Abstract
The human hippocampus is widely believed to be necessary for the rapid acquisition of new declarative relational memories. However, processes supporting on-line inferential word use (“fast mapping”) may also exercise a dissociable learning mechanism and permit rapid word learning without the hippocampus (Sharon et al. (2011) Proc Natl Acad Sci USA 108:1146–1151). We investigated fast mapping in severely amnesic patients with hippocampal damage (N = 4), mildly amnesic patients (N = 6), and healthy comparison participants (N = 10) using on-line measures (eye movements) that reflected ongoing processing. All participants studied unique word-picture associations in two encoding conditions. In the explicit-encoding condition, uncommon items were paired with their names (e.g., “This is a numbat.”). In the fast mapping study condition, participants heard an instruction using a novel word (e.g., “Click on the numbat.”) while two items were presented (an uncommon target such as a numbat, and a common distracter such as a dog). All groups performed fast mapping well at study, and on-line eye movement measures did not reveal group differences. However, while comparison participants showed robust word learning irrespective of encoding condition, severely amnesic patients showed no evidence of learning after fast mapping or explicit encoding on any behavioral or eye-movement measure. Mildly amnesic patients showed some learning, but performance was unaffected by encoding condition. The findings are consistent with the following propositions: the hippocampus is not essential for on-line fast mapping of novel words; but is necessary for the rapid learning of arbitrary relational information irrespective of encoding conditions.
Keywords: memory, hippocampus, vocabulary, lexicon, medial temporal lobe
Introduction
The medial temporal lobe (MTL) is necessary for declarative learning (Scoville and Milner, 1957; Cohen and Squire, 1980), and within MTL, hippocampus is necessary for rapid learning of new associations between arbitrarily related memoranda (Eichenbaum and Cohen, 2001; Davachi and Dobbins, 2008; Ranganath, 2010). Word knowledge exemplifies learned, arbitrary relations between phonology, orthography, objects, and concepts, and therefore word learning should benefit from hippocampal-dependent relational memory. Indeed, hippocampal damage causes memory deficits extending to vocabulary acquisition (Gabrieli et al., 1988; Postle and Corkin, 1998) and association of arbitrary verbal labels with visual stimuli (e.g., Duff et al., 2006). However, the brain contains multiple memory systems (e.g., McClelland et al., 1995), and non-hippocampal mechanisms may support new word learning under certain conditions (Vargha-Khadem et al., 1997; Sharon et al., 2011).
While hippocampus appears to be necessary for adult word learning, children learn thousands of words despite immature MTL memory systems (Overman et al., 1996; Bauer, 2005). Developmental word learning may therefore rely on mechanisms outside the hippocampus and processes distinct from explicit encoding (EE). Fast mapping (FM) may be one such process: it describes the immediate association of a novel word with an unfamiliar visual stimulus (Carey and Bartlett, 1978). In FM tasks, a novel word is used in reference to an unfamiliar item presented with familiar visual competitors (Halberda, 2006; Horst and Samuelson, 2008; Kucker and Samuelson, 2011), and this often promotes selection of the unfamiliar referent (Carey and Bartlett, 1978; Halberda, 2006; Spiegel and Halberda, 2011). Referent selection in FM implies association of new words with new items, prompting suggestions that FM may be related to word learning (Carey and Bartlett, 1978).
Building on developmental linguistics findings, Sharon et al. (2011) used a FM referent selection task to test FM in patients with hippocampal damage and reported no impairment. Surprisingly, Sharon et al. also found durable word learning by patients after only two FM exposures, and suggested that this learning might be supported by extrahippocampal regions, specifically left anterior temporal lobe. This finding is intriguing because recent developmental studies show that on-line FM associations do not necessarily produce rapid, durable word learning in children (Gershkoff-Stowe and Hahn, 2007; Horst and Samuelson, 2008; Friedrich and Friederici, 2011; Vlach and Sandhofer, 2012; Bion et al., 2013). In fact, FM may reflect on-line, inferential decision processes relying on existing knowledge (von Koss Torkildsen et al., 2008) that do not yield substantial new knowledge. Therefore successful FM performance (but not learning) would be predicted even for amnesic patients. Supporting this perspective, Smith et al. (2014) strictly replicated the methods of Sharon et al. with a unique group of amnesic patients (their Exp. 1), but did not observe robust learning. Given the small number of studies on this topic and their conflicting findings, additional research is merited. Meanwhile, any role of the hippocampus in the online processes underlying FM performance remains undetermined. Eye movements can provide a rich, on-line measure of FM that reflects ongoing processes driven by underlying word knowledge (Halberda, 2006; McMurray et al., 2012; Bion et al., 2013), and can measure memory implicitly (Hannula et al., 2010).
Our investigation adapted and refined the methods of Sharon et al. (2011) to thoroughly characterize the roles of the hippocampus and MTL in fast mapping. Our procedure used materials from Sharon et al. (2011), but an alternative FM format was used to facilitate on-line observation of eye movements (Halberda, 2006). We tested participants ranging from neuropsychologically normal to severely, neurologically amnesic using a variety of carefully controlled measures to determine the strength and flexibility of any learning due to fast mapping and any interactions with severity of memory impairment. We predicted that overt responses and on-line measures would show normal FM performance and impaired FM learning by severely amnesic patients, and that degree of memory impairment would be related to ability to learn new words irrespective of study format.
Experimental Methods
Terminology
“Fast mapping” has been used to refer to selection of a novel referent in response to a novel word form, learning from such exposure, or both (McMurray et al., 2012, p. 836). Here, we reserve the term FM for the first situation only and use “learning from FM” to specify subsequent learning.
Participants
Four groups of participants were recruited. (1) Severely amnesic (SA) patients (N = 4) with MRI-confirmed atrophy or lesion of the hippocampus, sometimes extending into the MTL (Table 1). (2) Mildly amnesic (MA) patients (N = 6) (Table 1). (3) Healthy comparison participants (NC) (N = 10) with no history of neurological or psychiatric disease, each matched to one of the SA or MA patients for sex, handedness, age, and education. These three groups completed the entire experimental protocol (see Procedure). (4) Naive healthy comparison participants (NNC) (N = 15) who completed only the familiarity-rating and 3AFC recognition test phases of the protocol [6M/9F; age, mean = 56.800 (SD = 8.187) years; education: 16.000 (2.420) years]. Patients were recruited from the Iowa Registry of Neurological Patients. Comparison participants were recruited from Iowa City and surrounding communities. This research was approved by the University of Iowa Human Subjects Office and by the Biomedical Institutional Review Board (ID#201112768), and was conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained from all participants prior to their first experimental session. Consent documents described the study's purpose as follows: “… to investigate whether certain regions of the brain participate in the learning and expression of names.” All participants were remunerated $15/h.
Table 1. Demographic and neuropsychological data characterizing participating amnesic patients.
