Abstract
Pain is fundamentally unpleasant, a feature that protects the organism by promoting motivation and learning. Relief of aversive states, including pain, is rewarding. The aversiveness of pain, as well as the reward from relief of pain, is encoded by brain reward/motivational mesocorticolimbic circuitry. In this Review, we describe current knowledge of the impact of acute and chronic pain on reward/motivation circuits gained from preclinical models and from human neuroimaging. We highlight emerging clinical evidence suggesting that anatomical and functional changes in these circuits contribute to the transition from acute to chronic pain. We propose that assessing activity in these conserved circuits can offer new outcome measures for preclinical evaluation of analgesic efficacy to improve translation and speed drug discovery. We further suggest that targeting reward/motivation circuits may provide a path for normalizing the consequences of chronic pain to the brain, surpassing symptomatic management to promote recovery from chronic pain.
Pain is a subjective individual experience composed of sensory, affective and cognitive dimensions. The sensory characteristics of pain, including the stimulus intensity, location, source (for example, deep organs or superficial structures) and quality (for example, burning, stabbing), are tightly linked to activation of nociceptors, nerves that signal mechanical, thermal or chemical insult1,2. The resulting pain experience, however, is affected by contextual and cognitive factors3. Nociceptive activation does not always produce pain, and conversely, pain can occur without an identifiable nociceptive input4. The aversiveness of pain (that is, its affective dimension) is important for survival by promoting withdrawal from tissue-damaging stimuli and by inhibiting actions that could further harm injured areas. Of critical importance for the organism is that pain aversiveness facilitates learning that affects future decisions in selecting actions that will prevent potential injury5. Congruent neural circuits that mediate unpleasantness and associated motivated behaviors and promote learning are therefore integral to the pain experience across species. In some individuals, for unknown reasons, pain that occurs following an acute injury persists beyond the natural healing process and no longer serves a protective role6,7.
Chronic pain can occur without an obvious, or identifiable, pathophysiology (as it does, for example, in fibromyalgia or irritable bowel syndrome)8. Despite the high prevalence of chronic pain in our population9, little is known about the mechanisms that underlie the transition from acute to chronic pain. Risk factors contributing to chronification include genetic predisposition, age, gender, previous pain experience and attitudes toward pain10,11. Cellular adaptations (for example, priming and sensitization mechanisms) of the nervous system can amplify nociceptive signaling and likely contribute to pain chronicity12. Mechanisms of amplification have been primarily characterized in peripheral nociceptors and in the spinal cord12 but increasingly are being studied in the brain13. Notably, chronic pain conditions are often accompanied by comorbid affective, emotional and cognitive disorders (for example, anxiety, depression, sleep disturbance, cognitive deficits14). Consistent with these observations, recent evidence from neuroimaging in patients demonstrates adaptations in reward/motivational circuits that may strengthen emotional and affective pain mechanisms13,15. Whether the brain changes observed in chronic pain patients represent an underlying intrinsic vulnerability for the transition to chronic pain or whether these alterations occur in response to sustained pain is not yet clear11. However, dysfunctional or maladaptive changes in aversive/motivational circuits likely contribute to the challenges of treatment of chronic pain.
Relief of acute persistent pain can be achieved clinically by pharmacological modulation of nociceptive input. Increased understanding of sensory neurobiology has been a primary focus of preclinical pain research and has resulted in promising approaches for new therapeutics (for example, anti–nerve growth factor and anti–calcitonin gene related peptide (CGRP) strategies, sodium channel subtype blockers16–18). Sustained blockade of sensory features of pain for control of chronic pain may not be desirable or clinically feasible. The aversive aspects of pain are bothersome to patients, and many clinically used drugs act, in part, by modulation of pain aversiveness. Recent studies have begun to explore the circuits that underlie the affective and motivational features of pain in nonverbal animals. Consistent with the imperative need of the organism to maintain homeostasis, pain can be considered a primordial, homeostatic emotion, analogous to hunger, thirst, desire to sleep, thermoregulation and maintenance of visceral functions19. Homeostatic mechanisms involve receptors that detect internal imbalance (that is, sensation) and an aversive emotion that demands a behavioral response (that is, motivation) to ensure the organism will take proper action to restore homeostasis20. Thus, pain produces strong motivational drive to promote escape or, in the case of chronic pain, to seek relief. The impact of chronic pain on reward/motivational circuits is relatively unknown. Evaluation of pain-motivated behaviors may provide a path for assessment of efficacy of new therapies for chronic pain with a high likelihood of translational relevance. Targeting aversive/motivational mechanisms may offer new opportunities for treatment of chronic pain or for preventing its occurrence.
Pain, relief and motivation
The evolutionary role of negative (pain) and positive (relief) affective states is to elicit motivations, respectively resulting in escape/avoidance and approach behaviors and to allow learning of how to predict dangerous or rewarding situations in the future21. Organisms often experience many competing or conflicting motivations simultaneously, but only one behavioral response can be selected5. During vertebrate evolution, the basal ganglia (including the caudate-putamen, subthalamic nucleus, globus pallidus and nucleus accumbens) and associated limbic cortex emerged to serve in evaluating the importance of competing motivational goals and selecting the action to achieve the best outcome5. Decision-making depends on previous experience and current options22. Conversely, the selected action and the difference between predicted and actual outcome (prediction error) will influence future decisions22.
In his motivation-decision model of pain23, Fields states that in the context of interacting motivations (for example, pain or danger from a predator) a neural decision is made on the basis of the value that a response provides to the organism either in terms of homeostatic needs or potential risks and benefits (for example, responding to pain to expedite healing or suppressing pain to escape). Accordingly, the pain experience can be modulated in a bidirectional fashion to facilitate the execution of the selected action and inhibit conflicting motivations23. Because, in daily life, pain is suppressed or enhanced by factors such as threat, predicted reward, attention or distraction, the relationship between activation of nociceptors and the pain experience is not linear24,25. Modulation of pain occurs from top-down, descending pathways that can inhibit or facilitate afferent nociceptive traffic to shape the pain experience. Disruption of these descending pain modulatory circuits to favor facilitation has been suggested as one possible mechanism of amplification that can promote chronic pain26. Chronic pain represents a constant demand for relief and can suppress other emotions, including natural rewards, potentially leading to anhedonia (for example, reward deficit state) and diminished quality of life27.
Relief of pain is rewarding
Pain research has conceptualized relief of pain as termination of negative affect and return to a normal (that is, neutral) state. Psychological investigations, however, suggest that satisfaction of an aversive state is rewarding and may be considered as one of the basic positive emotions28. Although the concept of pain relief as a reward has been observed in humans studies29,30, it has only recently been demonstrated in animals31–33. Analogous to observations in humans, relief of ongoing injury-induced pain produces a positive affective state (reward from pain relief) that can be reliably demonstrated by assessment of motivated behavior in rodents34. Such pain relief–motivated behaviors can be captured using classically established models (for example, conditioned place preference (CPP) learning) and are accompanied by activity in brain reward/motivational circuits31. Because of their fundamental role in survival, the basic brain circuits and mechanisms of reward, motivation and learning evolved early and are conserved across species35.
