaf epidermal cells: a genetic tool for the next generation. J Exp Bot 59:2499–2512. doi: 10.1093/jxb/ern114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sparkes I, Runions J, Hawes C, Griffing L. 2009. Movement and remodeling of the endoplasmic reticulum in nondividing cells of tobacco leaves. Plant Cell 21:3937–3949. doi: 10.1105/tpc.109.072249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparkes I. 2011. Recent advances in understanding plant myosin function: life in the fast lane. Mol Plant 4:805–812. doi: 10.1093/mp/ssr063. [DOI] [PubMed] [Google Scholar]
- 7.Avisar D, Abu-Abied M, Belausov E, Sadot E. 2012. Myosin XIK is a major player in cytoplasm dynamics and is regulated by two amino acids in its tail. J Exp Bot 63:241–249. doi: 10.1093/jxb/err265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge B, Hawes C. 1998. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant J 15:441–447. doi: 10.1046/j.1365-313X.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 9.Ueda H, Yokota E, Kutsuna N, Shimada T, Tamura K, Shimmen T, Hasezawa S, Dolja VV, Hara-Nishimura I. 2010. Myosin-dependent endoplasmic reticulum motility and F-actin organization in plant cells. Proc Natl Acad Sci U S A 107:6894–6899. doi: 10.1073/pnas.0911482107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harries PA, Schoelz JE, Nelson RS. 2010. Intracellular transport of viruses and their components: utilizing the cytoskeleton and membrane highways. Mol Plant Microbe Interact 23:1381–1393. doi: 10.1094/MPMI-05-10-0121. [DOI] [PubMed] [Google Scholar]
- 11.Amari K, Lerich A, Schmitt-Keichinger C, Dolja VV, Ritzenthaler C. 2011. Tubule-guided cell-to-cell movement of a plant virus requires class XI myosin motors. PLoS Pathog 7:e1002327. doi: 10.1371/journal.ppat.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avisar D, Prokhnevsky AI, Dolja VV. 2008. Class VIII myosins are required for plasmodesmatal localization of a closterovirus Hsp70 homolog. J Virol 82:2836–2843. doi: 10.1128/JVI.02246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan Z, Chen H, Chen Q, Omura T, Xie L, Wu Z, Wei T. 2011. The early secretory pathway and an actin-myosin VIII motility system are required for plasma-desmatal localization of the NSvc4 protein of Rice stripe virus. Virus Res 159:62–68. doi: 10.1016/j.virusres.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Cotton S, Grangeon R, Thivierge K, Mathieu I, Ide C, Wei T, Wang A, Laliberte JF. 2009. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. J Virol 83:799–809. doi: 10.1128/JVI.00819-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui X, Wei T, Chowda-Reddy RV, Sun G, Wang A. 2010. The Tobacco etch virus P3 protein forms mobile inclusions via the early secretory pathway and traffics along actin microfilaments. Virology 397:56–63. doi: 10.1016/j.virol.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Feng Z, Chen X, Bao Y, Dong J, Zhang Z, Tao X. 2013. Nucleocapsid of Tomato spotted wilt tospovirus forms mobile particles that traffic on an actin/endoplasmic reticulum network driven by myosin XI-K. New Phytol 200:1212–1224. doi: 10.1111/nph.12447. [DOI] [PubMed] [Google Scholar]
- 17.Harries PA, Palanichelvam K, Yu W, Schoelz JE, Nelson RS. 2009. The cauliflower mosaic virus protein P6 forms motile inclusions that traffic along actin microfilaments and stabilize microtubules. Plant Physiol 149:1005–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu JZ, Blancaflor EB, Nelson RS. 2005. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiol 138:1853–1865. doi: 10.1104/pp.105.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei T, Wang A. 2008. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. J Virol 82:12252–12264. doi: 10.1128/JVI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei T, Huang T, McNeil J, Laliberte JF, Hong J, Nelson RS, Wang A. 2010. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. J Virol 84:799–809. doi: 10.1128/JVI.01824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laliberte JF, Sanfaçon H. 2010. Cellular remodeling during plant virus infection. Annu Rev Phytopathol 48:69–91. doi: 10.1146/annurev-phyto-073009-114239. [DOI] [PubMed] [Google Scholar]