| ID | Amn. | Age | Edu. | Sex | Eti. | Chr. | Hand | FSIQ | VIQ | PIQ | DS | Read | BNT | GMI | DRI | AVLT | CFT C/R | BVRT | HcV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1846 | Sev. | 49 | 14 | F | An./SE | 19 | 100 | 84 | 88 | 86 | 10 | 102 | 43 | 57 | 62 | 7/3 | 28/6 | 8 | −4.23* |
| 1951 | Sev. | 60 | 16 | M | HSE | 32 | 100 | 106 | 107 | 116 | 9 | 105 | 49 | 57 | 63 | 9/2 | 32/4 | 5 | ≫ |
| 2308 | Sev. | 56 | 16 | M | HSE | 13 | −100 | 98 | 95 | 92 | 9 | 105 | 52 | 45 | 48 | 5/0 | 32/0 | 22 | ≫ |
| 2363 | Sev. | 56 | 18 | M | An. | 14 | 100 | 98 | 112 | 91 | 8 | 90 | 58 | 73 | 74.5 | 8/0 | 26/5 | 5 | −2.64* |
|
| |||||||||||||||||||
| Mean | 55.3 | 16.5 | 19.5 | 50.0 | 96.5 | 100.5 | 96.3 | 9 | 100.5 | 50.5 | 58.0 | 61.9 | 7.3/1.3 | 29.5/3.8 | 10.0 | ||||
| SD | 4.6 | 1.9 | 8.7 | 100.0 | 9.1 | 11.0 | 13.4 | 0.8 | 7.1 | 6.2 | 11.5 | 10.9 | 1.7/1.5 | 3.0/2.6 | 8.1 | ||||
|
| |||||||||||||||||||
| 1465 | Mild | 83 | 14 | M | HSE | 22 | 100 | 110 | 107 | 130 | 10 | 94 | 57 | 79 | 82 | 10/7 | 36/12 | 3 | −2.95† |
| 2571 | Mild | 49 | 16 | F | An. | 12 | 100 | 112 | 116 | 109 | 10 | 99 | 59 | 87 | 91.5 | 12/8 | 36/10 | 3 | −1.01* |
| 2997 | Mild | 64 | 14 | M | An. | 10 | 100 | 112 | 114 | 121 | 10 | 107 | 57 | 87 | 88.5 | 9/6 | 34/23 | 6 | 0.57‡ |
| 3139 | Mild | 60 | 20 | M | An. | 7 | 100 | 107 | 109 | 105 | 11 | 96 | 60 | 78 | 82 | 8/3 | 34/25 | 0 | NA |
| 3571 | Mild | 61 | 20 | M | An. | 18 | 10 | 141 | 134 | 142 | 14 | 126 | 58 | 88 | 88.5 | 9/5 | 32/5 | 4 | NA |
| 3633 | Mild | 50 | 16 | M | An. | 3 | 100 | 114 | 116 | 133 | 9 | 116 | 60 | 81 | 82 | 9/5 | 27/10 | 4 | 20.59‡ |
|
| |||||||||||||||||||
| Mean | 61.2 | 16.7 | 12.0 | 85.0 | 116.0 | 116.0 | 123.3 | 10.7 | 106.3 | 58.5 | 83.3 | 85.8 | 9.5/5.7 | 33.2/14.2 | 3.3 | ||||
| SD | 12.3 | 2.7 | 7.0 | 36.7 | 12.5 | 9.6 | 14.4 | 1.8 | 12.6 | 1.4 | 4.5 | 4.3 | 1.4/1.8 | 3.4/8.0 | 2.0 | ||||
Italicized scores for neuropsychological tests indicate significant normative impairment. For this project, our criterion for severe amnesia was a WMS-III GMI of 75 or less (i.e., 1.67 standard deviations below normative values). Score notes: 3633's IQ scores (underlined) are prorated from WAIS-IV subtests (Wechsler, 2008). Abbreviations: Amn., Amnesia severity; Sev., severely amnesic; Mild, mildly amnesic; Age, years; Edu., education, years; Chr., Chronicity, years since injury (2012); Hand, handedness (+100=fully right handed, −100= fully left handed); Eti., Etiology; Anoxia/An., anoxic/ischemic episode; SE, status epilepticus; HSE, herpes simplex encephalitis; FSIQ, WAIS-III full-scale IQ (Wechsler, 1997); VIQ, verbal IQ; PIQ, performance IQ; DS, WAIS 3/4 Digit Span; Read., WRAT- R (or 4, if underlined) standardized reading score (Jastak & Wilkinson, 1984); BNT, Boston Naming Task (Kaplan, Goodglass, & Weintraub, 1983); WMS-III GMI, general memory index (The Psychological Corporation, 1997); DRI, delayed recall index (average of the auditory delayed index and visual delayed index); AVLT, Rey Auditory Verbal Learning Task, trial 5/15-min. delay (Kaplan et al., 1983); CFT, Complex Figure Task copy/recall (Osterrieth, 1944; Rey, 1941); BVRT, Benton Visual Retention Test, errors (Sivan, 1992); HcV, bilateral hippocampal volumes per Allen et al. (2006). Volumes are Studentized residuals relative to normative expectations:
reported in Allen et al. (2006);
value not available, but near-complete bilateral hippocampal lesion (see Cavaco, Feinstein, van Twillert, & Tranel, 2012; Feinstein et al., 2010);
newly available hippocampal volumes derived using method of Allen et al. (2006);
near-complete right hippocampal lesion, but largely intact left hippocampus; NA, volumetric measurements unavailable due to contraindications for MRI (e.g., pacemakers).
SA patients had hippocampal damage and severe, focal deficits in declarative memory as shown by neuropsychological measures (Table 1). Results of the Boston Naming Test indicated that naming performance was normal for 2363 and 2308, mildly defective for 1951, and defective for 1846. However, 1846 performs normally when naming animals, fruits, and vegetables similar to the common items in our task (Warren et al., 2012b, p. 347), while 1951 performs normally when naming animals but poorly when naming fruits and vegetables (Feinstein et al., 2010, p. 93). All SA patients had bilateral, MRI-confirmed atrophy or lesion of the hippocampus. Two SA patients (1846 and 2363) had atrophy limited to the hippocampus. The two postencephalitic SA patients (1951 and 2308) had lesions extending further into the medial, lateral, and anterior temporal lobes (Allen et al., 2006; Feinstein et al., 2010; Cavaco et al., 2012; Warren et al., 2012b). Specifically, patient 1951 has a large lesion of the right temporal lobe (including the right temporal pole) and damage to the limbic system bilaterally, while patient 2308 has a large lesion of the left temporal lobe (including the left temporal pole).
A group of mildly amnesic patients was studied in order to examine whether any benefit of learning by fast mapping might interact with the severity of memory impairment. We used a neuropsychological criterion (WMS GMI > 75) to distinguish between the SA group and the MA group. Neuroanatomically, MRI exam (when available) revealed that MA patients showed less hippocampal atrophy (anoxic patients 2571, 2997, and 3633) or more restricted hippocampal lesions (postencephalitic patient 1465, unilateral right) than that of SA patients (Table 1).
Materials
Our experiment used stimulus materials based on those developed by Sharon et al. (2011). Sharon et al. trained participants on two 16-image sets of uncommon plants and animals (we did not employ eight uncommon item images used by Sharon et al. as study lures). We incorporated additional uncommon item images to produce two sets of 24 uncommon item images each. We also collected a supplementary image of each uncommon item for use in cued recall testing (see Procedure). Finally, images of 60 common items were collected to use as FM competitors and practice items. All images were obtained either from Sharon et al. or from the World Wide Web, and were utilized under the principle of fair use. Auditory materials were recorded by a female speaker in the English dialect of this study's locale.
Regarding nomenclature
Word-image associations were ecologically valid (i.e., associations were drawn from real-world languages), making pre-experimental exposure a potential concern. Measures were taken to control for this (see Procedure), and so stimuli are consistently described as: common (i.e., likely to be familiar); uncommon (i.e., unlikely to be familiar); or unfamiliar (i.e., found to be unfamiliar to a particular participant). Items that were not unfamiliar to a given participant were removed from analyses. All groups rated common words as very familiar and uncommon words as not familiar (see Results: Familiarity).
Equipment
Visual stimuli were presented on a 21-in LCD monitor (Multi-Sync 2190UXi, NEC Corporation of America, Irving, TX) at a distance of 550 mm. Behavioral responses were made verbally or with a computer mouse. During study and recognition phases, subjects placed their head in a padded chinrest/ headrest apparatus, and eye movements were monitored at a sampling rate of 1,000 Hz using an EyeLink 1000 remote infrared camera system (SR Research Ltd., Ontario). Calibration procedures ensured that gaze position was accurate to within 1° of visual angle. Audio and video were recorded with a Flip camera (Cisco Systems, San Jose, CA).
Procedure
Our protocol used a within-subjects design to thoroughly evaluate familiarity ratings, free recall, recognition memory, and cued recall performance in two different experimental conditions. The protocol is outlined in Figure 1 and Supporting Information Table S1. All participants completed the protocol twice (once in each study format), and the order of administration was fixed by design (i.e., FM in the first session and EE in the second). The two nonoverlapping item sets (see Materials) were assigned to different study conditions for different participants. Specifically, our counterbalancing was designed to control for item effects between participants by ensuring that the sets of items that were assigned to the FM condition (e.g., set A) and EE condition (e.g., set B) for one participant were reversed for the next participant (i.e., set B for FM, set A for EE). Amnesic patients and their matched comparisons completed the same counterbalancing conditions. Interactive computerized phases were implemented in Matlab 2007b (The Mathworks, Inc., Natick, MA) using the Psychophysics Toolbox (Brainard, 1997).
Figure 1.
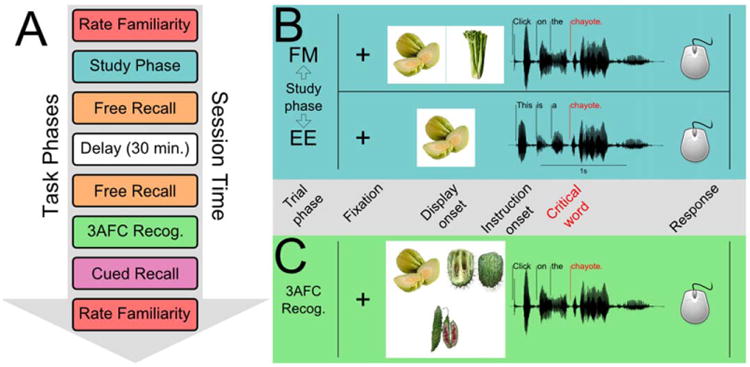
Task phases (A) and trial sequence of study (B) and 3AFC recognition (C) phases (labels between B&C are relevant for both). A) Task phase sequence for the protocol (see Experimental Methods and Supporting Information Table S1). B and C) All study and test trials began with central fixation followed by display onset, audio instruction playback (including a critical orienting word), and a response phase. Eye movements were monitored during all trials, and we used the critical target word (crit.) onset event to anchor timecourse analyses of eye movements (see Method and Results). B) Fast mapping (FM) and explicit encoding (EE) study formats were similar, but in the EE study format, only one uncommon item was presented, while in the FM study format, two items were presented and a choice was required. C) 3AFC recognition test format was the same after FM and EE encoding. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
After consenting, participants rated their familiarity with a list of common and uncommon words (24 common; 28 uncommon) according to the following instructions: “I'm going to ask about your familiarity with each of a list of words. Some of the words are likely to be familiar, while others may be unfamiliar. For each word, please first rate your familiarity with the word from: 1, not at all familiar, to 6, very familiar. Then, if you are familiar with the word, please give a brief description of the word's meaning.” Ratings greater than 1 elicited a request for a brief description. Notably, instructions in this phase did not include remembering the words in the list.
