Abstract
Drugs of abuse modulate the function and activity of the mesolimbic dopamine circuit. To identify novel mediators of drug-induced neuroadaptations in the ventral tegmental area (VTA), we performed RNA sequencing analysis on VTA samples from mice administered repeated saline, morphine, or cocaine injections. One gene that was similarly upregulated by both drugs was serum- and glucocorticoid-inducible kinase 1 (SGK1). SGK1 activity, as measured by phosphorylation of its substrate N-myc downstream-regulated gene (NDRG), was also increased robustly by chronic drug treatment. Increased NDRG phosphorylation was evident 1 but not 24 hours after the last drug injection. SGK1 phosphorylation itself was similarly modulated. To determine the role of increased SGK1 activity on drug-related behaviors, we overexpressed constitutively-active (CA) SGK1 in the VTA. SGK1-CA expression reduced locomotor sensitization elicited by repeated cocaine, but surprisingly had the opposite effect and promoted locomotor sensitization to morphine, without affecting the initial locomotor responses to either drug. SGK1-CA expression did not significantly affect morphine or cocaine conditioned place preference (CPP), although there was a trend towards increased CPP with both drugs. Further characterizing the role of this kinase in drug-induced changes in VTA may lead to improved understanding of neuroadaptations critical to drug dependence and addiction.
Keywords: dopamine, ventral tegmental area, morphine, cocaine, locomotor activity
Introduction
The mesocorticolimbic circuit plays a critical role in drug dependence and addiction. In particular, activity of the dopamine (DA) neurons in the ventral tegmental area (VTA) mediates the rewarding action of addictive drugs, in part through increased DA signaling in the nucleus accumbens (NAc) (Di Chiara & Imperato 1988). Opiate drugs such as morphine acutely activate VTA DA neurons in two ways: by disinhibition through hyperpolarization of local GABA interneurons that synapse onto VTA DA neurons (Johnson & North 1992); and through synaptic adaptation by decreasing long-term potentiation of GABAergic synapses (Niehaus et al. 2010) and increasing the strength of excitatory synapses (Saal et al., 2003) on VTA DA neurons. In contrast, stimulant drugs such as cocaine act primarily at the terminals of VTA DA neurons, where they block DA reuptake by the presynaptic dopamine transporter, thereby increasing DA levels and signaling in the NAc (Ritz et al. 1987). Cocaine also potentiates excitatory input to VTA DA neurons (Saal et al. 2003, Ungless et al. 2001). More recent work has established that these are long-lasting synaptic adaptations in the VTA, with enhancement evident even after 3 months of abstinence (Chen et al. 2008). Despite the prominent role of the VTA in drug action and in neuroadaptations underlying addiction, the signaling changes induced by drugs of abuse in the VTA, and their role in mediating behavioral changes, are not well defined.
We and others have highlighted changes in neurotrophic signaling in the VTA induced by morphine, including decreased AKT (Russo et al. 2007) and mTORC2 (Mazei-Robison et al. 2011) activity, and increased PLCgamma (Wolf et al. 1999, Wolf et al. 2007) and ERK (Berhow et al. 1996) activity. The effect of stimulants on neurotrophic signaling in the VTA has not been as thoroughly investigated, with most studies focusing on the NAc and striatum (Brami-Cherrier et al. 2002, McGinty et al. 2008, Shi & McGinty 2007, Perrine et al. 2008), although cocaine has been found to elicit an increase in VTA ERK activity similar to that induced by morphine (Berhow et al. 1996, Pan et al. 2011). Surprisingly, no genome-wide screen has compared the pattern of gene expression induced in the VTA by cocaine to that induced by morphine. While there is one published study that examined gene expression changes in the VTA induced by chronic morphine and withdrawal (McClung et al. 2005), no studies to date have completed a similar screen with chronic cocaine administration. Thus, we used RNA sequencing analysis to identify novel genes that may mediate both morphine and cocaine-induced neuroadaptations in the VTA. From this screen, we chose to focus on serum- and glucocorticoid-regulated kinase 1 (SGK1), one of the few genes upregulated by both drugs in the VTA.
SGK1 was initially identified as an immediate early gene induced by glucocorticoid and serum stimulation (Webster et al. 1993a, Webster et al. 1993b), and by cell shrinkage of cultured hepatoma and renal epithelial cells (Waldegger et al. 1997). SGK1 is a member of the AGC protein kinase family, which includes AKT and p70S6K. Similar to AKT activity, SGK1 kinase activity is activated by growth factors and insulin through phosphorylation at S422 by mTORC2 and at T256 by PDK1 (Park et al., 1999; Garcia-Martinez and Alessi, 2008). Phosphorylation at these two sites is known to increase SGK1 catalytic activity and increase phosphorylation of its substrates such as such as N-myc down-regulated gene (NDRG) (Kobayashi & Cohen 1999, Garcia-Martinez & Alessi 2008). A third site of phosphorylation, S78, has also been identified. Phosphorylation at this site is increased by EGF stimulation, BMK1/ERK5 activation, and MAPK/ERK (Hayashi et al. 2001, Lee et al. 2006). SGK1 plays an important role in ion balance, particularly in the renal system where one of its main targets is the epithelial Na+ channel (Arteaga & Canessa 2005). Similar regulation has recently been shown with the brain specific SGK1 isoform SGK1.1, and SGK1 has been implicated in learning and memory mediated via its actions in the hippocampus (Lee et al. 2003, Tsai et al. 2002, Lee et al. 2007). Here, we explore a role for SGK1 activity in the VTA in response to drug treatment. The objective of this study is to investigate the regulation of SGK1 expression and activity by cocaine and morphine and its potential influence on drug-elicited behaviors.