- 22.Niehl A, Heinlein M. 2011. Cellular pathways for viral transport through plasmodesmata. Protoplasma 248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 23.Fontana J, López-Montero N, Elliott RM, Fernández JJ, Risco C. 2008. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell Microbiol 10:2012–2028. doi: 10.1111/j.1462-5822.2008.01184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kikkert M, Lent J, Storms M, Bodegom P, Kormelink R, Goldbach R. 1999. Tomato spotted wilt virus particle morphogenesis in plant cells. J Virol 73:2288–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kormelink R, Garcia ML, Goodin M, Sasaya T, Haennie AL. 2011. Negative-strand RNA viruses: The plant-infecting counterparts. Virus Res 162:184–202. doi: 10.1016/j.virusres.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 26.Nayak DP, Balogun RA, Yamada H, Zhou ZH, Barman S. 2009. Influenza virus morphogenesis and budding. Virus Res 143:147–161. doi: 10.1016/j.virusres.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cros JF, Palese P. 2003. Trafficking of viral genomic RNA into and out of the nucleus: influenza, Thogoto and Borna disease viruses. Virus Res 95:3–12. doi: 10.1016/S0168-1702(03)00159-X. [DOI] [PubMed] [Google Scholar]
- 28.Kaukinen P, Vaheri A, Plyusnin A. 2005. Hantavirus nucleocapsid protein: a multifunctional molecule with both housekeeping and ambassadorial duties. Arch Virol 150:1693–1713. doi: 10.1007/s00705-005-0555-4. [DOI] [PubMed] [Google Scholar]
- 29.Ruigrok RW, Crêpin T, Kolakofsky D. 2011. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr Opin Microbiol 14:504–510. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Elbeaino T, Digiaro M, Martelli GP. 2009. Complete nucleotide sequence of four RNA segments of fig mosaic virus. Arch Virol 154:1719–1727. doi: 10.1007/s00705-009-0509-3. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa K, Maejima K, Komatsu K, Kitazawa Y, Hashimoto M, Takata D, Yamaji Y, Namba S. 2012. Identification and characterization of two novel genomic RNA segments of fig mosaic virus, RNA5 and RNA6. J Gen Virol 93:1612–1619. doi: 10.1099/vir.0.042663-0. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa K, Maejima K, Komatsu K, Netsu O, Keima T, Shiraishi T, Okano Y, Hashimoto M, Yamaji Y, Namba S. 2013. Fig mosaic emaravirus p4 protein is involved in cell-to-cell movement. J Gen Virol 94:682–686. doi: 10.1099/vir.0.047860-0. [DOI] [PubMed] [Google Scholar]
- 33.Appiano A, Conti M, Zini N. 1995. Cytopathological study of the double-membrane bodies occurring in fig plants affected by fig mosaic disease. Acta Hortic 386:585–592. [Google Scholar]
- 34.Martelli GP, Castellano MA, Lafortezza R. 1993. An ultrastructural study of fig mosaic. Phytopathol Mediterr 32:33–43. [Google Scholar]
- 35.Hashimoto M, Komatsu K, Maejima K, Okano Y, Shiraishi T, Ishikawa K, Takinami Y, Yamaji Y, Namba S. 2012. Identification of three MAPKKKs forming a linear signaling pathway leading to programmed cell death in Nicotiana benthamiana. BMC Plant Biol. 12:e103. doi: 10.1186/1471-2229-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Earley KW, Haag JR, Pontes O, Opper K, Juehne T, Song K, Pikaard CS. 2006. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 37.daSilva LLP, Snapp EL, Denecke J, Lippincott-Schwartz J, Hawes C, Brandizzi F. 2004. Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells. Plant Cell 16:1753–1771. doi: 10.1105/tpc.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voigt B, Timmers ACJ, Samaj J, Müller J, Baluska F, Menzela D. 2004. GFP-FABD2 fusion construct allows in vivo visualization of the dynamic actin cytoskeleton in all cells of Arabidopsis seedlings. Plant Physiol 84:595–608. doi: 10.1016/j.ejcb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Kalderon D, Roberts BL, Richardson WD, Smith AE. 1984. A short amino acid sequence able to specify nuclear location. Cell 39:499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- 40.Nelson BK, Cai X, Nebenführ A. 2007. Multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136. doi: 10.1111/j.1365-313X.2007.03212.x. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Yoshii A, Sakurai K, Hamada K, Yamaji Y, Suzuki M, Namba S, Hibi T. 2009. Identification of a novel tobacco DnaJ-like protein that interacts with the movement protein of tobacco mosaic virus. Arch Virol 154:959–967. doi: 10.1007/s00705-009-0397-6. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe T, Honda A, Iwata A, Ueda S, Hibi T, Ishihama A. 1999. Isolation from tobacco mosaic virus-infected tobacco of a solubilized template-specific RNA-dependent RNA polymerase containing a 126K/183K protein heterodimer. J Virol 73:2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakizawa S, Oshima K, Nishigawa H, Jung HY, Wei W, Suzuki S, Tanaka M, Miyata S, Ugaki M, Namba S. 2004. Secretion of immunodominant membrane protein from onion yellows phytoplasma through the Sec protein-translocation system in Escherichia coli. Microbiology 150:135–142. doi: 10.1099/mic.0.26521-0. [DOI] [PubMed] [Google Scholar]
- 44.Genovés A, Navarro JA, Pallás V. 2010. The intra- and intercellular movement of Melon necrotic spot virus (MNSV) depends on an active secretory pathway. Mol Plant Microbe Interact 3:263–272. doi: 10.1094/MPMI-23-3-0263. [DOI] [PubMed] [Google Scholar]
- 45.Yamaji Y, Kobayashi T, Hamada K, Sakurai K, Yoshii A, Suzuki M, Namba S, Hibi T. 2006. In vivo interaction between Tobacco mosaic virus RNA-dependent RNA polymerase and host translation elongation factor 1A. Virology 347:100–108. doi: 10.1016/j.virol.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 46.Elbeaino T, Digiaro M, Alabdullah A, De Stradis A, Minafra A, Mielke N, Castellano MA, Martelli GP. 2009. A multipartite single-stranded negative-sense RNA virus is the putative agent of fig mosaic disease. J Gen Virol 90:1281–1288. doi: 10.1099/vir.0.008649-0. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann C, Niehl A, Sambade A, Steinmetz A, Heinlein M. 2009. Inhibition of tobacco mosaic virus movement by expression of an actin-binding protein. Plant Physiol 149:1810–1823. doi: 10.1104/pp.108.133827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holweg CL. 2007. Living markers for actin block myosin-dependent motility of plant organelles and auxin. Cell Motil Cytoskeleton 64:69–81. doi: 10.1002/cm.20164. [DOI] [PubMed] [Google Scholar]
- 49.Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA. 1999. Stop-and-go movements of plant golgi stacks are mediated by the acto-myosin system. Plant Physiol 121:1127–1141. doi: 10.1104/pp.121.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schoelz JE, Harries PA, Nelson RS. 2011. Intracellular transport of plant viruses: finding the door out of the cell. Mol Plant 5:813–831. doi: 10.1093/mp/ssr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takeuchi M, Tada M, Saito C, Yashiroda H, Nakano A. 1998. Isolation of a tobacco cDNA encoding Sar1 GTPase and analysis of its dominant negative mutations in vesicular traffic using a yeast complementation system. Plant Cell Physiol 39:590–599. doi: 10.1093/oxfordjournals.pcp.a029409. [DOI] [PubMed] [Google Scholar]