Next, participants practiced the assigned study format (Fig. 1B). The following directions were presented on the display: “Listen to the instructions, then use the mouse to click on one item. Between trials, look at the dot.” Six practice trials were presented; stimuli were common items. In the main phase, 32 study trials were presented: 24 critical trials, including one uncommon target item (with a common item lure in the FM condition); and 8 catch trials, including a common item as a target (with another common item lure in the FM condition). Images subtended ∼8° of visual angle, and were presented centrally in the EE condition or at left and right in the FM condition. Trials were administered twice in a pseudorandom sequence that ensured one presentation of each trial before any trial repeated. In the FM condition, target location was counterbalanced across left and right positions (reversing upon repetition). Gaze position was calibrated immediately prior to the main phase.
Each trial began with central fixation, after which the stimulus display containing one (EE) or two (FM) images was presented. After 1 s, the condition-specific orienting phrase (EE: “This is [a or an] …”; FM: “Click on the …”) was presented, followed by the name of the target. The response phase began 1 s after the target name ended and continued for up to 15 s after which a time-out was recorded; a centered cursor appeared, and the participant responded by moving and clicking the mouse. All trial events were recorded and timestamped.
After the study phase, free recall was tested (3-min session, recorded) before and after a filled 30-min delay. Participants were first asked to recall unfamiliar words heard during the study phase in order to maximize recall performance for these potentially difficult items, and then asked to recall familiar words. Following the second free recall phase, a 3AFC recognition test was administered (see Fig. 1C). First, participants practiced the 3AFC format using common items. During each trial, an orienting phrase and the name of one uncommon item were presented auditorily while three studied uncommon items were displayed (one target and two lures). Trials were structured similarly to study trials, although a generic orienting phrase was used (“Click on the …”) irrespective of study condition. Participants were told to follow the auditory instructions, use the mouse to move a cursor and click on the target item, and to guess if unsure. In the main test phase, target position was counterbalanced across the three test locations. All 24 studied, uncommon items were targets in one trial during this phase (see Supporting Information Table S2). No catch trials were presented. Gaze position was calibrated before the main test phase; each trial began with central fixation. Response timing was the same as at study.
Next, the cued recall phase was administered. In the visual cued recall task participants viewed a randomly ordered series of novel visual exemplars of all 24 studied uncommon items, and were asked to name or pass on each. All responses were recorded, and correct responses were reinforced. Near-miss responses yielded an invitation to try again. The visual and auditory cued recall task was similar to the preceding task (same 24 items in a new random order), but the experimenter now provided the first sound of each pictured item's name unless the participant produced the name immediately. Both tasks were recorded. After the cued recall phase, each subject again rated her/his familiarity with a list of words using the same list and procedure as in the pre-test rating phase. This phase was recorded.
The entire protocol was administered to each participant twice in two separate 2.5-h sessions, first in the FM study condition and second in the EE study condition. Because of testing constraints, the interval between FM and EE sessions varied between participant groups. SA and MA patients typically completed single FM and EE sessions within the same day (e.g., morning and afternoon sessions) or on 2 consecutive days, while NC participants completed their two sessions at least 1 month apart due to concerns about proactive interference between sessions. NNC participants completed only the familiarity rating and 3AFC phases in a single, 1-h session. MA and NC participants completed both conditions once. SA patients completed both conditions once, and then a second time after a significant interval (patients 1846, 2308, and 2363 were tested a second time 3 months after their first session, while 1951 was tested again after 3 days due to scheduling constraints). Assignment of the item sets to the FM and EE study conditions was reversed for each SA patient between the first and second administrations, and none showed any overt evidence of prior exposure to the materials. Repeated testing of SA patients using unique counterbalancing conditions is a common means of addressing concerns about sample size or reproducibility (Ryan et al., 2000; Hannula et al., 2006, 2007).
Analysis
Data were aggregated using Matlab 2007b, Python 2.7, and Python's pandas module. Data were analyzed using R 3.0 and the nlme, multcomp, lattice, and Cairo libraries.
The data we collected belonged to two broad categories: behavioral responses and eye movements. Overt behavioral responses included familiarity ratings, free recall, cued recall, referent selection in the FM study condition, and 3AFC recognition in both conditions. Eye movement data complemented behavioral responses in the FM study phase and the 3AFC phase. All of our analyses used repeated-measures ANOVA tests implemented as linear mixed-effect (LME) models with subjects entered as a random effect. We tested planned comparisons using linear contrasts applied to the LME models (reported using the normally-distributed Z value), and P values were corrected for multiple comparisons (indicated by Pc; α = 0.05).
Behavioral measures
Word familiarity
Changes in familiarity ratings (post-rating minus pre-rating) were calculated for all words unfamiliar to each participant and averaged within participants. These mean change scores were analyzed using a repeated-measures ANOVA. Participants were a random effect and study condition was a within-subjects fixed effect (levels: FM and EE). Group membership (levels: SA, MA, or NC) was a between-subjects fixed effect.
Free recall
Number of words recalled (unfamiliar and familiar) was analyzed using a similar repeated-measures ANOVA, but included a delay factor (levels: pre- or post-delay).
Cued recall
Number of words recalled was analyzed using another, similar repeated-measures ANOVA, but included a cue-type factor (levels: visual only or visual and verbal).
FM study
Referent selection performance was summarized as proportion correct (i.e., number of correct responses/number of responses), and analyzed using a simple ANOVA with group membership as the sole factor.
3AFC recognition
Performance (proportion correct as above) was analyzed using a repeated-measures ANOVA with a within-subjects factor for study condition, and a group membership factor with four groups (SA, MA, NC, and NNC).
Response time
Response time (RT) for correct trials was analyzed in the same manner as other behavioral measures for both the study and 3AFC recognition phases. For all performance measures, the repeated-measures ANOVA was supplemented by planned comparisons between groups and (where possible) versus chance.
Eye-movement measures
Fixations were defined using two criteria: if eye velocity and acceleration were both less than their respective thresholds (30°/s and 8,000°/s2, respectively), the eye was deemed to be engaged in a fixation (else saccade or blink). We measured the position and timing of fixations to displays during the study and 3AFC test phases of our protocol. We analyzed these fixation data at three different levels. First, whole-display measures included number of fixations and time spent fixating a display. Second, we divided the displays into rectangular regions of interest (ROIs) bounding the images and coded each ROI either for contents (e.g., target item, competitor item) or for response (i.e., selected or non-selected). ROI measures then included the proportion of time spent fixating each ROI and the number of fixations to each ROI. Finally, we extended our ROI analysis by locking the time of eye movements to specific trial events. We analyzed data at each level as described below.
During FM study, fixation time and number of fixations were summarized by averaging across trials to obtain per-participant values. We analyzed those data using a simple ANOVA with a single, between-subjects group membership factor (levels: SA, MA, NC) to evaluate group differences, followed by planned comparisons. 3AFC fixation time and number of fixations were analyzed using a repeated-measures ANOVA with a within-subjects study condition factor (levels: EE or FM), an additional level for group (NNC), and planned comparisons.
Measures of proportion fixation time were calculated by determining the fixation time to each ROI, and then dividing those per-ROI fixation time values by the sum of all ROI fixation time values (i.e., the quotient was a proportion of total fixation time across ROIs). In the FM study phase, each trial contained two ROIs: the ROI containing the target image, and the ROI containing the competitor image. With only two ROIs, values of proportional fixation time were constrained and we limited our analyses to correct trials and the selected-target ROI. We analyzed these data using a simple ANOVA with a single group-membership factor (levels as before) and planned comparisons. In the 3AFC test phase, each trial contained three ROIs (only one of which could be selected): the ROI containing the target image, and two ROIs containing competitor images. We analyzed these data using a repeated-measures ANOVA with a between-subjects group-membership factor (levels: SA, MA, NC, and NNC), and within-subjects factors representing ROI type (levels: target or competitor), selection (levels: selected and non-selected), and study condition (levels: EE or FM), along with planned comparisons. This ANOVA was applied to data from both correct trials and incorrect trials.