Materials and Methods
Animals
All experiments were approved by the Institutional Animal Use and Care committees at Mount Sinai Medical Center and Michigan State University and adhered to the strict guidelines set in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Adult, male c57Bl/6J mice (8–10 weeks, Jackson labs) were used in all experiments. Mice were allowed to acclimate to the animal facility for at least 7 days before testing or drug treatment. Mice were group-housed in a temperature-controlled vivarium on a 12-hour light/dark cycle with food and water available ad libitum.
Experiment 1: RNA sequencing (Fig. 1A)
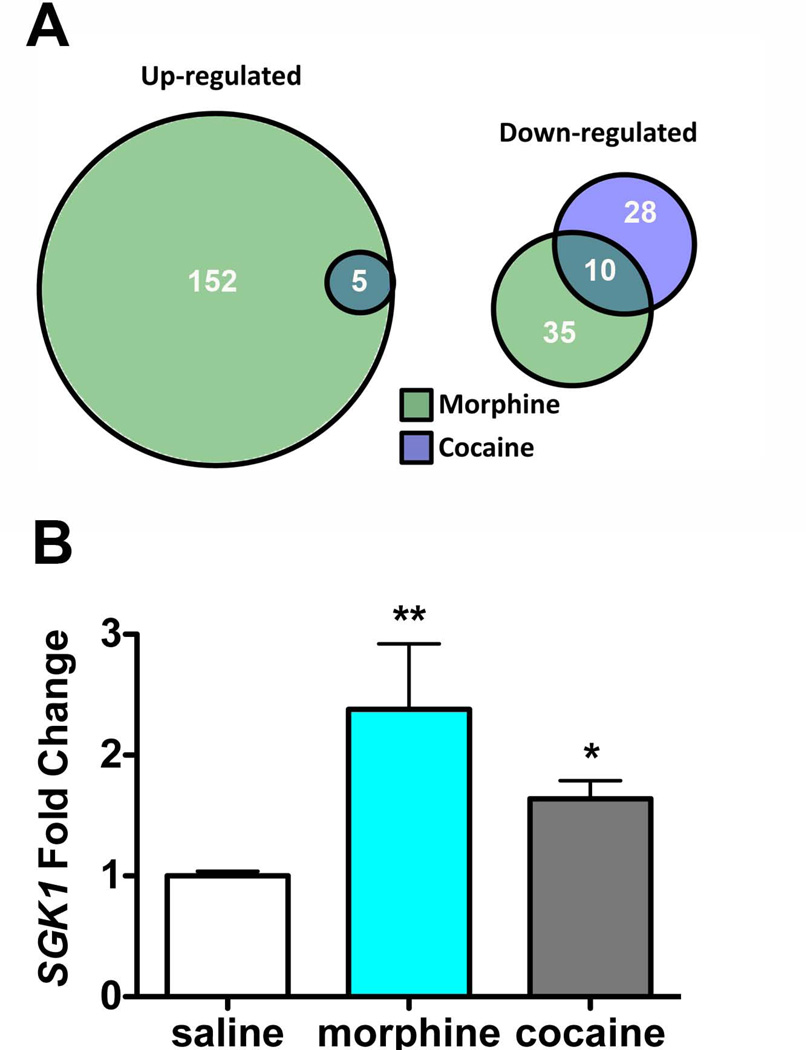
Figure 1. Comparison of gene expression changes induced by cocaine or morphine in the VTA shows upregulation of SGK1 mRNA by both drugs.
A. Mice were injected daily with saline, morphine, or cocaine (15 mg/kg) for 7 days. VTA was dissected and samples were pooled and processed for RNA sequencing. As illustrated by the Venn diagrams, more genes were regulated by morphine (187, green) than cocaine (33, blue). More genes were up-regulated by morphine treatment (152), while cocaine treatment primarily down-regulated genes in the VTA (28). B. Mice were injected daily with saline, morphine, or cocaine (15 mg/kg) for 7 days, RNA was isolated from VTA and RT-PCR was performed using primers specific for SGK1. Morphine and cocaine increased SGK1 gene expression compared to saline. Data are expressed as mean ± sem. n=4–8 mice/group, One-way ANOVA, Tukey’s multiple comparison test, *p<0.05 or **p<0.01 compared to saline control.
Mice (n=90) were given daily intraperitoneal (i.p.) injections of morphine sulfate (Sigma, 15 mg/kg), cocaine hydrochloride (Sigma, 15 mg/kg), or vehicle (sterile saline) for 7 days and were analyzed 24 hours after the last injection. RNA sequencing and analysis was completed as described in Warren et al. (2013). Briefly, total RNA was isolated from VTA punches (14 g, 1.25 mm diameter) via homogenization in Trizol and processed according to the manufacturer’s protocol (10 mice pooled/sample). RNA was then purified using RNAesy micro columns (Qiagen) and quality was assessed by spectroscopy. 4 µg was used for mRNA library construction using the Illumina mRNA sample prep kit. Verification of cDNA size and concentration of the cDNA libraries was completed on a bioanalyzer (Agilent) and high-depth sequencing (on triplicate independently generated samples/conditions) was performed on an Illumina HiSeq200 machine (Mount Sinai Genome Core Facility). The read counts per gene were summarized with custom Perl scripts. DESeq was then used to identify differentially expressed genes using default parameter values (Anders & Huber 2010). Genes that had a p adjusted value <0.05 for morphine or cocaine compared to saline are listed in Supplemental Table 1.
Experiment 2: Validation of SGK1 gene expression change by RT-PCR (Fig. 1B)
Mice (n=20) were given daily i.p. injections of morphine (15 mg/kg), cocaine (15 mg/kg), or vehicle (saline) for 7 days and were analyzed 24 hours after the last injection. Punches (14 g) from mouse VTA were homogenized in Trizol (samples were not pooled) and processed according to the manufacturer’s protocol. RNA was purified using RNAesy micro columns (Qiagen) and quality was assessed by spectroscopy. RNA was then reverse transcribed (iScript, BioRad) and quantified by semi-quantitative RT-PCR using SYBR green. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was utilized as a normalization control and all samples were run in triplicate and analyzed using the ΔΔCt method as described previously (Tsankova et al. 2006).