Brain neural systems for pain and motivation
How human pain is encoded in the brain is not well understood. Until recently, such information could be deduced only indirectly from observations of patients with accidental brain lesions36. Neuroimaging techniques have revolutionized our understanding of brain areas engaged in pain perception and pain modulation and how these networks interact with reward/motivation circuits to shape behavior37. Brain areas most commonly activated during acute pain include the thalamus, the primary and secondary somatosensory cortices (S1, S2), insula and the anterior cingulate cortex (ACC)38. These regions receive direct afferent nociceptive information from the spinal cord, and their neuronal activity correlates with the intensity of nociceptive stimulation14. Additional cortical and subcortical regions, including reward/motivation circuits, integrate internal and external signals and encode signal valuation, action selection and learning. These corticolimbic regions are engaged during anticipation of pain or expectation of relief (such as in placebo-induced analgesia39). Corticolimbic structures also serve in cognitive modulation of pain via opioid-sensitive descending endogenous (intrinsic) modulatory systems14.
Reward/motivation and decision circuits
Dopamine neurotransmission in the striatum and particularly in the nucleus accumbens (NAc) has long been recognized for its role in reward-motivated behavior. The NAc receives projections from dopaminergic neurons in the ventral tegmental area (VTA). Natural rewards, as well as rewarding drugs, activate these mesolimbic neurons and elicit dopamine release in the NAc. At the single-neuron level, mesolimbic and striatal neurons encode quantitative reward prediction error, which is important for learning and decision-making40. Reward-related information is also encoded in frontal lobe areas, specifically in the ACC, lateral orbitofrontal cortex (OFC), and ventromedial and anterior prefrontal cortices (PFC)41,42. Notably, the ACC and other prefrontal structures receive direct inputs from mesolimbic dopaminergic neurons43. The ACC performs critical functions in learning both appetitive and aversive outcomes. The ACC encodes information about the reward value of a chosen action and is therefore particularly important in future action selection22,41. Thus, differential reward coding in the NAc and cortical regions enables the integrated circuit to predict future outcomes on the basis of past and present experience and select an action necessary to achieve the desired goal44.
Pain and pain relief profoundly influence the activity of reward/motivation and decision circuits37 (Fig. 1). Blood oxygen level–dependent (BOLD) functional magnetic resonance imaging (fMRI) activations within the reward/motivation network, including the OFC, NAc and VTA, are often observed following an acute noxious stimulus45. Positron emission tomography (PET) imaging with dopamine receptor radiotracers demonstrates increased dopaminergic neurotransmission in the NAc in response to noxious stimulation46. Moreover, these paininduced NAc dopaminergic activations have been linked to variations in the individual ratings of negative affect during pain46.
Figure 1.
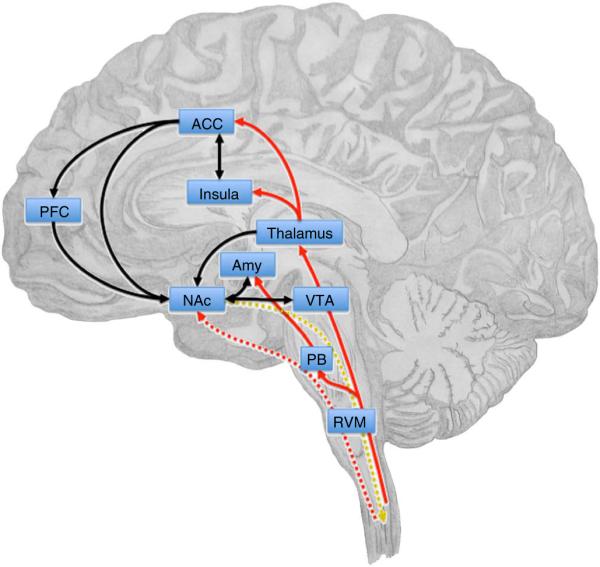
The corticolimbic circuit integrates motivationally salient information, including pain, and makes decisions about action selection. The NAc receives afferent nociceptive information through connections with the thalamus, parabrachial area (PB), amygdala (Amy) and ACC. Direct projections from the spinal cord to the NAc may be postulated on the basis of findings in rodents47 (red lines). VTA dopaminergic inputs to the NAc signal saliency, as well as the value of pain or relief. Corticostriatal connections from prefrontal, orbitofrontal and anterior cingulate cortices contribute to affective, emotional and cognitive control of pain perception and are involved in motivational decision-making. In the NAc, glutamatergic outputs from the amygdala converge on dopaminergic terminals from the VTA and influence motivated behavior in response to stress and anxiety (black lines). A descending pathway from the NAc that can modulate spinal nociceptive signals, possibly via the RVM, has been suggested109 (gold dotted line). Chronic pain states are characterized by anatomical and functional reorganization of the corticolimbic circuit, including changes in gray matter density in the PFC, ACC and NAc and increased functional connectivity between the PFC and NAc108.
Role of the NAc in pain-motivated behavior
The NAc receives pain information via projections from subcortical and cortical regions engaged in pain processing that include the amygdala, PFC and ACC. Direct projections of spinal dorsal horn neurons to the NAc are found in rodents47. The NAc does not seem to encode pain sensory information, but rather signals affective value and saliency of the stimulus40. Noxious stimuli depress the majority of mesolimbic dopamine neurons (value-coding neurons), but increased activity is regularly observed in a subset of neurons (salience-coding neurons)48. In the striatum, aversive stimuli usually increase dopamine concentrations measured by in vivo microdialysis. Recent studies, however, demonstrate that aversive and reward prediction signals are differentially encoded by specific patterns of dopamine responses in subregions of the NAc40. Investigations of phasic (subsecond) dopamine transients in the striatum using fast scan voltammetry demonstrate that, in anesthetized rats, painful tail pinch triggers dopamine release in the dorsal striatum and NAc core during the stimulus, whereas in the NAc shell dopamine is released after the stimulus termination49. The time course of activity in the NAc shell is consistent with the concept of pain offset as a reward. In agreement with the cellular responses, the fMRI BOLD signals that represent some aspects of neuronal activity also show a negative signal valence in the NAc at the onset of a prolonged thermal stimulation (pain) and a positive signal valence at the stimulus offset (relief)50. The same pattern of responses was observed in both humans and rodents32, supporting the idea that the activity in the NAc represents an evolutionarily conserved mechanism encoding both positive and negative salient stimuli. In healthy subjects, NAc activation at the offset of acute thermal stimulus was found to predict the magnitude of pain relief reward51.