We analyzed proportional fixation-time measures time-locked to the onset of the critical target word (“crit.”). A 6-s epoch including the crit. event was analyzed, stretching from 2 s before playback began to 4 s after. The 6-s epoch was split into twelve 500 ms timebins, and these timebins were a within-subjects factor.
Study
Fixation data from the FM condition within the ROI of correctly-selected targets were analyzed (EE displays contained only one item). Group membership was a between-subjects factor.
3AFC recognition
Study condition (levels: FM or EE) was added as a within-subjects factor, and the NNC group was added to the group factor. Additionally, ROI was added a within-subjects factor for separate analyses of selected ROI data (levels: selected-target and selected competitor) and non-selected ROI data (levels: non-selected target, non-selected competitor/miss, and non-selected competitor/hit).
Correlation measures
We tested whether FM and EE conditions were differently related to performance on neuropsychological tests. We calculated correlation coefficients (Pearson's r) between 3AFC recognition performance of amnesic patients (i.e., SA and MA groups) under each study condition and declarative memory measures including: the Rey Auditory Verbal Learning Test (1st presentation, 5th presentation, and 15-min delay), the Weschler Memory Scale-III General Memory Index, the Rey-Osterrieth Complex Figure Test (CFT, delay), and the Benton Visual Retention Test (correct responses). We also calculated correlations for tests not thought to rely on declarative memory including digit span, CFT copy, and reading proficiency (Wide Ranging Achievement Test). We normalized all scores prior to calculation, and compared FM and EE correlation coefficients using Fisher's z transform.
Results
Study Phase
Behavior
After the familiarity-rating phase, participants completed a study phase in which uncommon words were presented along with novel images in either a FM or EE format (Fig. 1B). FM task comprehension was good, reflected in near-perfect performance on catch trials in which both images and the spoken word were common (eight trials tested twice per FM session; only two misses across all participants). This suggested that all participants had naming abilities sufficient to support good task performance. All groups also performed well on non-catch FM trials (each group >90% correct; Fig. 2A, left), but SA patients fast mapped less often than MA patients (Z = 2.988, Pc =0.014) and NC participants (Z = 2.843, Pc =0.021); the NC and MA groups performed comparably (Z = 0.514, Pc =0.951). The poorer performance of the SA group was not driven by any single patient (see Supporting Information Table S3). Even so, SA patients performed near the range of the NC and MA groups (Fig. 2A, left) and well above chance (Z = 25.628, Pc < 0.001), suggesting that their ability to fast map unfamiliar words to unfamiliar items on-line was largely intact. In order to account for poor encoding from incorrect FM study trials, all subsequent analyses of FM-format data were conducted twice, once with all unfamiliar items, and once with only unfamiliar items that were correctly selected during both exposures in the FM study phase. No qualitative differences were observed between the two analyses, and so the more inclusive analyses are reported.
Figure 2.
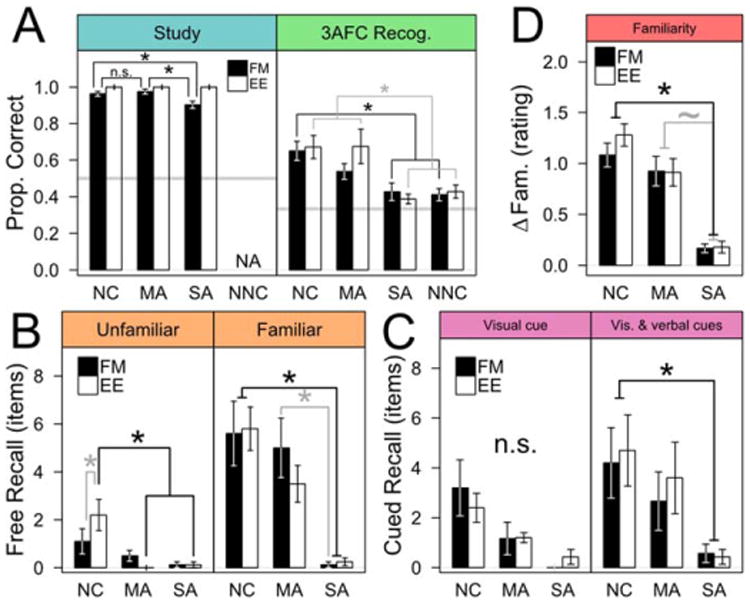
Behavioral performance at study (A, left) was similar for all groups, while at test (all others) SA patients showed no learning in either condition. Note that panels are labeled counterclockwise. Bars show group means and whiskers are s.e.m. (*P<0.05; ∼P<0.1). A) Left panel: all participants performed FM well above chance at study, but NC (N = 10) and MA (N = 6) groups performed better than SA patients (N = 4). Right panel: in the 3AFC recognition task, NC and MA groups performed well above chance, but SA patients performed no better than chance and much like the NNC group. Neither EE nor FM study affected recognition in SA patients. B) Left panel: free recall of unfamiliar items was poor for all groups, but the SA and MA groups averaged fewer than one recalled item. Right panel: free recall of familiar items was better than that of unfamiliar items for the NC and MA groups, but SA patients recalled very few items. C) Left panel: cued recall based on a novel visual exemplar of a studied item was performed best by the NC group, while SA patients recalled no words on average. Right panel: adding a verbal cue improved performance of the NC and MA groups, but SA patients were still near floor. Neither EE nor FM encoding affected cued recall. D) Post-test minus pre-test familiarity rating differences. NC and MA participants both reliably increased their familiarity ratings of unfamiliar items, while SA patients did not; EE and FM encoding produced similar results. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Eye movements
All groups viewed FM study displays similarly, fixating correctly-selected targets more later in trials (Fig. 3A), reflecting an on-line selection effect. Here (and in the 3AFC results) we considered the timecourse of proportional fixation time locked to specific trial events. In this analysis, displays were divided into regions of interest (ROIs) containing items, and trial time was split into 500-ms timebins anchored to the playback of the uncommon word (“critical word” or “crit.”; see Fig. 1B, top). No between-group differences were found [crit.-locked group-by-timebin interaction, F(24,240) = 1.231, P = 0.216; all pair-wise between-group, within-timebin planned comparisons were n.s.: Z < 2.9, Pc >0.10].
Figure 3.