Experiment 3: Determination of SGK1 protein changes following 7-day injection protocol (Fig. 2)
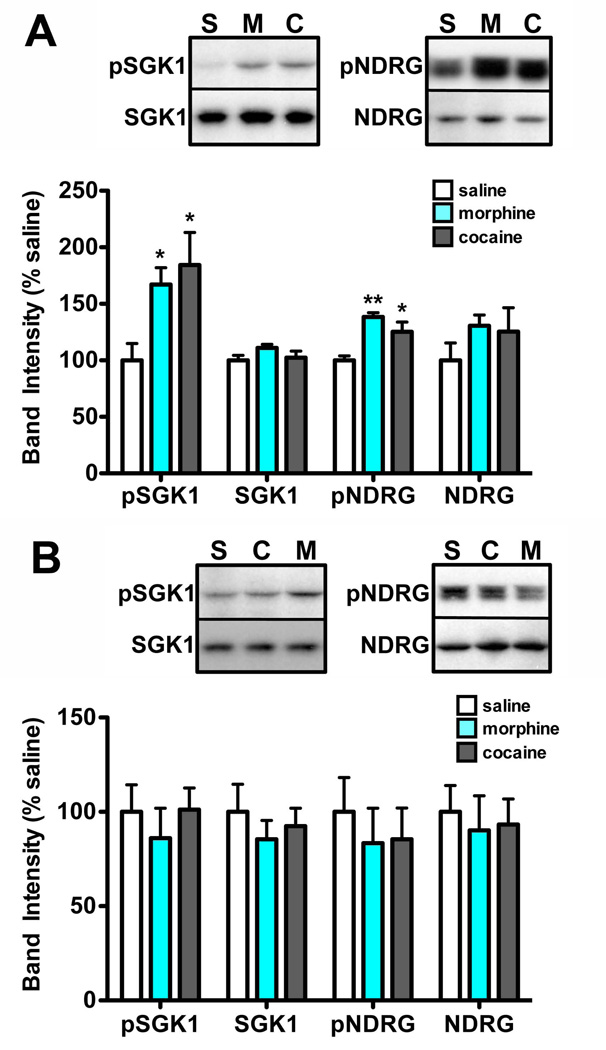
Figure 2. SGK1 phosphorylation and activity in the VTA are increased 1 hour, but not 24 hours, after repeated morphine or cocaine injections.
Mice were injected daily with saline, morphine, or cocaine (15 mg/kg) for 7 days. VTA was dissected 1 hour or 24 hours after the last injection, and tissue was processed for western blot analysis. A. Morphine and cocaine administration increase SGK1 phosphorylation at S78 and phosphorylation of the SGK1 substrate NDRG. Data are expressed as mean ± sem. n=8 mice/group, One-way ANOVA, Tukey’s multiple comparison test, *p<0.05 or **p<0.01 compared to saline control. B. No significant differences in protein expression were observed 24 hours after the last drug injection. Data are expressed as mean ± sem. n=8 mice/group.
Mice (n=48) were given daily i.p. injections of morphine (15 mg/kg), cocaine (15 mg/kg), or vehicle (saline) for 7 days and were analyzed 1 hour (Fig. 2A) or 24 hours (Fig. 2B) after the last injection. Western blot analysis was completed as described in Mazei-Robison et al. (2011). Briefly, brains were removed, sectioned at a 1 mm thickness by brain matrix, and VTA punches (14 g) were collected from each mouse and stored at −80°C. Tissue was sonicated in RIPA buffer, centrifuged 20 minutes at 14,000 rpm, and supernatants were removed and protein concentration was determined by Lowry assay. Samples (5–25 µg protein, samples were not pooled) were then electrophoresed on 4–15% precast SDS gradient gels, transferred to PVDF membranes, and blocked with 5% milk-Tris-buffered saline, 0.1%-Tween-20 (TBST) at 1 hour at 25°C. Blots were incubated in primary antibodies overnight at 4°C, washed with TBST and incubated with secondary antibody conjugated to horseradish peroxidase for 1 hour. After washing, bands were visualized using enhanced chemiluminescence. All data presented are normalized to the loading control, GAPDH. Primary antibodies were purchased from Millipore: SGK1 (07–315) and Cell Signaling Technology: phospho-SGK1 (5599), phospho-NDRG (3217), NDRG (5196), GAPDH (2118).
Experiment 4: Determination of SGK1 protein changes following 1-day injection protocol (Fig. 3)
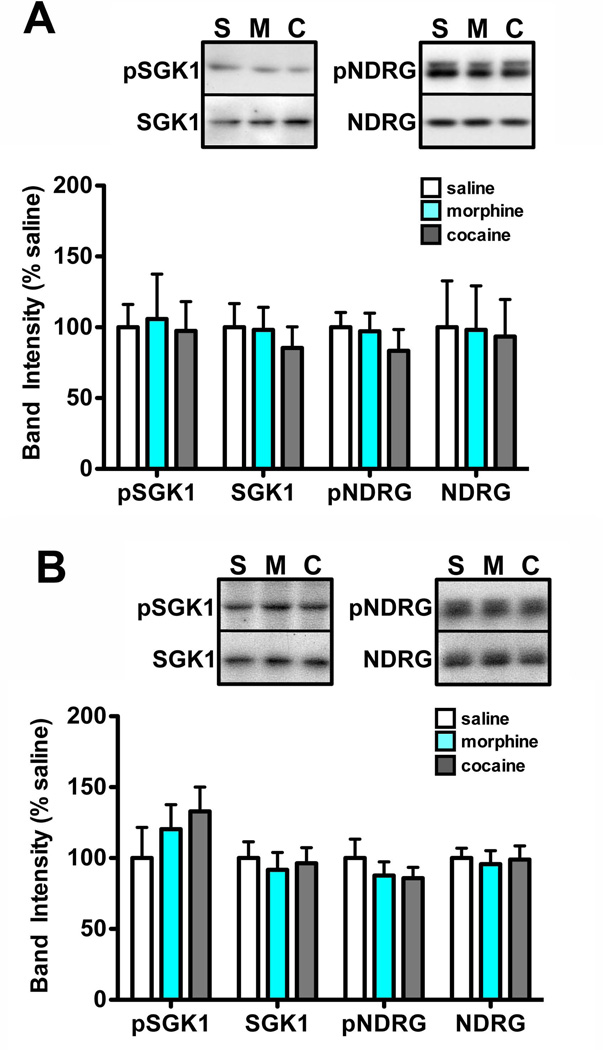
Figure 3. SGK1 phosphorylation and activity in VTA are not altered following a single drug exposure.