Role of the ACC in pain affect
The ACC receives nociceptive signals from the medial thalamus via the spinothalamic pathway. Numerous animal studies as well as human imaging support the role of the ACC in the evaluation of the aversiveness of pain14,52. Electrophysiological recordings from the ACC in freely moving rats show long-lasting pain-induced neuronal activity with wide bilateral receptive fields consistent with the role of these neurons in encoding pain unpleasantness53. Accordingly, microinjection of excitatory amino acids into the rostral ACC (Brodmann areas 24 and 32) of uninjured rats produces conditioned place aversion (CPA) without effects on sensory thresholds52. In contrast, lesion of the rostral ACC abolish pain-induced aversive behavior but do not change evoked pain responses54,55. In humans, using hypnotic suggestions to alter pain unpleasantness while maintaining the intensity of the noxious stimulation, Rainville demonstrated activation of the ACC but not somatosensory cortex56. Pain aversiveness ratings, but not perceived intensity of a phasic thermal stimulus, can be selectively modulated by pleasant or unpleasant odors57. Reduced pain unpleasantness is associated with reduced ACC activity57. Importantly, the amount of pain reduction elicited by pleasant odor positively correlates with increased activity in the ventral striatum, implicating this region in analgesic (that is, pain relieving) effects of positive mood (that is, manipulation of pain affect).
Preclinical measures of pain affect
Chronic pain patients often suffer from allodynia: that is, pain resulting from normally innocuous stimuli such as touch or cold58. Much preclinical research has explored mechanisms promoting evoked hypersensitivity, laying the groundwork for the discovery of therapies to help such patients. More often, however, chronic pain patients experience ongoing (that is, stimulus-independent) pain and emotional suffering. At present, an individual’s emotional component of pain cannot be objectively and quantitatively measured, although some investigations utilize surrogate physiological measures such as heart rate or skin conductance59. In humans, the emotional impact of pain is usually assessed by subjective and personal self-reports using standardized questionnaires. Assessment of analogous affective aspects of pain in nonverbal animals has been challenging. Several preclinical approaches have been employed for this purpose, including vocalization after discharge in rats following electrical tail shock60 and evaluation of facial grimace that detects pain affect of short and intermediate, but not longer, duration61. Recently, operant measures have been developed that use pain-motivated behavior to capture affective features of pain in animals.
Measures of pain-motivated behavior
Operant measures are based on the principle that animals have a strong motivation to maintain a homeostasis. This motivation facilitates learning to avoid situations and contexts associated with pain or injury (avoidance/escape behavior) and to achieve pain relief (approach behavior). In operant assays, animals learn to perform a behavior (for example, choose a preferred place or press a lever) to achieve the desired goal. Recent efforts have advanced methods that allow the assessment of rodent motivated behavior resulting from evoked pain stimuli or from ongoing (non-evoked, spontaneous) pain (Table 1).
Table 1.
Operant measures of the affective component of pain
| Operant model | Pain measure | Pain stimulus | Response measure | Refs. |
|---|---|---|---|---|
| Place escape/avoidance paradigm (PEAP) |
Mechanical sensitivity | Tactile stimulation of injured paw | Time in compartment where uninjured paw is stimulated |
63 |
| Thermal sensitivity | Thermally regulated floor surface | Latency and duration of escape to a platform | 62 | |
| Operant orofacial pain assessment device (OPAD) |
Orofacial thermal sensitivity | Thermal stimulation of face while drinking |
Pressing a thermode to obtain a drink reward |
64 |
| Analgesic drug self-administration | Ongoing neuropathic pain (spinal nerve ligation) |
No evoked stimulation | Self-administration of intrathecal clonidine only in neuropathic rats |
65,66 |
| Stimulation-induced analgesia | Ongoing neuropathic pain (spinal cord injury) |
No evoked stimulation | CPP to motor cortex stimulation in SCI rats | 74 |
| Conditioned place preference (CPP) | Ongoing pain | No evoked stimulation | CPP to non-opioid analgesics | 34,67 |
Operant measures of evoked pain
Place escape/avoidance paradigms (PEAP) allow simultaneous measurements of sensory and affective responses to evoked tactile or thermal stimulation. These assays are based on conflict involving a choice between a compartment where the animal receives noxious stimulation and escape to a usually not favored (for example, illuminated) compartment or platform62,63. A modified protocol, the orofacial pain assessment device (OPAD)64, uses a reward/pain conflict, where the animal is required to choose between receiving a rewarding drink by pressing its face into a temperature-controlled thermode or avoiding painful contact with the thermode and forgoing the reward. These assays are able to evaluate complex pain behavior in response to evoked stimulation and explore the effects of sustained inflammatory or neuropathic pain and drug treatments.
Operant measures of ongoing pain
Drug self-administration65,66 and CPP34,67 allow evaluation of the affective aspects of ongoing pain. These assays are based on the motivation of animals with ongoing pain to obtain relief by either self-administration of an analgesic drug or demonstrating preference for a context that is associated with pain relief. In humans and animals, intrathecal administration of the α2-adrenergic agonist clonidine produces effective analgesia. Rats with experimental neuropathic pain will self-administer intrathecal clonidine while uninjured rats will not65. This observation establishes that a nonaddictive drug given at a site in the neuraxis separate from the reward system can produce motivated behavior, suggesting a primary goal of alleviation of ongoing pain.
Conditioned place preference
Historically, CPP has been used to assess rewarding effects of addictive drugs, but it has recently been applied to study reward from pain relief 34,68 (Box 1 and Fig. 2). The CPP assay allows evaluation of (i) the time-dependent nature of ongoing pain in rodent pain models, (ii) the neural mechanisms underlying pain relief–motivated behavior and (iii) the efficacy of molecular targets for relieving ongoing pain67. Studies in rats demonstrate that relief of ongoing pain, regardless of the injury model, produces CPP. For example, in a rat model of postsurgical pain, incision of the hindpaw elicited thermal hyperalgesia and guarding behavior that was observed on day 1 and 4 after surgery31. Peripheral nerve blockade with popliteal fossa lidocaine alleviated thermal hyperalgesia at day 1 and 4 after incision. In contrast, peripheral nerve blockade produced CPP only at day 1 and not at day 4 (Fig. 2), indicating that in time-dependent pain conditions, such as postsurgical injury, evoked and ongoing pain may have different time dependencies31. Similarly, in rats with neuropathic pain but not sham-treated rats, CPP was demonstrated following treatments that block evoked hypersensitivity, including intrathecal clonidine, N-type calcium channel blockers or inactivation of descending pain facilitation pathways by administration of local anesthetic within the rostral ventromedial medulla (RVM)34,69. Importantly, lesions of the ACC abolish pain relief–induced CPP without disruption of learning69, supporting the link between pain aversiveness and motivated behavior.
