Abstract
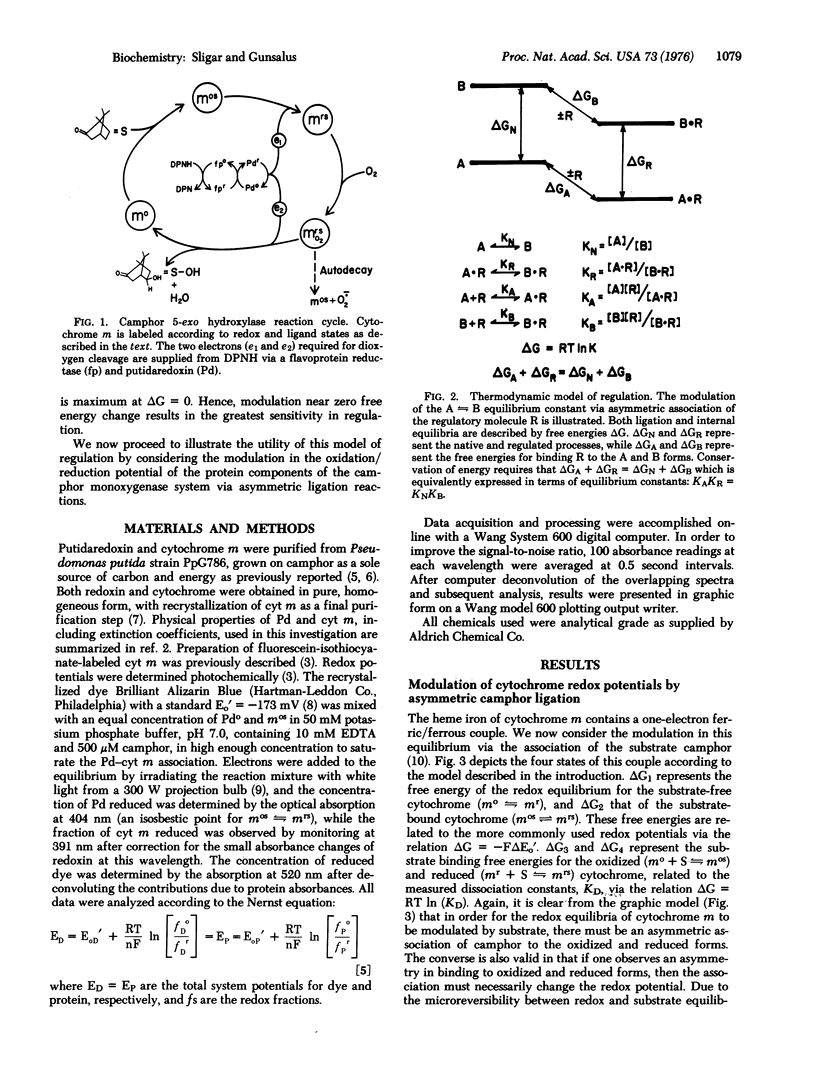
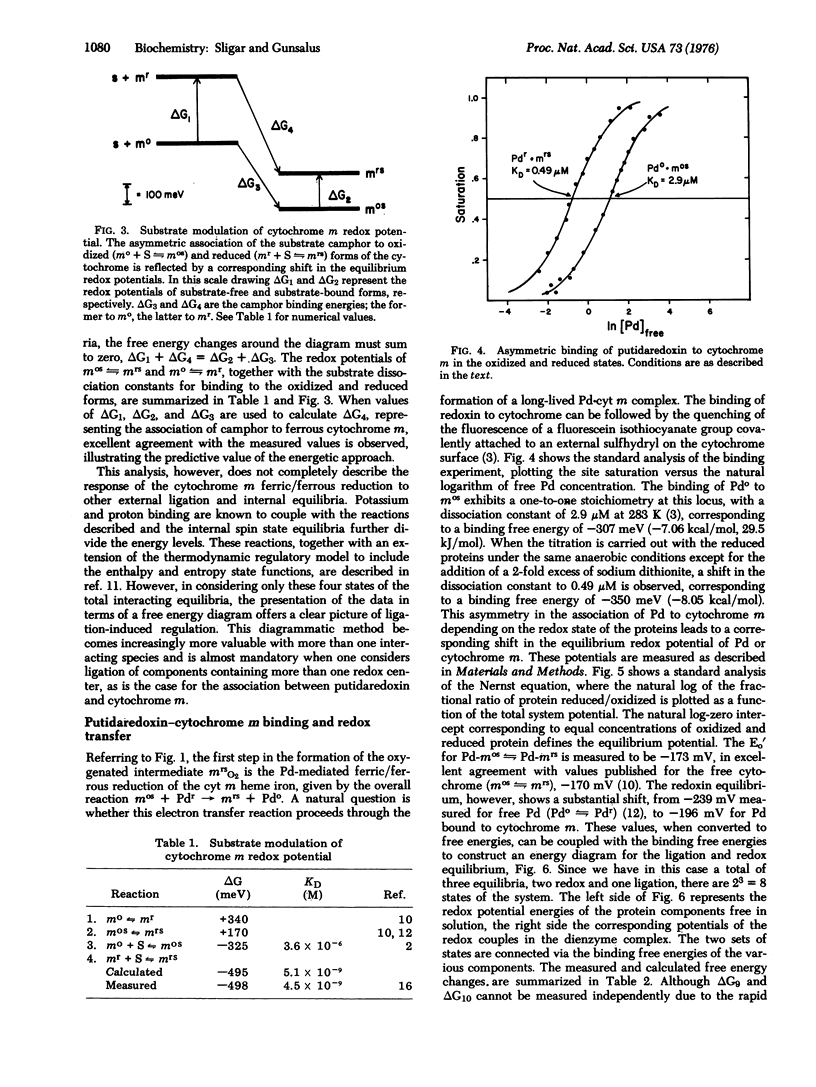
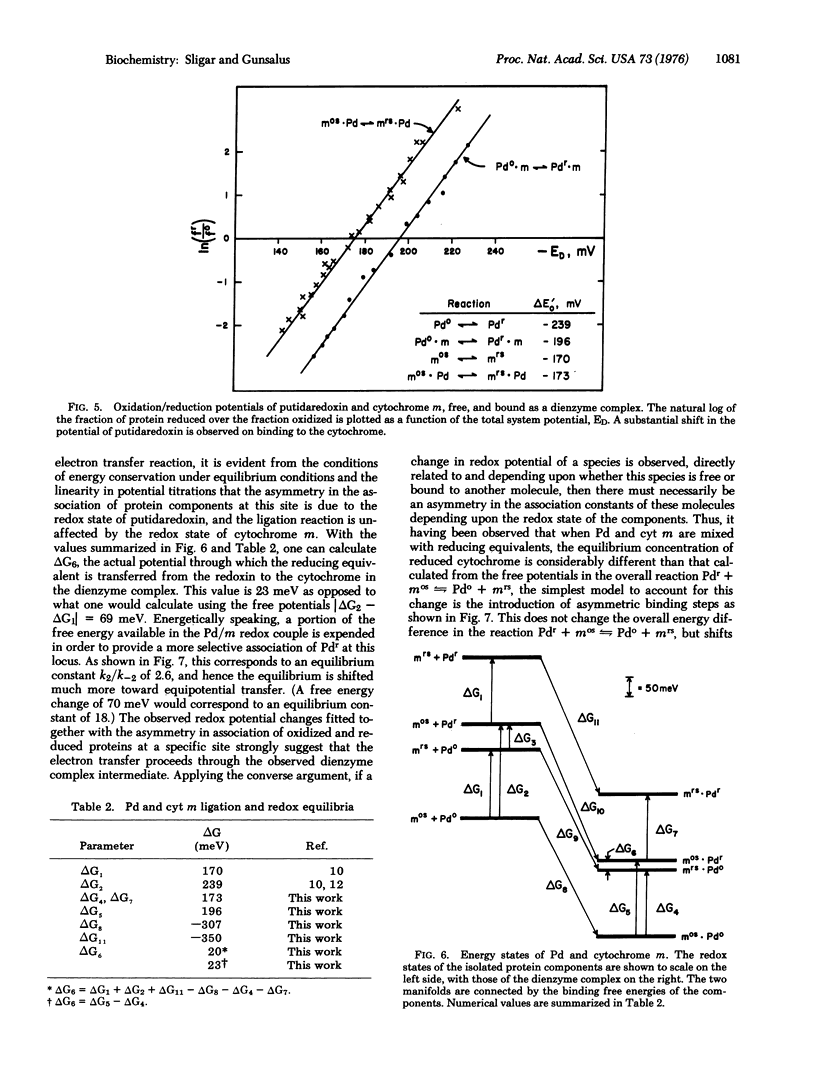
Regulation of biological phenomena occurs in all types of systems, being manifested in many different reaction types, from allosteric behavior in proteins, through modulation in energy and information transfer, to the control of growth and differentiation in cells, organelles, and organisms. In this communication, a modulation in oxidation/reduction potential via ligation of substrate and protein components in the camphor 5-exo-monoxygenase system is described in terms of a four-state system using as fundamental parameters the transition free energies between equilibrium states. This approach provides a concise description of the data and is useful for describing many aspects of regulatory phenomena.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Greenbaum E., Austin R. H., Frauenfelder H., Gunsalus I. C. Photoreduction of NADP + sensitized by synthetic pigment systems. Proc Natl Acad Sci U S A. 1972 May;69(5):1273–1276. doi: 10.1073/pnas.69.