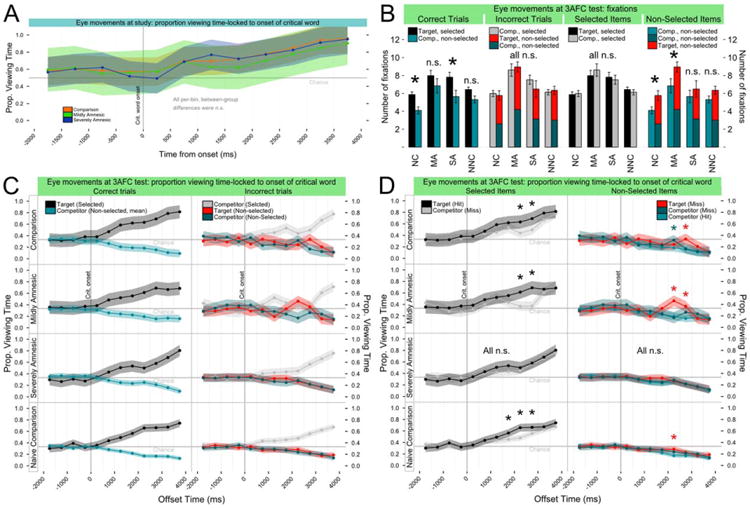
On-line eye-movement measures of all groups were similar during study (A), but differed at 3AFC test (B and C) reflecting learning by some groups. In A,C,&D, points and lines indicate group mean proportion fixation time (PFT) per 500 ms timebin (shading shows 95% confidence intervals); in B, bars show group means of fixation counts and whiskers show s.e.m. (*: adjacent bars differed at P < 0.05). C and D plot the same data in different ways to emphasize specific patterns of viewing over time. A) During correct FM study trials, a selection effect was evident for all groups: viewing of the selected target increased after playback of the critical word. B) During 3AFC test trials, differences in fixation (after critical word onset) of nonselected items corresponded to better memory. All groups fixated selected items equally often (center right), whether those items were correctly-selected targets or incorrectly-selected competitors. However, the NC and MA groups fixated non-selected items more in incorrect trials (far right), potentially reflecting uncertainty in recognition processes. Meanwhile, all groups fixated correctly-selected target items (numerically) more than competitors in correct trials (far left), but in incorrect trials incorrectly-selected competitors were fixated as frequently as all non-selected items combined (center left). C) Increased viewing of targets by NC and MA participants at test appeared to be an on-line expression of prior learning. PFT is plotted to targets and competitors in 3AFC test displays before and after the critical word during correct (left) and incorrect (right) trials (group labels on left ordinate). Correct trials: All groups viewed selected target items (black) more later in the trial. Incorrect trials: The SA and NNC groups also steadily increased viewing of an incorrectly-selected competitor item (gray) later in incorrect trials. However, the NC and MA groups differed, showing increased viewing of non-selected items later in the trial, particularly the non-selected target (red). In these groups, memory appeared to modulate the on-line selection effect. D) Knowledge increased viewing of target items versus competitors whether selected or not. NC and MA groups showed a temporally-localized increase in proportion fixation time to selected targets (black) versus selected competitors (gray) (left). SA patients did not exhibit any differences in PFT for correct and incorrect selection (*: PFT Target>PFT Comp., P <0.05). Meanwhile, NC and MA groups viewed non-selected targets more than non-selected competitors (right), reflecting prior learning from FM and EE study trials (*: PFT Target/Comp. Miss>PFT Comp. Hit, P<0.05). A similar pattern in the NNC group may be attributable to within-test learning. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
3AFC Associative Recognition
Behavior
In contrast to the persistent benefits in delayed recognition performance of SA patients after FM study reported by Sharon et al. (2011), we found no influence of FM study on 3AFC recognition for SA patients (Fig. 2A, right). Significant group-level differences in recognition were evident, but there was no interaction with study format [ME of group: F(3,35) = 9.682, P < 0.001; ME of study format: F(1,35) = 1.009, P = 0.322]. SA patients performed similarly after both FM and EE encoding (Z = 0.734, Pc = 0.993), and in neither condition did they perform better than chance (each Z < 1.8, each Pc >0.45) or than a group of naïve NC participants (NNC) who did not have the benefit of study exposure (each Z < 0.65, Pc > 0.99). The main NC group performed well above chance after both study formats (ZFM = 6.642, Pc < 0.001; ZEE = 7.082, Pc < 0.001), as did MA patients (ZFM = 3.320, Pc = 0.007; ZEE = 5.542, Pc < 0.001), and these two groups did not differ (each Z< 1.5, each Pc > 0.75). Numerically, NC and MA patients both performed better after the EE study format, but this difference was not significant (Pc > 0.2). There were group differences in response time (RT) at 3AFC test [ME of group, F(3,35) = 6.167, P < 0.005], but no differences related to study format [ME of study format, F(1,35) = 1.375, P > 0.20]. The NC and NNC groups responded most quickly, and significantly faster than the MA patients (each Z > 2.8, each Pc < 0.025), while only the NC group responded significantly more quickly than the SA group (Z = 2.857, Pc = 0.022). The SA and MA groups did not differ (Z = 1.308, Pc > 0.50). There was no evidence of a speed-accuracy tradeoff as RT of the least accurate groups (i.e., NNC and SA patients) fell between those of the more accurate groups (i.e., slower than NC participants, faster than MA patients).
We evaluated any effects of etiology and lesion extent in the SA group, and no significant effects on 3AFC recognition were observed. Within the SA group, etiology corresponded with the extent of temporal lobe damage: both anoxic SA patients had focal hippocampal atrophy; and both post-encephalitic SA patients had lesions extending further into the temporal lobes. 3AFC performance did not differ by etiology (no main effects or interactions: each F < 1.0, each P > 0.30). Sharon et al. (2011) suggested that left anterior temporal lobe might support learning by FM, and patient 2308 had lesion damage to this region. We controlled for this by analyzing the 3AFC recognition performance of the remaining SA patients. As in the main analysis, this subgroup not differ from the NNC group after the FM study format [T(19) = 0.993, P = 0.333].
Individual performance of the SA patients was considered, and as reported in a preliminary conference presentation (Warren and Duff, 2012) SA patients occasionally recognized unfamiliar words above chance by one-tailed binomial test. However, such recognition was uniformly inconsistent or marginal. Neither patient 1846 (anoxic) nor patient 2308 (post-encephalitic) exceeded chance after FM or EE study in either of two sessions. Patient 2363 (anoxic), who had good 3AFC recognition after his first FM encoding session (prop. correct = 0.667, P < 0.001), was not above chance in his second FM session (prop. correct = 0.417, P > 0.10). Intriguingly, 2363 was just above chance after EE study of the same item set in the second session (prop. correct = 0.5, P = 0.028), suggesting an idiosyncratic material-specific effect. Patient 1951 (post-encephalitic) performed above chance after both FM sessions (prop. correct = 0.5, P = 0.028), but just, recognizing 12 of 24 items, the minimum value to exceed chance. For additional perspective, NNC participants also occasionally performed well above chance (prop. correct range = 0.167–0.739) without the benefit of study. Critically, the SA and NNC groups did not differ significantly from chance or from each other.
Eye movements
Eye movements can reveal prior experience not observed in explicit responses (Ryan et al., 2000; Hannula et al., 2010). We analyzed the number of fixations made after playback of the critical word and the timecourse of fixations during 3AFC recognition, and expected increases in fixation of targets (selected or not) to reflect word learning. While the FM and EE conditions did not differ on any measure (and were collapsed for all analyses), the eye movement data provide useful insight into ongoing memory processes as detailed below.
Fixations
All groups fixated items they selected with similar frequency during both correct trials (target selected) and incorrect trials (competitor selected) (each Z≤ 1.1, each Pc >0.98), but during incorrect trials the NC and MA groups fixated non-selected items (i.e., the missed target and another competitor) more frequently than during correct trials (each Z>3.7, each Pc <0.005). These patterns suggest a selection effect reflected in increased fixation of selected items by all groups, and a knowledge-related modulation of that selection effect in the NC and MA groups when incorrect (see Fig. 3B).
Fixation timecourse
The NC and MA groups viewed targets more than competitors in both correct and incorrect trials, indicating that associative knowledge substantially influenced eye movements during 3AFC recognition. Meanwhile, viewing by SA patients was influenced by on-line selection but not by studied associations, much like NNC participants. Specifically, during correct trials all groups viewed selected targets more after playback of the critical word (Fig. 3C, left) (all groups above chance by 1,000 ms: each Pc< 0.005). During incorrect trials (Fig. 3C, right) SA patients and NNC participants showed similar viewing patterns to selected competitors (SA and NNC groups above chance by 1,500 ms: each Pc < 0.05). Despite similar early viewing of selected competitors by the NC and MA groups (first above-chance viewing, each Pc < 0.005: NC, 1,500 ms; MA, 1,000 ms), both groups showed decreased viewing of the selected competitor later in the trial coupled with increased viewing of the non-selected target (Fig. 3C, right). Neither the SA nor the NNC group showed this pattern of viewing. Direct comparison of selected-item viewing (i.e., target vs. competitor; see Fig. 3D, left) revealed significantly more target viewing for the NC and MA groups in two timebins (both groups, 2,000 and 2,500 ms: each Z > 3.0, each Pc < 0.025), but no corresponding pattern in the SA group (each Z < 2.0, each Pc >0.39). Additionally, the NC and MA groups viewed ROIs containing non-selected targets significantly more than non-selected competitors (NC: 2,500 ms, Z = 3.622, Pc < 0.0025; MA: 2,000 and 2,500 ms, each Z > 4.1, each Pc< 0.001) (Fig. 3D, right). The NNC group occasionally showed similar, significant effects of smaller magnitude, which may have been driven by within-test learning. Meanwhile, SA patients did not show any increases in viewing of non-selected targets (each Z < 2.5, each Pc >0.08). Increased late viewing reflected a selection effect that was modulated by knowledge for the NC and MA groups, but not the SA group.
Free Recall
We tested free recall of studied words twice during our protocol (immediately and after a 30-min. delay prior to 3AFC recognition), and SA patients were impaired irrespective of delay, word familiarity, or study format. Recall of unfamiliar studied words was generally poor and did not differ by delay [main effect (ME) of delay; F(1,63) = 0.447, P = 0.506], so our analysis focused solely on the delayed recall data. Both SA and MA patients recalled <1 unfamiliar words on average, while NC participants recalled 1.8 unfamiliar words on average (see Fig. 2B); this difference was significant [ME group factor: F(2,21) = 4.795, P = 0.019] and driven by group differences after EE encoding. Recall of familiar words after the 30 min delay was markedly better than unfamiliar words for the NC and MA groups, as both recalled several words on average (NC: mean = 5.700 words, s.e.m. = 0.788; MA: mean = 4.250, s.e.m. = 0.730). SA patients were again near floor [mean = 0.188 words, s.e.m. = 0.101; main effect of group, F(2,21) = 16.568, P < 0.001]. Study format was not significant as a main effect or as an interaction (each P > 0.5), and all groups performed similarly across study formats (each Z < 1.1, each Pc > 0.8). NC participants recalled more words than SA patients (both EE and FM; each Z > 4.300, each Pc < 0.001), while MA patients recalled more words than SA patients after the FM study format only (ZFM = 3.367, Pc = 0.006); NC and MA groups performed comparably (each Z < 1.7, each Pc > 0.4). These differences in free recall were entirely congruent with our expectations based on the well-characterized memory status of the SA and MA patients (Table 1).