Box 1. Conditioned place preference procedure.
CPP can be used to unmask the presence of pain and to screen mechanisms that can elicit relief. CPP can be performed with either multitrial or a single-trial conditioning protocols, as previously described34,67. CPP boxes contain two conditioning chambers distinguished by visual and sensory cues that are connected by a middle (neutral) chamber. Rats are conditioned to associate vehicle treatment with one of the chambers and drug treatment with the opposite pairing chamber. On test day, rats are placed in the CPP box with access to all chambers and time spent in each chamber is recorded and analyzed for chamber preference. On the test day animals are placed in the chambers drug free; therefore, any possible motor or sedative effects of the drug do not affect chamber preference. The difference score, calculated as increased time spent in the drug-paired chamber on test day compared to the day before conditioning, indicates preference for the drug treatment. Single-trial protocols are appropriate when the treatments employed produce rapid change in pain affect (for example, peripheral nerve block, intrathecal clonidine, RVM lidocaine, electrical stimulation of motor cortex)67,72,74. The effects of slow-acting drugs can be revealed if the drug is given as a pretreatment that may produce relief of the pain-induced aversive state and prevent CPP to a rapidly acting pain reliever.
Figure 2.
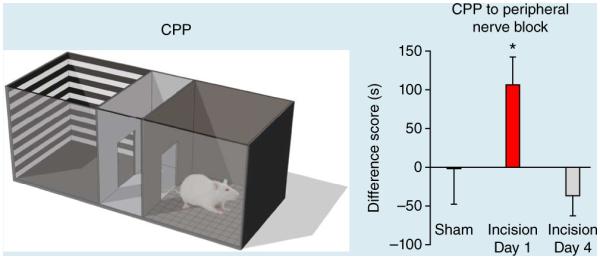
Relief of ongoing pain produces CPP. Left, CPP box. Right, in rats with incision of the hindpaw peripheral nerve blockade of the injured limb with popliteal fossa lidocaine produced CPP at 1 d but not 4 d after incision, indicating time-dependent resolution of ongoing postsurgical pain31. Data are means ± s.e.m. *P < 0.05; Student’s paired t-test. Right panel adapted from ref. 31.
In addition to experimental neuropathic and incisional pain, CPP to pain-relieving treatments has been demonstrated in other experimental pain conditions, including ongoing inflammatory, osteoarthritic and cephalic pain69–75. Importantly, CPP has been demonstrated in injured rats following pain-relieving treatments that do not produce preference in uninjured animals. Thus, analgesic treatments including peripheral nerve block, intrathecal administration of ω-conotoxin or clonidine, or RVM lidocaine may become rewarding in the presence of ongoing pain31,34,67,72,74. This conclusion is consistent with the concept of negative reinforcement elicited by relief of an aversive state. Conversely, positive reinforcing properties of some analgesics including opiates or antidepressants can represent a limitation of the use of CPP to evaluate their antiaversive effects, as the intrinsically rewarding effects of such drugs cannot easily be dissociated from antiaversive effects. An additional concern that must be controlled for is the potential influence of drugs on the animal’s ability to learn.
Activation of reward circuits by pain relief
A potential relationship between motivated behavior following relief of ongoing pain (CPP) and the activity of dopaminergic mesolimbic circuits was recently investigated in rats31 (Fig. 3). The study demonstrated that the same treatment with peripheral nerve blockade that relieves ongoing postsurgical pain and produces CPP also activates the mesolimbic dopaminergic circuit and stimulates efflux of dopamine in the NAc shell. Furthermore, inhibition of dopaminergic neurons in the VTA or blockade of dopamine signaling in the NAc prevents pain relief–induced CPP, providing direct evidence for a causal relationship between activation of the mesostriatal reward circuit and pain-motivated behavior. Activation of dopaminergic neurotransmission in the NAc by pain relief was also investigated in a rat model of cephalic pain72. Treatments effective at alleviating headache in humans, including sumatriptan or a CGRP receptor antagonist, CGRP8–37, elicited CPP and dopamine efflux in the NAc only in rats with presumed cephalic pain. Thus the activity in the reward/motivation circuit reflects motivated behavior to seek relief of pain and may therefore represent an output measure (that is, a ‘biomarker’) of analgesic efficacy76.
Figure 3.
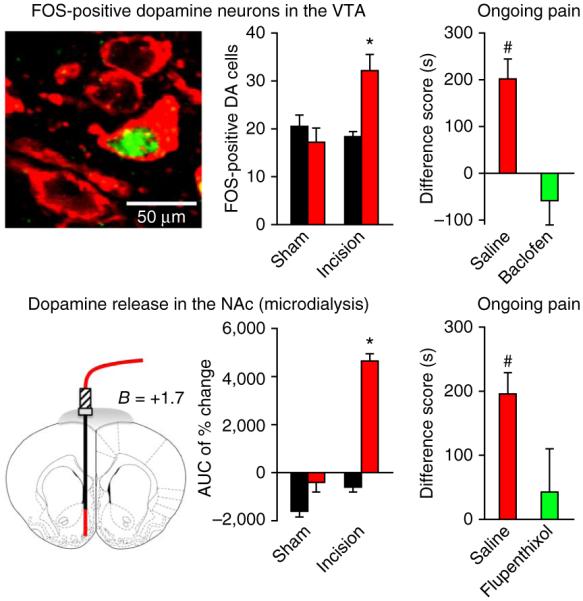
Pain relief produces CPP through activation of mesolimbic reward/motivation circuitry. In rats with ongoing postincisional pain, peripheral nerve blockade of the injured limb with popliteal fossa lidocaine resulted in the activation of FOS in dopaminergic (tyrosine hydroxylase positive; DA) neurons in the VTA (top left; FOS, green; tyrosine hydroxylase, red) and release of dopamine in the NAc measured by in vivo microdialysis (bottom left; area under the time curve (AUC) of percentage change in dopamine). In incised rats at 1 day after surgery, CPP to peripheral nerve blockade (Fig. 1) was prevented by inactivation of dopaminergic neurons in the VTA with the GABAB receptor agonist baclofen (top right). Inhibition of dopamine neurotransmission in the NAc by the dopamine receptor antagonist flupenthixol also prevented CPP in rats 1 d after incision surgery Student’s paired t-test. Data are means ± s.e.m. Adapted from ref. 31.