- 52.Burkhard P, Stetefeld J, Strelkov SV. 2001. Fluorescent protein and COPI antisera coiled coils: a highly versatile protein folding motif. Trends Cell Biol 11:82–88. doi: 10.1016/S0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- 53.Martínez-Turiño S, Hernández C. 2010. Identification and characterization of RNA-binding activity in the ORF1-encoded replicase protein of Pelargonium flower break virus. J Gen Virol 91:3075–3084. doi: 10.1099/vir.0.023093-0. [DOI] [PubMed] [Google Scholar]
- 54.Rajendran KS, Nagy PD. 2003. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J Virol 77:9244–9258. doi: 10.1128/JVI.77.17.9244-9258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fagarasanu A, Mast FD, Knoblach B, Jin Y, Brunner MJ, Logan MR, Glover JN, Eitzen GA, Aitchison JD, Weisman LS, Rachubinski RA. 2009. Myosin-driven peroxisome partitioning in S. cerevisiae. J Cell Biol 186:541–554. doi: 10.1083/jcb.200904050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peremyslov VV, Morgun EA, Kurth EG, Makarova KS, Koonin EV, Dolja VV. 2013. Identification of myosin XI receptors in Arabidopsis defines a distinct class of transport vesicles. Plant Cell 25:3022–3038. doi: 10.1105/tpc.113.113704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agbeci M, Grangeon R, Nelson RS, Zheng H, Laliberte JF. 2013. Contribution of host intracellular transport machineries to intercellular movement of turnip mosaic virus. PLoS Pathog 9:e1003683. doi: 10.1371/journal.ppat.1003683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harries PA, Park JW, Sasaki N, Ballard KD, Maule AJ, Nelson RS. 2009. Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proc Nat Acad Sci U S A 106:17594–17599. doi: 10.1073/pnas.0909239106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walter CT, Barr JN. 2011. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol 92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro D, Goldbach R, Kormelink R. 2009. Requirements for ER-arrest and sequential exit to the Golgi of tomato spotted wilt virus glycoproteins. Traffic 10:664–672. doi: 10.1111/j.1600-0854.2009.00900.x. [DOI] [PubMed] [Google Scholar]
- 61.Ribeiro D, Jung M, Moling S, Borst JW, Goldbach R, Kormelink R. 2013. The cytosolic nucleoprotein of the plant-infecting bunyavirus tomato spotted wilt recruits endoplasmic reticulum-resident proteins to endoplasmic reticulum export sites. Plant Cell 25:3602–3614. doi: 10.1105/tpc.113.114298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoffmann A, Nebenführ A. 2004. Dynamic rearrangements of transvacuolar strands in BY-2 cells imply a role of myosin in remodeling the plant actin cytoskeleton. Protoplasma 224:201–210. doi: 10.1007/s00709-004-0068-0. [DOI] [PubMed] [Google Scholar]
- 63.Hussey PJ, Yuan M, Calder G, Khan S, Lloyd CW. 1998. Microinjection of pollen-specific actin-depolymerizing factor, ZmADF1, reorients F-actin strands in Tradescantia stamen hair cells. Plant J 14:353–357. doi: 10.1046/j.1365-313X.1998.00122.x. [DOI] [Google Scholar]
- 64.Shimmen T, Hamatani M, Saito S, Yokota E, Mimura T, Fusetani N, Karaki H. 1995. Roles of actin filaments in cytoplasmic streaming and organization of transvacuolar strands in root hair cells of Hydrocharis. Protoplasma 185:188–193. doi: 10.1007/BF01272859. [DOI] [Google Scholar]
- 65.Staiger CJ, Yuan M, Valenta R, Shaw PJ, Warn RM, Lloyd CW. 1994. Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr Biol 4:215–219. doi: 10.1016/S0960-9822(00)00050-6. [DOI] [PubMed] [Google Scholar]
- 66.Griffing LR, Gao HT, Sparkes I. 2014. ER network dynamics are differentially controlled by myosins XI-K, XI-C, XI-E, XI-I, XI-1, and XI-2. Front Plant Sci 5:218. doi: 10.3389/fpls.2014.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.