Cued Recall
We tested cued recall using novel visual exemplars of studied unfamiliar items: first alone; then combined with the beginning of the item's name as a verbal cue. SA patients were impaired relative to the NC group irrespective of study format. We observed significant effects of cue type and group on cued recall performance (Fig. 2C), but no effect of study format [ME of cue type, F(1,58) = 16.390, P < 0.001; ME of group, F(2,20) = 3.600, P = 0.046; ME of study format, F(1,58) = 0.002, P > 0.95]. Both NC and MA groups benefited from combined visual-verbal cues (NC: Z = 3.545, Pc = 0.003; MA: Z = 3.042, Pc = 0.018). NC participants recalled more items than SA patients when given combined cues (Z = 3.094, Pc = 0.015), although MA patients did not (Z = 1.684, Pc = 0.454). Combined cues did not benefit SA patients (Z = 0.514, Pc > 0.99), who did not recall any items on average in either cue condition (each Z < 0.6, Pc > 0.98).
Familiarity
Participants rated their familiarity with uncommon words twice (beginning and end of session), allowing us to identify pre-experimentally familiar words and to measure any changes in familiarity. Only the NC and MA groups showed significant changes in familiarity ratings. Pre-experimentally, uncommon words were very unfamiliar to all participants, while common words were extremely familiar (see Supporting Information Table S4). Each group had greater familiarity with common than uncommon words [ME of commonality, F(1,113) = 9721.219, P < 0.001], and all groups were similarly unfamiliar with the uncommon words (n.s. pairwise differences: each Z < 2.6, each Pc > 0.1). Surprisingly, SA patients rated common words as being less familiar than the NC and MA groups (each Z > 4.5, each Pc < 0.001), while NC and MA ratings were similar (Z = 1.058, Pc = 0.948). Review of SA patient word descriptions suggested unusually conservative ratings of common words rather than defective knowledge, but the result is intriguing.
Post-experimentally, we found that non-associative familiarity of unfamiliar words among SA patients was largely unaffected by repeated exposure throughout the session, while the NC and MA groups both increased their familiarity ratings [ME of group, F(2,21) = 9.834, P = 0.001] (Fig. 2D). There were no significant effects of study format [ME of study format, F(1,20) = 0.695, P = 0.414]. Both NC and MA groups rated previously unfamiliar items as more familiar post-experimentally (NC, Z = 7.499, Pc < 0.001; MA, Z = 4.127, Pc < 0.001; n.s. different, Z = 1.329, Pc = 0.519), while SA patients rated items similarly (Z = 0.984, Pc = 0.739). Familiarity ratings by NC participants changed more than those of SA patients (Z = 4.266, Pc < 0.001). MA patients also differed from the SA group, but not significantly (Z = 2.476, Pc = 0.058).
Word Learning Related to Neuropsychological Measures
Learning from fast mapping has been hypothesized to rely on extrahippocampal regions including left anterior temporal lobe (Sharon et al., 2011), which suggests that measures of declarative memory may not be strongly related to learning from fast mapping. Our SA group did not demonstrate robust learning, but there was sufficient individual variability within the SA and MA groups to test correlations between 3AFC recognition (after FM and EE study formats) and neuropsychological measures of memory. These correlations were uniformly positive (Fig. 4), and while the strength (range: r = 0.38–0.75) and significance of the correlations varied, in no case were the correlations between FM or EE performance and a given measure reliably different [each z(14) < 1.0, each P > 0.3]. In contrast, measures thought to be unrelated to declarative memory, including digit span, reading performance, and complex figure copy were all uncorrelated with 3AFC recognition after either study format (each r < 0.35, each P > 0.25). This pattern suggests that rapid word learning depends on mechanisms supporting declarative memory.
Figure 4.
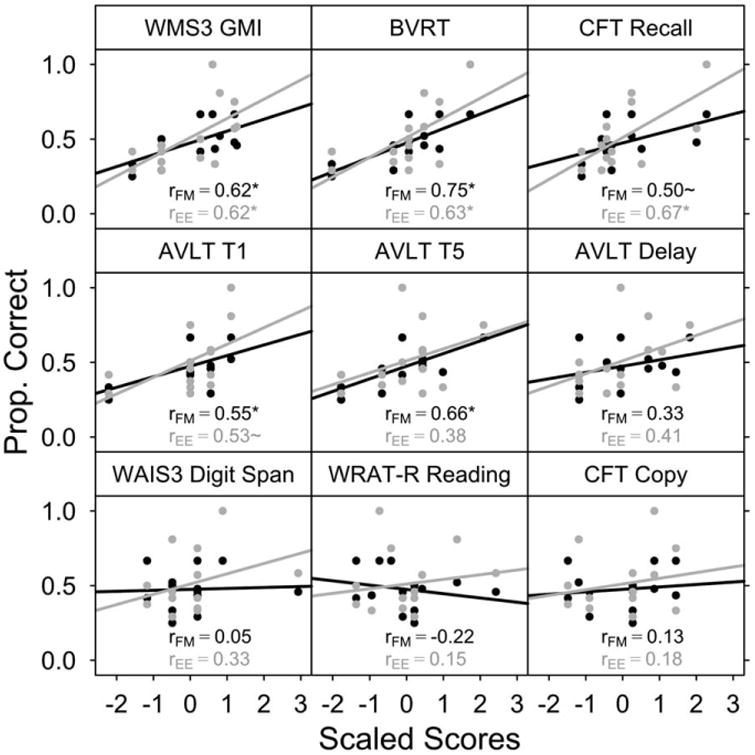
Neuropsychological measures of memory were positively correlated with 3AFC performance in amnesic patients after both FM and EE study. Correlations were calculated between several neuropsychological measures (normalized within a combined SA/MA group) and 3AFC task performance in the FM and EE conditions. Best-fit slopes and correlation coefficients are shown in black for FM and in gray for EE. The top and middle rows incorporate neuropsychological measures related to declarative memory, and correlation coefficients were uniformly positive, frequently significant (*P<0.05), and did not differ between FM and EE study conditions. The bottom row incorporates measures not thought to be related to declarative memory, none of which were significantly correlated with 3AFC task performance. This pattern suggests that word learning may be supported by typical declarative memory mechanisms.
Discussion
Fast mapping has been hypothesized to permit rapid learning of arbitrary relations without hippocampal involvement (Sharon et al., 2011). Here we found that NC participants were able to rapidly learn arbitrary new word information under FM and EE conditions, but SA patients were unable to learn from either condition. This was reflected in each of our explicit measures of memory performance, including 3AFC recognition, free recall, cued recall, and familiarity rating changes. Additionally, we observed on-line evidence of learning in the eye movements of NC and MA participants, but not in those of SA patients. Taken as a whole, our results suggest that learning words by FM is no different than learning words by any other means. Further, the hippocampal damage common to all SA patients (focally hippocampal in two cases) makes our findings consistent with the proposition that the hippocampus is necessary for the rapid acquisition of arbitrary new relational knowledge. At the same time, hippocampus was not critical for FM referent selection at study, and this dissociation mirrors a new perspective on FM from the developmental linguistics literature.
On-line Processing During Fast Mapping
The study-time FM task provided novel insight into how existing knowledge influences on-line performance. Despite impaired learning, SA patients performed the FM task well above chance both in our study and in Sharon et al. (2011). We found that the eye movements of SA patients during correct FM trials were no different than those of the NC or MA groups, exhibiting a selection effect (increased viewing of selected items) much like previous reports (Halberda, 2006; Bion et al., 2013). Good FM performance and poor learning parallels the developmental language literature: young children (with putatively immature MTL memory systems) perform FM tasks well but do not necessarily learn much from them (Gershkoff-Stowe and Hahn, 2007; Horst and Samuelson, 2008; Bion et al., 2013). Intriguingly, SA patients did not perform the FM task as well as the NC or MA groups. While acknowledging the limitations of our neuropsychological methodology regarding anatomical specificity, we hypothesize that the hippocampus and other MTL regions may be necessary to consistently bind together complex displays and incorporate existing knowledge on-line. As further evidence, hippocampal damage has been shown to impair on-line processing in many contexts (Warren et al., 2011, 2012a; Voss et al., 2011; Rubin et al., 2011), and older children with putatively more mature MTL memory systems may learn more from FM performance (Bion et al., 2013). Notably, even when any failures of on-line processing were controlled for by limiting analysis to items that were accurately fast mapped, 3AFC recognition of the SA group was no different than naive NCs or chance.