Modulation of pain and reward/motivation pathways
Many psychological and cognitive factors, including attention, emotion, expectations and prior experience, influence the experience of pain. The presence of persistent pain alters motivated behavior and decision-making and could modify the way patients perceive acute pain stimulation51. The transition of acute pain into a chronic, maladaptive state is characterized in some patients by abnormal anatomical and functional modifications of brain networks, including those mediating affective/motivational aspects of pain. Therefore, behavior is affected by both competing motivations and long-term neurological adaptations via modulation of reward/motivation circuits.
Emotional and cognitive control of pain and motivation
The psychological and emotional state of an individual can have a powerful impact on pain perception (see refs. 14,77 for review). Further, humans continually create predictions about future (expected) pain or relief, and these expectations influence the actual pain or analgesia we experience. For example, positive treatment expectancy produces heightened analgesia (placebo analgesia)78 while negative expectancy may exaggerate pain (nocebo)79. Thus, painful stimuli are interpreted on the basis of previous experiences, importance in the current context and probabilities of expected outcomes. Both sensory and affective aspects of pain can be influenced by emotional and cognitive factors.
Recent BOLD-fMRI measurements in the human spinal cord have demonstrated that expectation of pain relief (placebo analgesia) can directly reduce nociceptive processing in the dorsal horn of the spinal cord, presumably via intrinsic descending inhibitory mechanisms80. Animal research supports the notion that these mechanisms comprise descending projections from the prefrontal regions (including the ACC) to the periaqueductal gray area (PAG) and ultimately outflow from the RVM to modulate the nociceptive signal at the spinal cord level26,81. These top-down modulatory pathways can either inhibit or facilitate afferent pain transmission. Supporting the engagement of the descending modulatory mechanisms, placebo manipulations have shown increased neuronal responses in cortical regions (for example, dorsolateral PFC, OFC, ACC) and in the PAG during anticipation of reduced pain39,82. PET studies of placebo analgesia using specific opioid radiotracers demonstrate increased opioid activity in similar brain regions, consistent with the involvement of endogenous opioid neurotransmission in the descending pain pathway83. Thus, psychological factors can cause neurobiological changes in pain pathways at the spinal and supraspinal levels to alter pain experience.
PET imaging has identified increased placebo-induced opioid and dopamine neurotransmission in the basal ganglia, particularly in the NAc84,85. During placebo analgesia opioid and dopamine activity in the NAc correlates with reduced pain scores and with improvement in the Positive and Negative Affectivity Scale (PANAS) ratings83. Variations in placebo-induced activity in the NAc between subjects have been linked to differences in the subjects’ reward responding84. As in placebo analgesia, the ventral striatum and descending mechanisms involving the anterior cingulate and prefrontal cortices and the PAG are also engaged during emotional pain modulation25,57. The magnitude of the BOLD fMRI responses to noxious stimuli in the reward circuits predicts subsequent opioid-induced analgesia45, providing further support for the role of central endogenous opioid mechanisms in pain modulation. Notably, pain modulation may not require a change in perception but may occur during reappraisal in later phases of the pain experience. Wager and colleagues86 recently demonstrated that the prefrontal cortex communicates with subcortical regions that include the NAc and amygdala to mediate cognitive reappraisal of emotional experiences. These findings indicate that emotional and cognitive factors can alter pain perception, in part by modulating afferent nociceptive signaling via descending pathways and in part by re-evaluating pain unpleasantness within cognitive and affective circuits.
Motivational circuits during tonic pain
Most human neuroimaging studies have investigated the effects of acute noxious stimuli (phasic pain) typically involving cutaneous heat, cold, electric shock, ischemia or visceral distention. Clinical pain, however, is functionally and neuroanatomically distinct from phasic nociceptive pain38. Clinical conditions are characterized by prolonged ongoing pain (that is, non-evoked pain at rest) and evoked hypersensitivity (that is, allodynia and hyperalgesia) that may be caused by peripheral or central sensitization12. A meta-analysis comparing fMRI studies using various experimental and clinical pain protocols, including thermal and mechanical stimulation, allodynia and hyperalgesia, or clinical pain in patients suggests many similarities but also differences in neuronal representations related to different types of acute, tonic and chronic pain87. Although different noxious stimuli activate similar pain-related regions, the precise spatial loci within the brain regions may differ (see ref. 38 for review).
Differential engagement of motivational circuits has been observed between phasic and tonic pain, consistent with their different affective and motivational functions (namely, arousal of defensive behavior associated with phasic pain and avoidance and protective behavior associated with chronic pain). Studies in rodents88 suggest a key role of the NAc in the mediation of pain and analgesia during persistent pain. Investigations of brain activity related to tonic pain in humans have used capsaicin to elicit cutaneous allodynia and hyperalgesia89–91. Lorentz89 evaluated the effects of heat stimulation of normal and capsaicin-sensitized skin and found that, at matching pain intensities, heat allodynia produced greater unpleasantness than normal pain and was represented by distinct brain activations. The findings of a distinct activity in prefrontal cortex and ventral striatum during tonic pain may reflect adaptations of motivational and cognitive functions in the context of tissue injury89. Seymour and colleagues investigated the neural coding of reward-guided decision making and learning in the presence of capsaicin-induced tonic pain91 and found different coding of prediction errors during tonic pain, involving opposing engagement of aversive and appetitive motivational networks.
Adaptations of motivation circuits in chronic pain
Chronic pain demands continuous engagement of brain motivational and emotional circuitry and shifts behavioral goals toward achieving relief. The tonic long-lasting motivational burden could result in time-dependent adaptations leading to chronification92. The impact of chronic pain on functional, anatomical or molecular changes in the brain was investigated in patients across many conditions, including chronic back pain, neuropathic pain, fibromyalgia, irritable bowel syndrome, headache, complex regional pain syndrome and osteoarthritis, and in rats with experimental neuropathic pain93. These investigations identified extensive changes in gray matter94,95, abnormalities in the white matter connectivity95, and neurochemical modifications in glutamate, opioid and dopamine neurotransmission96,97 (see refs. 8,15 for review). Recent reports demonstrate that the cortical changes may be reversed when pain is relieved, suggesting that some brain abnormalities may indeed develop as a consequence of ongoing nociceptive input98,99. This conclusion is supported by a longitudinal study in rats with experimental neuropathic pain revealing decreased frontal cortex volume that develops several months after the injury and coincides with the onset of anxiety-like behavior93. In contrast, other neuroanatomical and functional modifications of the brain circuits may represent genetic or epigenetic predispositions, conferring enhanced vulnerability for development of chronic pain10,11.