Mice were injected once with saline, morphine, or cocaine (15 mg/kg). VTA was dissected 1 hour or 24 hours after the injection and tissue was processed for western blot analysis. A. Morphine and cocaine administration did not alter SGK1 or NDRG1 phosphorylation 1 hour after an acute injection. Data are expressed as mean ± sem. n=8 mice/group. B. No significant differences in protein expression or phosphorylation were observed 24 hours a single drug injection. Data are expressed as mean ± sem. n=8 mice/group.
Mice (n=48) were given a single i.p. injection of morphine (15 mg/kg), cocaine (15 mg/kg), or vehicle (saline) and were analyzed 1 hour (Fig. 3A) or 24 hours (Fig. 3B) after the last injection. Western blot analysis was completed as described in Experiment 3.
Experiment 5: Determination of SGK1 protein changes following morphine pellet administration (Fig. 4A)
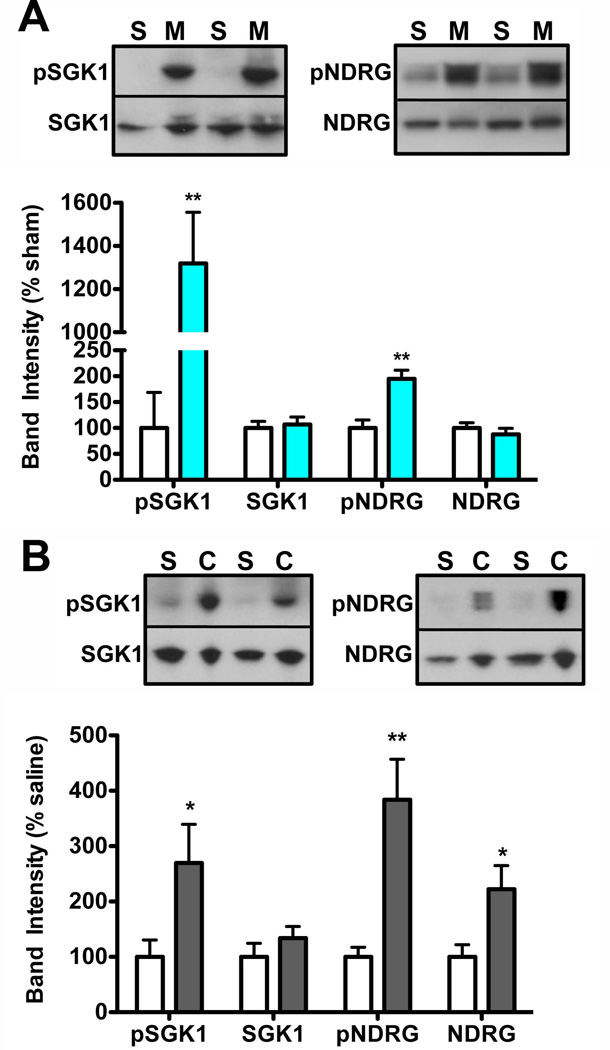
Figure 4. SGK1 activity and phosphorylation in VTA are increased by morphine pellet and cocaine binge paradigms.
A. Mice were implanted (sc) with 25 mg morphine pellets on day 1 and 3 and were analyzed on day 5. VTA was dissected and tissue was processed for western blot analysis. Chronic morphine administration increased phospho-SGK1 and phospho-NDRG in the mouse VTA. Student’s t-test, *p<0.05, **p<0.01, n=14–19 mice/group. B. Mice were injected with cocaine in a “binge” protocol (5 × 20mg/kg over 3 days), VTA was dissected 4 hours after the last injection, and tissue was processed for western blot analysis. Binge cocaine administration increased phospho-SGK1, phospho-NDRG, and total NDRG in the mouse VTA. Student’s t-test, *p<0.05, **p<0.01, n=9–10 mice/group.
Mice (n=37) were lightly anesthetized with isofluorane and implanted subcutaneously (s.c.) with 2–25 mg pellets (NIDA drug supply program) 48 hours apart, then analyzed 48 hours following the last pellet implantation, per published reports (Mazei-Robison et al. 2011, Fischer et al. 2008). Western blot analysis was completed as described in Experiment 3.
Experiment 6. Determination of SGK1 protein changes following “binge” cocaine administration (Fig. 4B)
Mice (n=20) were given 5 i.p. injections of cocaine (20 mg/kg) over 3 days in a “binge” treatment or vehicle (saline) as described previously (Russo et al., 2009). Mice were analyzed 4 hours after the last injection and western blot analysis was completed as described in Experiment 3.