Word Learning as an Extended Process
Single-exposure learning from FM is intriguing, but concentrating on the effectiveness of encoding during the first encounter with a new word ignores the protracted process of incorporating that word into the mental lexicon (McMurray et al., 2012). Word learning requires the binding together of many arbitrarily related features, including concepts, phonology, orthography, and objects. While a healthy, mature hippocampal memory system may be sufficient to rapidly combine those features after a single exposure, adding a new entry to the lexicon is a slow process described as “extended mapping … that takes place over months or years” (Carey, 2010). The deliberate pace of extended mapping suggests slow learning typical of neocortex (McClelland et al., 1995; Norman and O'Reilly, 2003) perhaps localized to lateral portions of the temporal lobes that are necessary for normal word knowledge (Damasio et al., 2004) and that are activated during word learning (Davis and Gaskell, 2009; Shtyrov, 2012). Meanwhile, deficits in rapid learning of arbitrary associations caused by hippocampal damage are likely to also affect the extended mapping process and to impede word learning. Attempts to teach severely amnesic patients new vocabulary items have revealed profound impairments in such learning (Gabrieli et al., 1988; Postle and Corkin, 1998; Duff et al., 2006), but some new learning may occur for items that enter common usage (e.g., in the media) (Kitchener et al., 1998; O'Kane et al., 2004). However, any semantic learning is greatly diminished relative to normative expectations (Bayley and Squire, 2005).
Hippocampus, Relational Memory, and Fast Mapping
Hippocampal amnesia has been repeatedly shown to disrupt new associative learning. Amnesic patients are impaired when learning item-location associations, face-scene associations, rich spatial contexts, or other examples of complex, relational information (Chun and Phelps, 1999; Ryan et al., 2000; Hannula et al., 2006, 2007; Hartley et al., 2007; Konkel et al., 2008). The relations that amnesic patients can learn are instead non-arbitrary (Duff et al., 2006), rely primarily on emotional responses to specific stimuli (Tranel and Damasio, 1993), or are inflexible and expressed only in performance of a specific task (Milner, 1972; Glisky, 1992; Bayley et al., 2005). In this context, failure to learn new words irrespective of study format is not surprising. A strict replication of the Sharon et al. methodology (Exp. 1 of Smith et al., 2014) that also incorporated elements found in a preliminary report of this work (Warren and Duff, 2012) (e.g., expanded tests of flexible learning, study lists of 24 items in Exp. 2) found no evidence that FM study improved recognition performance of amnesic patients beyond that of naive comparisons.
Our findings are consistent with this larger literature of associative memory and the hippocampus, but at odds with the post-FM learning described by Sharon et al. (2011). In that study as well as ours, amnesic patients were able to fast map unfamiliar words to unfamiliar images on-line. The two sets of results chiefly differ regarding learning from FM. Comparing the two outcomes directly is challenging because we did not intend to replicate the procedures of Sharon et al. precisely, instead adopting a FM format that facilitated the simultaneous collection of eye-movement data.
Several factors may have contributed to the differences between our results and those of Sharon et al. One factor is stimulus modality and timing during FM. Our task used a multimodal presentation format resembling a developmental learning context: simultaneous auditory presentation of an unfamiliar word and visual presentation of familiar and unfamiliar objects (e.g., Carey and Bartlett, 1978; Markson and Bloom, 1997; Halberda, 2006); while Sharon et al. presented auditory and written words followed by visual images. A strict replication of the Sharon et al. task (Smith et al., 2014) did not observe learning by fast mapping in amnesic patients, suggesting that matching the Sharon et al. study format alone is not sufficient to drive learning. Additionally, near-ceiling FM referent selection by all groups in our study and Sharon et al. suggests limited effects of study-time format differences including modality and stimulus timing. However, combining eye-movement measures with a strict replication of Sharon et al. is a potential future direction that would control these factors.
Other factors included list length: we tested 24 unfamiliar items for more precise estimates of recognition; Sharon et al. tested 16. Effects of list length on recognition memory are debated (cf. Dennis and Humphreys, 2001), but 16 versus 24 memoranda should not have affected performance substantially (Smith et al., 2014). However, future work should study this in a FM context. Another difference between the Sharon et al. study and our work was the specific orienting phrase used in the FM study format: in our study, participants were instructed to “Click on the numbat” (Halberda, 2006); while Sharon et al. instructed participants to respond to perceptual questions such as “Is the numbat's tail pointing up?” While different, both sets of instructions adhere to the methodology of prior work on fast mapping (Carey and Bartlett, 1978; Markson and Bloom, 1997; Halberda, 2006) by defining a task that requires perceptual judgment without explicitly encouraging memorization of the associations. Similarly, global task orientation may have differed slightly between our study and that of Sharon et al. as our consent procedure avoided deception by describing the project as a study of name learning. The effects of possible differences in global task orientation on learning by fast mapping deserve careful further consideration. Given that subtle features of an experiment can significantly alter participants' mental set, future studies should approach these issues with great care. Individual differences between patient samples could also contribute to different outcomes, and one of the post-encephalitic SA patients in our study (2308) had damage extending into the left anterior temporal lobe region that Sharon et al. suggested might support learning by FM. However, the remaining SA patients whom we tested (N = 3) performed 3AFC recognition no better than naive comparisons irrespective of study format.
Finally, our design carefully controlled categorical information during 3AFC recognition. Competitors in our 3AFC recognition test matched the superordinate category of the target (e.g., a plant target trial used plant competitors; see Supporting Information Table S2), potentially requiring more specific knowledge of word-image associations than the original task. Importantly, amnesia does not prevent normal or near-normal category learning in some tasks (Knowlton and Squire, 1993; Reed et al., 1999; cf. Zaki, 2004), and contrastive FM may promote categorization of novel word forms. Indeed, Reed et al. (1999) reported amnesic categorization performance similar to the FM advantage reported by Sharon et al. Notably, the amnesic group reported by Smith et al. (2014) did not show similar benefits, but future investigations should examine whether recognition performance is improved by the opportunity to express category knowledge.
In summary, we found no evidence that successful fast mapping performance provided any unusual benefits to learning by severely amnesic patients with hippocampal damage. Amnesic patients failed to recognize studied items above chance and demonstrated no explicit or implicit evidence of associative learning despite good on-line fast mapping performance. Our findings coincide with recent work in developmental linguistics indicating that good fast mapping performance is not necessarily tied to rapid associative learning. These findings are consistent with the following propositions: that while that the hippocampus is not necessary for good fast mapping performance, fast mapping does not necessarily produce rapid, robust learning of new words or arbitrary relations in severely amnesic patients; and that the hippocampus is necessary for normal word learning by adults.
Supplementary Material
Acknowledgments
The authors thank R. Clark, S. Jones, J. Bruss, and S. Kirk for their assistance with this project, A. Gilboa for providing materials used by Sharon et al. (2011), and B. McMurray for commenting on an earlier draft of this article. The authors also thank the participating patients and their families for facilitating our testing.