Many of the brain changes observed in patients involve affective and motivational circuits95. Apkarian and colleagues51 investigated the motivational aspects of pain in chronic conditions and observed that NAc responses at the offset of an acute thermal stimulation differ between healthy volunteers and patients with chronic back pain. In healthy subjects, a positive phasic NAc signal reflected prediction of reward associated with pain relief, while in patients a negative signal was consistent with punishment, as the patients turned attention to their ongoing chronic pain. Indeed, the magnitude of NAc activity at the stimulus offset positively correlated with the subject’s ratings of ongoing back pain. These findings suggest that the motivational value of acute pain may be distorted in chronic pain states. Further, patients demonstrate strengthening of functional connectivity between NAc and PFC that correlates with the magnitude of the individual’s ongoing pain and predicts transition to chronic pain100. This was interpreted as a shift in pain perception from sensory to emotional brain regions51. Therefore, anatomical and functional changes in reward/motivation circuits in chronic pain may lead to the comorbid affective and cognitive disorders observed in these patients101.
Implications for drug discovery
Since the isolation of morphine in 1804 and synthesis of aspirin in 1899, opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) remain the first-line classes of analgesics. While these drugs are effective in many patients, they are also associated with potentially serious side effects and societal problems. More than 100,000 patients are hospitalized annually for NSAID-related gastrointestinal complications, and about 16,500 NSAID-related deaths occur each year among arthritis patients alone102. Prescription opioid abuse is the fastest growing drug problem in the United States, resulting in approximately 12,000 unintentional medication overdose deaths in 2007 (Center for Disease Control and Prevention, Morbidity and Mortality Weekly Report, 61 (01), 10–13; 2012). Preclinical research has identified new disease mechanisms and analgesic targets; however, few new pain therapies have been introduced for treatments to patients. Many obstacles can be identified in the translation of efficacy from preclinical studies in animal pain models to successful clinical trials in pain patients. One impediment has been the difficulty in capturing affective dimensions of pain. Increased understanding of the role of reward/motivational circuits in pain could expedite analgesic drug discovery.
Measures of affective/motivational aspects of pain and pain relief in animals could improve translation to human pain conditions
In humans, pain is often accompanied by physical or psychological impairments that may result in disability and alter motivations. Reflexive pain measures in rodents do not sufficiently capture these aspects of pain. In contrast, assessment of pain-motivated behavior depends on the affective dimensions of ongoing pain and may better reflect some features of clinical pain. Studies in rat pain models show that relief of ongoing pain with treatments validated to produce analgesia in patients elicits CPP67. Notably, treatments shown ineffective for ongoing pain in humans do not produce CPP in animal pain models. The findings so far suggest that in injured rats CPP to treatments used in humans faithfully reflects clinically validated outcomes.
Brain activity in the reward/motivation circuit could be an objective neurochemical measure of analgesic efficacy
It has been suggested that fMRI techniques may be able to objectively measure relevant mechanisms subserving the experience of chronic pain in patients. Building on machine learning analyses, Wager and colleagues developed an fMRI-based measure to identify a pattern of activity (‘neural pain signature’) that is sensitive and specific to acute thermal pain in healthy subjects103. Whether a similar approach can be extended to clinical pain in patients is not yet clear. Work by Apkarian and others suggests that specific chronic pain conditions are associated with distinct patterns of brain reorganization15. These maladaptations develop over time, but changes in functional connectivity can be detected early and, remarkably, can predict progression from acute to chronic pain13. It is plausible that by measuring individual brain activation responses in cortico-limbic structures, neuroimaging may be able to evaluate analgesic effects of treatments in individual patients, as has been shown in healthy controls45. In rat pain models, responses in dopaminergic reward/motivational circuits to pain relief have been shown to correlate with behavioral measures. Specifically, dopamine release in the NAc was observed only after treatments that also elicited CPP in the same animal pain model31,72. Therefore, in animal pain models, dopamine efflux in the NAc may be an objective and quantifiable output measure (biomarker) of pain relief reward that reflects analgesic efficacy76. However, increased mesolimbic dopaminergic neurotransmission can be elicited by natural rewards and by rewarding drugs, resulting in activation of apparently the same circuitry irrespective of the pain state. Thus, pain relief reward specificity must be demonstrated by the lack of treatment effect in uninjured animals.
Targeting of reward/motivational circuits could relieve intractable chronic pain
Given the role of the aversive and motivational networks in pain, direct manipulation of the activity within these regions may offer pain relief. Some patients with intolerable pain achieve relief following lesions of the cingulate cortex36,104,105. These patients experience diminished pain-related unpleasantness but are still able to discriminate intensity and location of a noxious stimulus. Notably, in rats with neuropathic pain, lesion of the rostral ACC eliminates pain-induced aversiveness without reducing evoked hypersensitivity69. Recently, less invasive neuromodulatory techniques to target the ACC for pain management have been employed. For example, pain relief can be achieved in selected patients with transcranial magnetic stimulation (TMS) or deep brain stimulation (DBS) of the ACC106. An attractive technique demonstrating potential for pain management uses real-time fMRI with feedback to train patients to control activation of their ACC and modulate their own pain107. Although the methods for selective modulation of a specific brain region are still used rarely, they highlight the benefits of this approach and hold promise for future pain management.
Could pain-induced neural adaptations in reward/motivational circuits be reversed?
Increasing evidence shows pain-induced reorganization of the reward/motivational circuitry13 and contributions to the transition from acute to chronic pain93,108 One prominent observation is the apparent shrinkage of cortical volume in patients with chronic pain94,95. Whether pain-induced changes in cortical volume reflect neuronal loss is unknown, but these changes appear to be time related and reversible88,89. These observations suggest that pain-induced reorganization in reward/motivational circuits may also be reversible, or perhaps preventable. Pharmacological or other interventions could (i) prevent the anatomical and functional reorganization of brain circuits to protect the patient against the development of comorbid affective and cognitive impairments or delay the trajectory for their development and (ii) reverse pain-induced neural maladaptations in these circuits to restore function.
Conclusion
Affective/emotional features of pain are of fundamental importance to patients. It is recognized that understanding how pain aversiveness and its relief occur in the brain deserves increased attention in both preclinical and clinical arenas. In animal models of pain, behavioral measures that capture affective and motivational aspects of pain appear to accurately reflect the effectiveness of treatments that are useful clinically (bedside to bench translation). Neuroimaging studies in humans, and recently also in animals, have identified evolutionarily conserved neural mechanisms and brain networks comprising reward/motivational mesocorticolimbic circuits that are modulated by clinically useful treatments. These observations suggest that evaluation of activity within this circuitry in response to pain relief may provide an objective measure (biomarker) of analgesic efficacy in animals that may improve predictive success for target relevance in humans (bench to bedside translation).
Chronic pain is frequently associated with affective and cognitive impairments, such as anxiety, depression, anhedonia and learning deficits. Whether the anatomical and functional reorganization of brain reward/motivation circuits that may underlie pain-related comorbidities correlates with pain chronicity is not yet clear. However, targeting these circuits not only might provide new therapies for symptomatic management of pain but also could alter the trajectory of outcomes related to chronic pain. Pain-induced maladaptations of reward/motivation circuits may be reversible with therapy, raising the possibility of recovery from chronic pain and restoration of normal affective/emotional and cognitive function. Finally, preemptive treatments could prevent anatomical and functional reorganization of brain reward/motivation circuits to inhibit the progression from acute to chronic pain, as well as associated comorbidities.