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. W., Peterson J. A. Camphor binding by Pseudomonas putida cytochrome P-450. Kinetics and thermodynamics of the reaction. Biochemistry. 1972 Dec 5;11(25):4740–4746. doi: 10.1021/bi00775a017. [DOI] [PubMed] [Google Scholar]
- Gunsalus I. C., Pederson T. C., Sligar S. G. Oxygenase-catalyzed biological hydroxylations. Annu Rev Biochem. 1975;44:377–407. doi: 10.1146/annurev.bi.44.070175.002113. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Anglin J. R., Bobrik M. A., Davison A., Holm R. H. Synthetic analogs of the active sites of iron-sulfur proteins. IX. Formation and some electronic and reactivity properties of Fe4S4 Glycyl-L-cysteinylglycyl oligopeptide complexes obtained by ligand substitution reactions. J Am Chem Soc. 1974 Sep 18;96(19):6042–6048. doi: 10.1021/ja00826a014. [DOI] [PubMed] [Google Scholar]
- Sligar S. G., Debrunner P. G., Lipscomb J. D., Namtvedt M. J., Gunsalus I. C. A role of the putidaredoxin COOH-terminus in P-450cam (cytochrome m) hydroxylations. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3906–3910. doi: 10.1073/pnas.71.10.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G. Energetics of ligand binding to proteins. Adv Protein Chem. 1975;29:1–83. doi: 10.1016/s0065-3233(08)60410-6. [DOI] [PubMed] [Google Scholar]
- Weber G. Ligand binding and internal equilibria in proteins. Biochemistry. 1972 Feb 29;11(5):864–878. doi: 10.1021/bi00755a028. [DOI] [PubMed] [Google Scholar]
- Wilson G. S., Tsibris J. C., Gunsalus I. C. Electrochemical studies of putidaredoxin and its selenium analog. J Biol Chem. 1973 Sep 10;248(17):6059–6061. [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C. Crystalline cytochrome P-450cam. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1431–1436. doi: 10.1016/0006-291x(70)90027-6. [DOI] [PubMed] [Google Scholar]
- Yu C., Gunsalus I. C., Katagiri M., Suhara K., Takemori S. Cytochrome P-450cam. I. Crystallization and properties. J Biol Chem. 1974 Jan 10;249(1):94–101. [PubMed] [Google Scholar]