Experiment 7. Validation of Herpes Simplex Virus (HSV)-SGK1 constructs (Fig. 5)
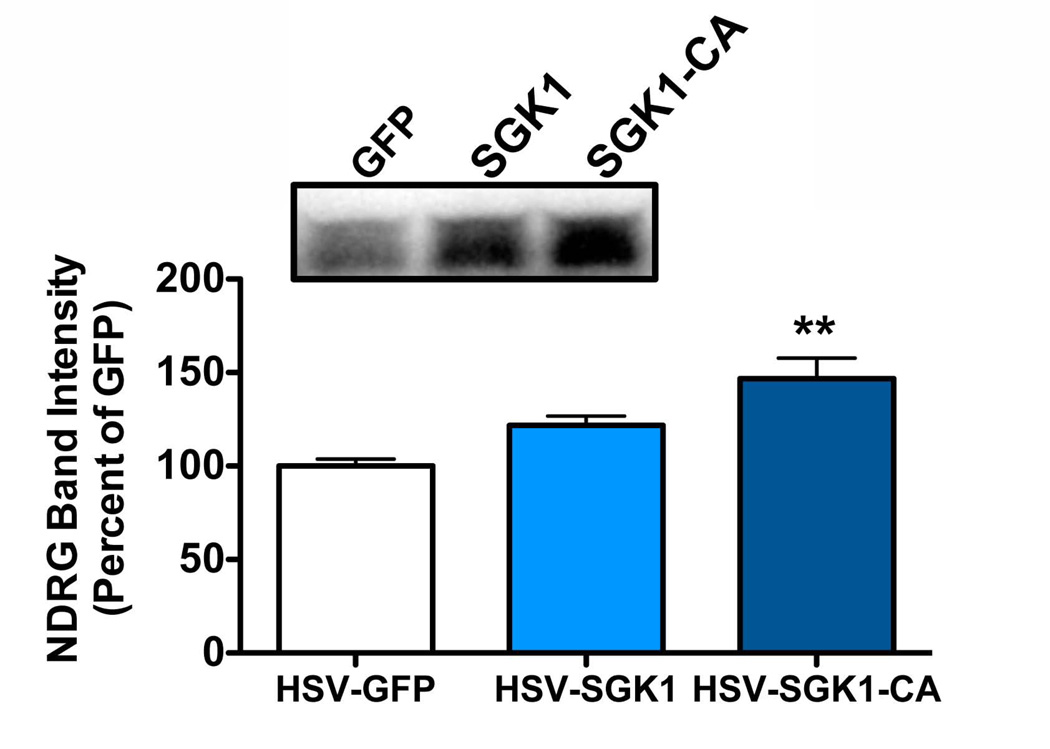
Figure 5. HSV-mediated overexpression of SGK1-CA increases phosphorylation of NDRG in the VTA.
Wild-type SGK1 cDNA or SGK1 cDNA containing the S422D mutation (SGK1-CA) was subcloned into an HSV vector. HSV-GFP, HSV-SGK1, or HSV-SGK1-S422D was bilaterally infused into the VTA. VTA was dissected 3 days later, when transgene expression is maximal, and processed for western blot analysis. Overexpression of SGK1-CA, but not wild-type SGK1, increased phosphorylation of NDRG. One-way ANOVA, F (2,8)=12.70, p<0.01, Tukey’s multiple comparison test, **p<0.01 compared to GFP control.
The HSV vector encoding GFP has been previously used and validated (Mazei-Robison et al. 2011). SGK1 cDNA (wild-type and the S422D mutant) was provided by Dr. Michael Greenberg (Harvard) and was cloned into the p1005 HSV vector using KpnI (3’) and BamHI (5’). Correct p1005 insertion was verified by dideoxysequencing.
For stereotaxic surgeries, mice (n=11) were anaesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) and VTA was targeted using established coordinates (-3.2 mm A/P, +1.0 mm M/L, −4.6 mm D/V). Infusions (0.5 µL) were bilateral via 33 g Hamilton syringe at a rate of ∼0.1 µL/min and placements were verified by standard histological methods. Mice were analyzed 3 days following surgery and tissue was processed for western blot analysis as described in Experiment 3.
Experiment 8. Determination of the effect of VTA SGK1 overexpression on cocaine locomotor behavior (Fig. 6A)
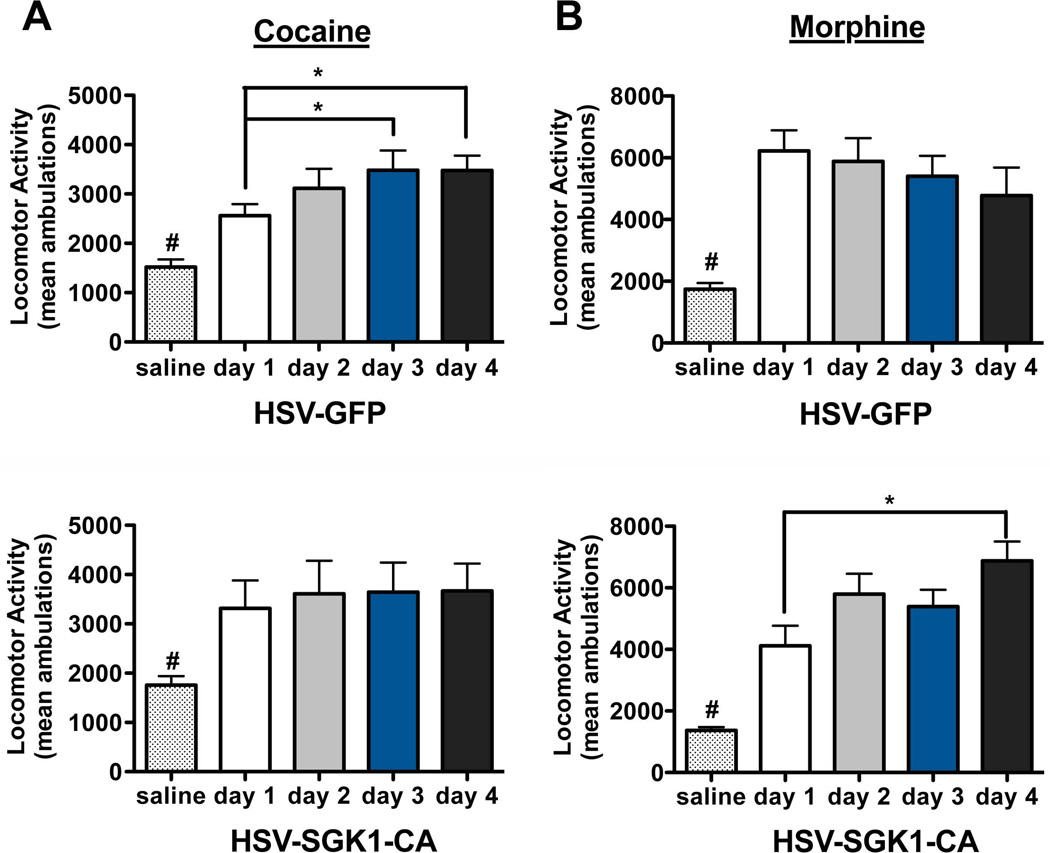
Figure 6. Overexpression of SGK1-CA in VTA alters locomotor sensitization to cocaine and morphine.