ACKNOWLEDGMENTS
We gratefully acknowledge comments and suggestions from I. Tracey and H. Fields. We thank P. Navratilova for help with illustrations. This work was funded by grants NS066958 and DA034975 from the US National Institutes of Health.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat. Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fields HL. Pain: an unpleasant topic. Pain. 1999;6:S61–S69. doi: 10.1016/S0304-3959(99)00139-6. suppl. [DOI] [PubMed] [Google Scholar]
- 3.Lee MC, Tracey I. Imaging pain: a potent means for investigating pain mechanisms in patients. Br. J. Anaesth. 2013;111:64–72. doi: 10.1093/bja/aet174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee MC, Tracey I. Unravelling the mystery of pain, suffering, and relief with brain imaging. Curr. Pain Headache Rep. 2010;14:124–131. doi: 10.1007/s11916-010-0103-0. [DOI] [PubMed] [Google Scholar]
- 5.Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res. Rev. 2008;58:322–339. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ, Ma Q. Nociceptors–noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–1625. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]
- 8.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J. Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J. Pain. 2010;11:1230–1239. doi: 10.1016/j.jpain.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 10.van Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. Br. J. Anaesth. 2013;111:13–18. doi: 10.1093/bja/aet123. [DOI] [PubMed] [Google Scholar]
- 11.Denk F, McMahon SB, Tracey I. Pain vulnerability: a neurobiological perspective. Nat. Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013;26:360–367. doi: 10.1097/WCO.0b013e32836336ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013;14:502–511. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog. Neurobiol. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger JV, et al. Cellular and molecular insights into neuropathy-induced pain hypersensitivity for mechanism-based treatment approaches. Brain Res. Rev. 2011;67:282–310. doi: 10.1016/j.brainresrev.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Burgess G, Williams D. The discovery and development of analgesics: new mechanisms, new modalities. J. Clin. Invest. 2010;120:3753–3759. doi: 10.1172/JCI43195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodick DW, et al. Safety and efficacy of LY2951742, a monoclonal antibody to calcitonin gene-related peptide, for the prevention of migraine: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Neurol. 2014;13:885–892. doi: 10.1016/S1474-4422(14)70128-0. [DOI] [PubMed] [Google Scholar]
- 19.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 20.Denton DA, McKinley MJ, Farrell M, Egan GF. The role of primordial emotions in the evolutionary origin of consciousness. Conscious. Cogn. 2009;18:500–514. doi: 10.1016/j.concog.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Wiech K, Tracey I. Pain, decisions, and actions: a motivational perspective. Front. Neurosci. 2013;746 doi: 10.3389/fnins.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nat. Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- 23.Fields HL. In: 11th World Congress on Pain. Flor H, Kalso E, Dostrovsky JO, editors. IASP Press; Seattle: 2006. pp. 449–459. [Google Scholar]
- 24.Sprenger C, et al. Attention modulates spinal cord responses to pain. Curr. Biol. 2012;22:1019–1022. doi: 10.1016/j.cub.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Villemure C, Bushnell MC. Mood influences supraspinal pain processing separately from attention. J. Neurosci. 2009;29:705–715. doi: 10.1523/JNEUROSCI.3822-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 27.Simons LE, Elman I, Borsook D. Psychological processing in chronic pain: a neural systems approach. Neurosci. Biobehav. Rev. 2014;39:61–78. doi: 10.1016/j.neubiorev.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levenson RW. Basic emotion questions. Emot. Rev. 2011;3:379–386. [Google Scholar]
- 29.Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: a psychophysical investigation. Eur. J. Neurosci. 2008;28:794–801. doi: 10.1111/j.1460-9568.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 30.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS ONE. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navratilova E, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc. Natl. Acad. Sci. USA. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Becerra L, Navratilova E, Porreca F, Borsook D. Analogous responses in the nucleus accumbens and cingulate cortex to pain onset (aversion) and offset (relief) in rats and humans. J. Neurophysiol. 2013;110:1221–1226. doi: 10.1152/jn.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerber B, et al. Pain-relief learning in flies, rats, and man: basic research and applied perspectives. Learn. Mem. 2014;21:232–252. doi: 10.1101/lm.032995.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.King T, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat. Neurosci. 2009;12:1364–1366. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreatta M, et al. Onset and offset of aversive events establish distinct memories requiring fear and reward networks. Learn. Mem. 2012;19:518–526. doi: 10.1101/lm.026864.112. [DOI] [PubMed] [Google Scholar]
- 36.Brotis AG, Kapsalaki EZ, Paterakis K, Smith JR, Fountas KN. Historic evolution of open cingulectomy and stereotactic cingulotomy in the management of medically intractable psychiatric disorders, pain and drug addiction. Stereotact. Funct. Neurosurg. 2009;87:271–291. doi: 10.1159/000226669. [DOI] [PubMed] [Google Scholar]
- 37.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 38.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–1167. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- 40.Schultz W. Updating dopamine reward signals. Curr. Opin. Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Noonan MP, et al. Separate value comparison and learning mechanisms in macaque medial and lateral orbitofrontal cortex. Proc. Natl. Acad. Sci. USA. 2010;107:20547–20552. doi: 10.1073/pnas.1012246107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Narita M, et al. Implication of dopaminergic projection from the ventral tegmental area to the anterior cingulate cortex in mu-opioid-induced place preference. Addict. Biol. 2010;15:434–447. doi: 10.1111/j.1369-1600.2010.00249.x. [DOI] [PubMed] [Google Scholar]
- 44.Lim SL, O’Doherty JP, Rangel A. The decision value computations in the vmPFC and striatum use a relative value code that is guided by visual attention. J. Neurosci. 2011;31:13214–13223. doi: 10.1523/JNEUROSCI.1246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wanigasekera V, et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc. Natl. Acad. Sci. USA. 2012;109:17705–17710. doi: 10.1073/pnas.1120201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J. Neurosci. 2006;26:10789–10795. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein R, Giesler GJ., Jr. Retrograde labeling of neurons in spinal cord that project directly to nucleus accumbens or the septal nuclei in the rat. Brain Res. 1989;497:149–154. doi: 10.1016/0006-8993(89)90981-5. [DOI] [PubMed] [Google Scholar]
- 48.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. USA. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budygin EA, et al. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience. 2012;201:331–337. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur. J. Pain. 2008;12:866–869. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat. Neurosci. 2004;7:398–403. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 53.Wang JY, Luo F, Chang JY, Woodward DJ, Han JS. Parallel pain processing in freely moving rats revealed by distributed neuron recording. Brain Res. 2003;992:263–271. doi: 10.1016/j.brainres.2003.08.059. [DOI] [PubMed] [Google Scholar]