Grant sponsor: NIH; Grant numbers: P50-NS19632, R01-DC011755, and R01-MH062500.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Allen JS, Tranel D, Bruss J, Damasio H. Correlations between regional brain volumes and memory performance in anoxia. J Clin Exp Neuropsychol. 2006;28:457–476. doi: 10.1080/13803390590949287. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Developments in declarative memory. Psychol Sci. 2005;16:41–47. doi: 10.1111/j.0956-7976.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Frascino JC, Squire LR. Robust habit learning in the absence of awareness and independent of the medial temporal lobe. Nature. 2005;436:550–553. doi: 10.1038/nature03857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley PJ, Squire LR. Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus. 2005;15:273–280. doi: 10.1002/hipo.20057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bion RA, Borovsky A, Fernald A. Fast mapping, slow learning: Disambiguation of novel word-object mappings in relation to vocabulary learning at 18, 24, and 30 months. Cognition. 2013;126:39–53. doi: 10.1016/j.cognition.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard D. The psychophysics toolbox. Spatial Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Carey S. Beyond fast mapping. Lang Learn Dev. 2010;6:184–205. doi: 10.1080/15475441.2010.484379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey S, Bartlett E. Acquiring a single new word. Proc Stanford Child Lang Conf. 1978;15:17–29. [Google Scholar]
- Cavaco S, Feinstein JS, van Twillert H, Tranel D. Musical memory in a patient with severe anterograde amnesia. J Clin Exp Neuropsychol. 2012;34:1089–1100. doi: 10.1080/13803395.2012.728568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MM, Phelps EA. Memory deficits for implicit contextual information in amnesic subjects with hippocampal damage. Nat Neurosci. 1999;2:844–847. doi: 10.1038/12222. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Davachi L, Dobbins IG. Declarative Memory. Curr Dir Psychol Sci. 2008;17:112–118. doi: 10.1111/j.1467-8721.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH, Gaskell MG. A complementary systems account of word learning: neural and behavioural evidence. Philos Trans R Soc Lond B Biol Sci. 2009;364:3773–3800. doi: 10.1098/rstb.2009.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S, Humphreys MS. A context noise model of episodic word recognition. Psychol Rev. 2001;108:452–478. doi: 10.1037/0033-295x.108.2.452. [DOI] [PubMed] [Google Scholar]
- Duff MC, Hengst J, Tranel D, Cohen NJ. Development of shared information in communication despite hippocampal amnesia. Nat Neurosci. 2006;9:140–146. doi: 10.1038/nn1601. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York, NY: Oxford University Press; 2001. [Google Scholar]
- Feinstein JS, Rudrauf D, Khalsa SS, Cassell MD, Bruss J, Grabowski TJ, Tranel D. Bilateral limbic system destruction in man. J Clin Exp Neuropsychol. 2010;32:88–106. doi: 10.1080/13803390903066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M, Friederici AD. Word learning in 6-month-olds: fast encoding-weak retention. J Cogn Neurosci. 2011;23:3228–3240. doi: 10.1162/jocn_a_00002. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, Cohen NJ, Corkin S. The impaired learning of semantic knowledge following bilateral medial temporal-lobe resection. Brain Cogn. 1988;7:157–177. doi: 10.1016/0278-2626(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Gershkoff-Stowe L, Hahn ER. Fast mapping skills in the developing lexicon. J Speech Lang Hear Res. 2007;50:682–697. doi: 10.1044/1092-4388(2007/048). [DOI] [PubMed] [Google Scholar]
- Glisky EL. Acquisition and transfer of declarative and procedural knowledge by memory-impaired patients: A computer data-entry task. Neuropsychologia. 1992;30:899–910. doi: 10.1016/0028-3932(92)90034-j. [DOI] [PubMed] [Google Scholar]
- Halberda J. Is this a dax which I see before me? Use of the logical argument disjunctive syllogism supports word-learning in children and adults. Cognit Psychol. 2006;53:310–344. doi: 10.1016/j.cogpsych.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Althoff RR, Warren DE, Riggs L, Cohen NJ, Ryan JD. Worth a glance: Using eye movements to investigate the cognitive neuroscience of memory. Front Hum Neurosci. 2010;4:166. doi: 10.3389/fnhum.2010.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula DE, Ryan JD, Tranel D, Cohen NJ. Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J Cogn Neurosci. 2007;19:1690–1705. doi: 10.1162/jocn.2007.19.10.1690. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. J Neurosci. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Bird CM, Chan D, Cipolotti L, Husain M, Vargha-Khadem F, Burgess N. The hippocampus is required for short-term topographical memory in humans. Hippocampus. 2007;17:34–48. doi: 10.1002/hipo.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst JS, Samuelson LK. Fast mapping but poor retention by 24-month-old infants. Infancy. 2008;13:128–157. doi: 10.1080/15250000701795598. [DOI] [PubMed] [Google Scholar]
- Jastak S, Wilkinson G. The Wide Range Achievement Test: Manual of instructions. Wilmington, DE: Jastak Associates; 1984. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- Kitchener EG, Hodges JR, McCarthy R. Acquisition of post-morbid vocabulary and semantic facts in the absence of episodic memory. Brain. 1998;121:1313–1327. doi: 10.1093/brain/121.7.1313. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR. The learning of categories: Parallel brain systems for item memory and category knowledge. Science. 1993;262:1747–1749. doi: 10.1126/science.8259522. [DOI] [PubMed] [Google Scholar]
- Konkel A, Warren DE, Duff MC, Tranel DN, Cohen NJ. Hippocampal amnesia impairs all manner of relational memory. Front Hum Neurosci. 2008;2:15. doi: 10.3389/neuro.09.015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucker SC, Samuelson LK. The first slow step: Differential effects of object and word-form familiarization on retention of fast-mapped words. Infancy. 2011;17:295–323. doi: 10.1111/j.1532-7078.2011.00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markson L, Bloom P. Evidence against a dedicated system for word learning in children. Nature. 1997;385:813–815. doi: 10.1038/385813a0. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning-systems in the hippocampus and neocortex - Insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McMurray B, Horst JS, Samuelson LK. Word learning emerges from the interaction of online referent selection and slow associative learning. Psych Rev. 2012;119:831–877. doi: 10.1037/a0029872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B. Disorders of learning and memory after temporal lobe lesions in man. Clin Neurosurg. 1972;19:421–446. doi: 10.1093/neurosurgery/19.cn_suppl_1.421. [DOI] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: A complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O'Kane G, Kensinger EA, Corkin S. Evidence for semantic learning in profound amnesia: an investigation with patient H.M. Hippocampus. 2004;14:417–425. doi: 10.1002/hipo.20005. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complexe. Arch Psychol. 1944;30:206. [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behav Neurosci. 1996;110:1205–1228. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Postle BR, Corkin S. Impaired word-stem completion priming but intact perceptual identification priming with novel words: evidence from the amnesic patient H.M. Neuropsychologia. 1998;36:421–440. doi: 10.1016/s0028-3932(97)00155-3. [DOI] [PubMed] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Reed JM, Squire LR, Patalano AL, Smith EE, Jonides J. Learning about categories that are defined by object-like stimuli despite impaired declarative memory. Behav Neurosci. 1999;113:411–419. doi: 10.1037//0735-7044.113.3.411. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Arch Psychol. 1941;28:286. [Google Scholar]
- Rubin RD, Brown-Schmidt S, Duff MC, Tranel D, Cohen NJ. How do I remember that I know you know that I know? Psychol Sci. 2011;22:1574–1582. doi: 10.1177/0956797611418245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatr. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon T, Moscovitch M, Gilboa A. Rapid neocortical acquisition of long-term arbitrary associations independent of the hippocampus. Proc Natl Acad Sci USA. 2011;108:1146–1151. doi: 10.1073/pnas.1005238108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtyrov Y. Neural Bases of Rapid Word Learning. Neuroscientist. 2012;18:312–319. doi: 10.1177/1073858411420299. [DOI] [PubMed] [Google Scholar]
- Sivan AD. Benton Visual Retention Test. New York, NY: The Psychological Corporation; 1992. [Google Scholar]
- Smith CN, Urgolites ZJ, Hopkins RO, Squire LR. Comparison of explicit and incidental learning strategies in memory-impaired patients. Proc Natl Acad Sci USA. 2014;111:475–479. doi: 10.1073/pnas.1322263111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel C, Halberda J. Rapid fast-mapping abilities in 2-year-olds. J Exp Child Psychol. 2011;109:132–140. doi: 10.1016/j.jecp.2010.10.013. [DOI] [PubMed] [Google Scholar]
- The Psychological Corporation. WAIS-III-WMS-III Technical Manual. San Antonio, TX: Harcourt & Brace; 1997. [Google Scholar]
- Tranel D, Damasio AR. The covert learning of affective valence does not require structures in hippocampal system or amygdala. J Cogn Neurosci. 1993;5:79–88. doi: 10.1162/jocn.1993.5.1.79. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- Vlach HA, Sandhofer CM. Fast mapping across time: Memory processes support children's retention of learned words. Front Psychol. 2012;3:46. doi: 10.3389/fpsyg.2012.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Koss Torkildsen J, Svangstu JM, Hansen HF, Smith L, Simonsen HG, Moen I, Lindgren M. Productive vocabulary size predicts event-related potential correlates of fast mapping in 20-month-olds. J Cogn Neurosci. 2008;20:1266–1282. doi: 10.1162/jocn.2008.20087. [DOI] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc Natl Acad Sci USA. 2011;108:E402–E409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC. Proceedings of 2012 CNS Meeting. Cognitive Neuroscience Society; 2012. Associative recognition without hippocampus: Fast mapping can create novel, inflexible mnemonic representations. [Google Scholar]
- Warren DE, Duff MC, Jensen U, Tranel D, Cohen NJ. Hiding in plain view: lesions of the medial temporal lobe impair online representation. Hippocampus. 2012a;22:1577–1588. doi: 10.1002/hipo.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Magnotta V, Capizzano AA, Cassell MD, Tranel D. Long-term neuropsychological, neuroanatomical, and life outcome in hippocampal amnesia. Clin Neuropsychol. 2012b;26:335–369. doi: 10.1080/13854046.2012.655781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Duff MC, Tranel D, Cohen NJ. Observing degradation of visual representations over short intervals when medialtemporal lobe is damaged. J Cogn Neurosci. 2011;23:3873. doi: 10.1162/jocn_a_00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- Zaki SR. Is categorization performance really intact in amnesia? A meta-analysis. Psychon Bull Rev. 2004;11:1048–1054. doi: 10.3758/bf03196735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