A. While cocaine (7.5 mg/kg) increases locomotor activity in HSV-GFP and HSV-SGK1-CA mice, only HSV-GFP mice exhibit cocaine sensitization. Repeated measures ANOVA, Tukey’s multiple comparison test, *p<0.05 compared to cocaine day 1, #p<0.05 compared to all cocaine treatment days, n=8,10 mice/group. B. Morphine (10 mg/kg) increases locomotor activity in both groups, but only HSV-SGK1-CA mice exhibit morphine sensitization. Repeated measures ANOVA, Tukey’s multiple comparison test, *p<0.01 compared to morphine day 1, #p<0.05 compared to all morphine treatment days, n=8 mice/group.
Mice (n=18) underwent stereotaxic surgery as described in Experiment 7 and were allowed to recover for 24 hours before starting behavioral experiments. Locomotor behavior was measured per published protocols (Kelz et al. 1999) with minor modifications. Activity was assessed in the x and y planes for horizontal ambulations in 75 cm2 chambers using Ethovision XT (Noldus). On each day of testing, mice were injected i.p. with saline and analyzed for 20 minutes, then immediately injected with cocaine (7.5 mg/kg) and analyzed for 45 min. A lower dose of cocaine was used in behavioral studies as the mice would have likely exhibited a ceiling effect with 15 mg/kg dose, and we would not be able to detect any potential increase in behavior induced by the overexpression of SGK1-CA. Data are presented as total ambulations in the x+y axes.
Experiment 9. Determination of the effect of VTA SGK1 overexpression on morphine locomotor behavior (Fig. 6B)
Mice (n=16) underwent stereotaxic surgery as described in Experiment 7 and were allowed to recover for 24 hours before starting behavioral experiments. Locomotor behavior was measured as described in Experiment 8. On each day of testing, mice were injected i.p. with saline and analyzed for 20 minutes, then immediately injected with morphine (10 mg/kg) and analyzed for 45 min. As explained in Experiment 8, a lower dose of morphine was used in behavioral studies in order to detect potential increases in locomotor behavior. Data are presented as total ambulations in the x+y axes.
Experiment 10. Determination of the effect of VTA SGK1 overexpression on cocaine conditioned place preference (CPP) (Fig. 7A)
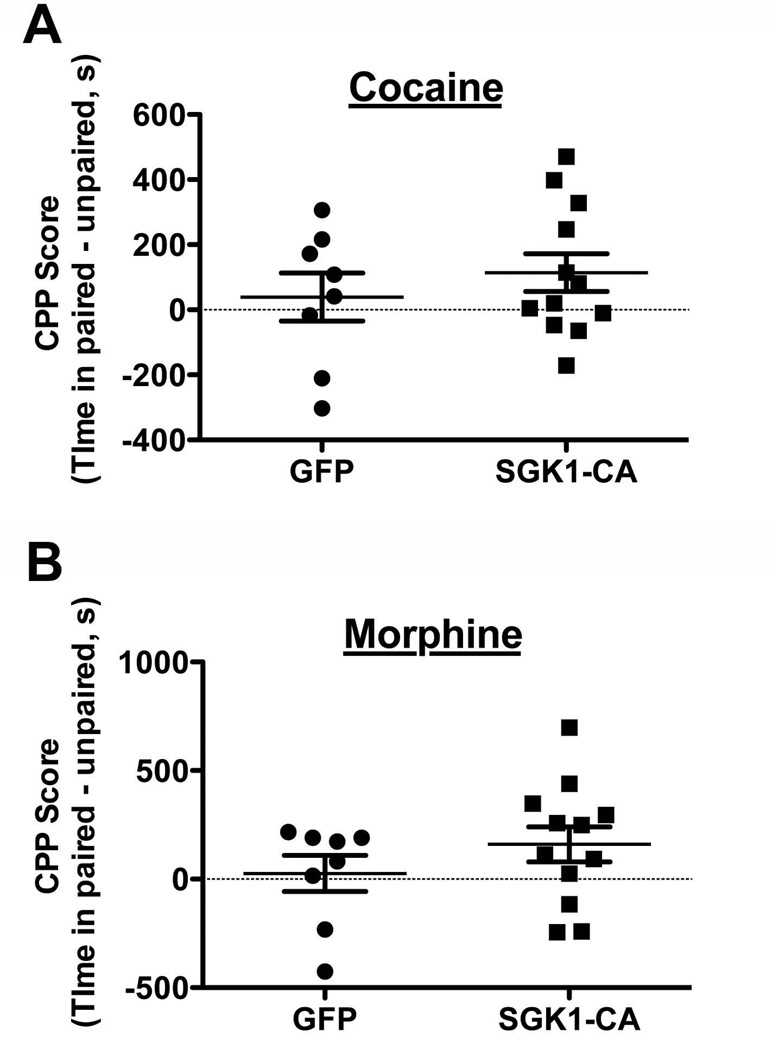
Figure 7. Overexpression of SGK1-CA in VTA does not significantly affect cocaine or morphine reward.