- 54.LaGraize SC, Labuda CJ, Rutledge MA, Jackson RL, Fuchs PN. Differential effect of anterior cingulate cortex lesion on mechanical hypersensitivity and escape/avoidance behavior in an animal model of neuropathic pain. Exp. Neurol. 2004;188:139–148. doi: 10.1016/j.expneurol.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc. Natl. Acad. Sci. USA. 2001;98:8077–8082. doi: 10.1073/pnas.141218998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 57.Villemure C, Laferriere AC, Bushnell MC. The ventral striatum is implicated in the analgesic effect of mood changes. Pain Res. Manag. 2012;17:69–74. doi: 10.1155/2012/371362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J. Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gjerstad AC, Wagner K, Henrichsen T, Storm H. Skin conductance versus the modified COMFORT sedation score as a measure of discomfort in artificially ventilated children. Pediatrics. 2008;122:e848–e853. doi: 10.1542/peds.2007-2545. [DOI] [PubMed] [Google Scholar]
- 60.Kender RG, Harte SE, Munn EM, Borszcz GS. Affective analgesia following muscarinic activation of the ventral tegmental area in rats. J. Pain. 2008;9:597–605. doi: 10.1016/j.jpain.2008.01.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotocinal SG, et al. The Rat Grimace Scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King CD, Devine DP, Vierck CJ, Mauderli A, Yezierski RP. Opioid modulation of reflex versus operant responses following stress in the rat. Neuroscience. 2007;147:174–182. doi: 10.1016/j.neuroscience.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 63.LaBuda CJ, Fuchs PN. A behavioral test paradigm to measure the aversive quality of inflammatory and neuropathic pain in rats. Exp. Neurol. 2000;163:490–494. doi: 10.1006/exnr.2000.7395. [DOI] [PubMed] [Google Scholar]
- 64.Anderson EM, et al. Use of the Operant Orofacial Pain Assessment Device (OPAD) to measure changes in nociceptive behavior. J. Vis. Exp. 2013 doi: 10.3791/50336. doi:10.3791/50336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin TJ, Kim SA, Eisenach JC. Clonidine maintains intrathecal self-administration in rats following spinal nerve ligation. Pain. 2006;125:257–263. doi: 10.1016/j.pain.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 66.Ewan EE, Martin TJ. Analgesics as reinforcers with chronic pain: evidence from operant studies. Neurosci. Lett. 2013;557A:60–64. doi: 10.1016/j.neulet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Navratilova E, Xie JY, King T, Porreca F. Evaluation of reward from pain relief. Ann. NY Acad. Sci. 2013;1282:1–11. doi: 10.1111/nyas.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sufka KJ. Conditioned place preference paradigm: a novel approach for analgesic drug assessment against chronic pain. Pain. 1994;58:355–366. doi: 10.1016/0304-3959(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 69.Qu C, et al. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011;152:1641–1648. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okun A, et al. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol. Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okun A, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012;153:924–933. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Felice M, et al. Capturing the aversive state of cephalic pain preclinically. Ann. Neurol. 2013;74:257–265. doi: 10.1002/ana.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J. Pain. 2012;13:598–607. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Davoody L, et al. Conditioned place preference reveals tonic pain in an animal model of central pain. J. Pain. 2011;12:868–874. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park HJ, et al. Persistent hyperalgesia in the Cisplatin-treated mouse as defined by threshold measures, the conditioned place preference paradigm, and changes in dorsal root Ganglia activated transcription factor 3: the effects of gabapentin, ketorolac, and etanercept. Anesth. Analg. 2013;116:224–231. doi: 10.1213/ANE.0b013e31826e1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie JY, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: A potential biomarker of efficacy. Pain. 2014;155:1659–1666. doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. 2009;47:987–994. doi: 10.1016/j.neuroimage.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 78.Atlas LY, Wager TD. How expectations shape pain. Neurosci. Lett. 2012;520:140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 79.Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 2010;16:1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- 80.Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 81.Fields H. State-dependent opioid control of pain. Nat. Rev. Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 82.Watson A, et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145:24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott DJ, et al. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- 84.Scott DJ, et al. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55:325–336. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 85.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J. Neurosci. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lanz S, Seifert F, Maihofner C. Brain activity associated with pain, hyperalgesia and allodynia: an ALE meta-analysis. J. Neural Transm. 2011;118:1139–1154. doi: 10.1007/s00702-011-0606-9. [DOI] [PubMed] [Google Scholar]
- 88.Altier N, Stewart J. The role of dopamine in the nucleus accumbens in analgesia. Life Sci. 1999;65:2269–2287. doi: 10.1016/s0024-3205(99)00298-2. [DOI] [PubMed] [Google Scholar]
- 89.Lorenz J, et al. A unique representation of heat allodynia in the human brain. Neuron. 2002;35:383–393. doi: 10.1016/s0896-6273(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 90.Wiech K, et al. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27:59–69. doi: 10.1016/j.neuroimage.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 91.Seymour B, et al. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- 92.Apkarian AV. The brain in chronic pain: clinical implications. Pain Manag. 2011;1:577–586. doi: 10.2217/pmt.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seminowicz DA, et al. MRI structural brain changes associated with sensory and emotional function in a rat model of long-term neuropathic pain. Neuroimage. 2009;47:1007–1014. doi: 10.1016/j.neuroimage.2009.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 95.Geha PY, et al. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60:570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Harris RE, et al. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wood PB, et al. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J. Pain. 2007;8:51–58. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J. Neurosci. 2009;29:13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seminowicz DA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J. Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baliki MN, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat. Neurosci. 2012;15:1117–1119. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res. Rev. 2009;60:226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. Am. J. Med. 1998;105:31S–38S. doi: 10.1016/s0002-9343(98)00072-2. [DOI] [PubMed] [Google Scholar]
- 103.Wager TD, et al. An fMRI-based neurologic signature of physical pain. N. Engl. J. Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yen CP, et al. Stereotactic bilateral anterior cingulotomy for intractable pain. J. Clin. Neurosci. 2005;12:886–890. doi: 10.1016/j.jocn.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 105.Foltz EL, White LE., Jr. Pain “relief” by frontal cingulumotomy. J. Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 106.Mohseni HR, et al. MEG can map short and long-term changes in brain activity following deep brain stimulation for chronic pain. PLoS ONE. 2012;7:e37993. doi: 10.1371/journal.pone.0037993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci. Lett. 2012;520:174–181. doi: 10.1016/j.neulet.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Exp. Neurol. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