HSV-GFP or HSV-SGK1-CA was bilaterally infused into the VTA and CPP training was completed (6 mg/kg cocaine, 10 mg/kg morphine). A. There was no significant difference in cocaine CPP between groups, n=8,12 mice/group. B. There was also no significant difference observed in morphine CPP. n=8,12 mice/group.
Mice (n=20) underwent stereotaxic surgery as described in Experiment 7 and were allowed to recover for 24 hours before starting behavioral experiments. CPP was completed as described previously (Kelz et al. 1999). Briefly, mice were placed in a three-chambered CPP box for 20 minutes to assess pre-test preferences and ensure that there was no chamber bias. For the next two days, mice were restrained to one chamber for 30 minutes in both the morning (saline) and the afternoon (6 mg/kg cocaine). On test day, mice were placed in the center chamber and allowed to move throughout for 20 minutes. Data are represented as time spent in the paired – time spent in the unpaired chamber.
Experiment 11. Determination of the effect of VTA SGK1 overexpression on morphine conditioned place preference (CPP) (Fig. 7B)
CPP was completed as described previously as in Experiment 10. The slight modifications include three pairing days and mice (n=20) were restrained to one chamber for 45 minutes in both the morning (saline) and the afternoon (10 mg/kg morphine).
Statistics
All values reported are mean ± SEM. Unpaired Student t-tests were used for the analysis of studies with two experimental groups. One-way ANOVA was used for analysis of three or more groups, followed by a Tukey’s multiple comparison test, when appropriate. Main effects were considered significant at p<0.05. For the locomotor sensitization data, repeated measures one-way ANOVAs were completed, followed by Tukey’s post-test, if appropriate.
Results
To identify novel genes regulated by morphine and cocaine in the VTA, we completed RNA sequencing analysis on VTA samples from mice treated for 7 days with either saline or drug. We found that more genes were regulated in the VTA by morphine treatment compared to cocaine treatment 24 hour after the last injection (Fig. 1A; morphine=187, cocaine=33; Supp. Table 1). The pattern of regulation also varied between the two drugs as morphine predominately up-regulated genes (152-up, 35-down) while cocaine treatment generally decreased gene expression (28-down, 5-up) in the VTA. We chose to focus our analysis on genes that were similarly regulated by morphine and cocaine, and were particularly interested in SGK1, as it was one of the few genes up-regulated by cocaine administration. We first verified that SGK1 expression was significantly increased in the VTA by chronic morphine and cocaine administration using quantitative RT-PCR analysis in a separate cohort of mice. We observed a significant induction in SGK1 mRNA expression in the VTA of morphine- and cocaine-treated mice compared to saline controls (Fig. 1B; one-way ANOVA, F (2,17)=9.12, p<0.01, post-hoc Tukey’s test).
Given the induction in SGK1 gene expression in the VTA by morphine and cocaine, we next sought to determine whether SGK1 activity was also increased. The most well-established substrate of SGK1 is NDRG, which is phosphorylated by SGK1 at multiple sites (Murray et al. 2004). One hour after the last injection of morphine or cocaine, we found a significant increase in phosphorylation of NDRG compared to saline-injected controls (Fig. 2A; one-way ANOVA, F (2,21)=11.32, p<0.001, post-hoc Tukey’s multiple comparison test), consistent with increased SGK1 activity. We also observed a robust increase in SGK1 phosphorylation at S78 (Fig. 2A, one-way ANOVA, F (2,21)=4.65, p<0.05, Tukey’s multiple comparison test), and it has been suggested that this site may increase SGK1 catalytic activity (Hayashi et al. 2001) potentially explaining the increase in NDRG phosphorylation. This increase in phospho-SGK1 was not due to an increase in total SGK1 protein (Fig. 2A, one-way ANOVA, F (2,21)=1.611, p=0.22). In contrast to the results at the 1-hour time-point, we do not observe any changes in phospho- or total NDRG or SGK1 levels 24 hours after the last injection (Fig. 2B), which is the time-point at which we observe increased SGK1 mRNA expression (Fig. 1B). Such a transient change in activity suggests that regulation of SGK1 phosphorylation may be mediated independently of its expression and perhaps only when drug is on board.
Given SGK1’s initial identification as an immediate early gene (Webster et al. 1993b, Webster et al. 1993a), we next wanted to determine whether a single drug exposure was sufficient for the induction of SGK1 activity. We found no differences in phospho- or total NDRG or SGK1 either 1 hour (Fig. 3A) or 24 hours (Fig. 3B) after a single injection of morphine or cocaine. These data support the conclusion that chronic, but not acute, drug administration increases SGK1 activity in the VTA.
While rodent studies typically utilize a once daily injection paradigm for biochemical studies, we have also utilized alternative chronic treatment paradigms in our study of drug-induced neuroadaptations. Specifically, we have utilized a subcutaneous pellet method in both rats (Russo et al. 2007, Sklair-Tavron et al. 1996) and mice (Mazei-Robison et al. 2011) that provides a more stable, continuous blood level of morphine, and leads to well-characterized biochemical changes in the VTA. Using the pellet paradigm, we observe highly significant increases in both phospho-NDRG (unpaired Student’s t-test, t(35)=4.15, p<0.001) and phospho-SGK1 (unpaired Student’s t-test, t(27)=4.80, p<0.001) in morphine-pelleted mice compared to sham-pelleted controls (Fig. 4A) 48 hours after the last pellet, the time-point at which we have previously observed biochemical changes (Mazei-Robison et al. 2011). As with the acute and chronic injection paradigms, we do not observe any changes in total NDRG or SGK1.
To take advantage of the short time course of HSV-mediated gene overexpression, in previous studies we have also utilized a binge cocaine regimen for investigation of the molecular mechanisms underlying cocaine-induced neuroadaptations in the NAc (Russo et al. 2009). Using this binge protocol, in which mice are given 5 – 20 mg/kg injections of cocaine over 3 days and analyzed 4–6 hours following the last injection, we observe significantly higher levels of phospho-NDRG (unpaired Student’s t-test, t(17)=3.61, p<0.01) and phospho-SGK1 (unpaired Student’s t-test, t(18)=2.23, p<0.05) in the cocaine-treated mice compared to saline-injected controls (Fig. 4B). Additionally, we found a significant increase in total NDRG protein (Fig. 4B; unpaired Student’s t-test, t(17)=2.45, p<0.05), that we did not observe under any other drug treatment conditions.
In order to determine whether increased SGK1 activity plays a role in drug responses, we generated HSV constructs to overexpress either wild-type or constitutively-active (CA) SGK1 (S422D) in the brain. We injected these constructs into the VTA of adult male mice and found that SGK1-CA, but not wild-type protein, induced a significant increase in NDRG phosphorylation (Fig. 5; one-way ANOVA, F (2,8)=12.70, p<0.01, Tukey’s multiple comparison test). We next infused either HSV-GFP or HSV-SGK1-CA into the VTA and assessed drug-induced locomotor behavior. As shown in Fig. 6A, we observed locomotor sensitization to cocaine (7.5 mg/kg) in HSV-GFP mice, with increased locomotion observed on days 3 and 4 compared to day 1 (Repeated measures ANOVA, F (4,9)=16.88, p<0.001, post-hoc Tukey’s multiple comparison test), but this sensitization was absent in HSV-SGK1-CA mice (Repeated measures ANOVA, F (4,7)=8.27, p<0.001, post-hoc Tukey’s multiple comparison test, no differences between cocaine treatment days). However, it appears that the HSV-SGK-CA mice had a near maximal locomotor response to cocaine on day 1, which might have limited the ability to observe sensitization. Despite this trend for an increased day 1 response in HSV-SGK-CA mice, there was no statistical difference between HSV-GFP and HSV-SGK1-CA mice with respect to their baseline locomotor activity (HSV-GFP=1513±156, HSV-SGK1-CA=1755±184, unpaired t-test: t(16)=1.0, p=0.33) or initial locomotor response to cocaine. In striking contrast, SGK1-CA exerted the opposite effect on morphine-induced locomotor behavior: repeated morphine (10 mg/kg) did not produce sensitized locomotor responses in control animals (Fig. 6B; Repeated measures ANOVA, F (4,7)=7.70, p<0.001, post-hoc Tukey’s multiple comparison test, no differences between morphine treatment days), but such sensitization became apparent upon SGK1-CA overexpression (HSV-SGK1-CA: Repeated measures ANOVA, F (4,7)=26.41, post-hoc Tukey’s multiple comparison test). Again, there was no significant different in baseline activity (HSV-GFP=1745±195, HSV-SGK1-CA=1367±109, unpaired t-test: t(14)=1.7, p=0.11) or initial locomotor responses to morphine between HSV-GFP and HSV-SGK1-CA mice.
In addition to locomotor sensitization, we also evaluated cocaine and morphine CPP to assess whether increasing SGK1 activity in VTA altered drug reward. We chose lower doses of both cocaine (6 mg/kg) and morphine (10 mg/kg) for pairing, in an attempt to detect either an increase or decrease in CPP with HSV-SGK1-CA overexpression. While we observed a trend for increased preference for both cocaine (Fig. 7A, GFP: 39.2±74.1, SGK1-CA: 114.1±58.2) and morphine (Fig. 7B, GFP: 26.2±83.0, SGK1-CA: 160.0±80.9) we did not observe any significant differences between GFP and SGK1-CA with either drug (cocaine, unpaired Student’s t-test, t(18)=0.80, p=0.42; morphine, t(18)=1.11, p=0.28). Together, these data suggest that increasing NDRG phosphorylation in the VTA through canonical SGK1 activation (S422D) does not strongly influence the rewarding properties of drugs, but may influence sensitized drug responses.
Discussion
The results of the present study demonstrate that SGK1 activity is robustly increased in the VTA by chronic morphine or cocaine exposure. Surprisingly, SGK1 was one of the few genes identified in an unbiased screen that was similarly regulated by both drugs in this brain region (Fig. 1; Supp. Table 1). The upregulation of SGK1 mRNA in the VTA by repeated morphine injections is consistent with microarray results from mice given subcutaneous morphine pellets, where SGK1 was increased in the VTA by both chronic morphine and morphine withdrawal (McClung et al. 2005). A recent study found that the SGK1 transcript is also upregulated in the VTA in response to physical or emotional stress using a similar RNA sequencing approach as that used here (Warren et al. 2013), although this increase was not validated by RT-PCR analysis and contrasts with results from a previous screen using microarray technology that did not identify SGK1 regulation in the VTA of physically stressed or resilient mice (Krishnan et al. 2007). Befort and colleagues (2008) found that SGK1 mRNA was significantly increased in response to a chronic, escalating dose morphine paradigm in the extended amygdala in wild-type, but not mu-opioid receptor knock-out mice, and that this SGK1 upregulation did not occur after a single, acute injection of morphine. These results are consistent with our protein data, where we observed robust increases in NDRG- and SGK1-phosporylation after chronic (Figs. 2 and 4) but not acute drug treatment (Fig. 3).
In addition to SGK1 transcript changes in the VTA, SGK1 regulation has also been noted to occur in additional brain structures not only in response to drugs of abuse, but also in response to antipsychotic drugs, behavioral tasks and training, and neuronal injury (reviewed in (Lang et al. 2010, Lang et al. 2006)). More specifically, SGK1 mRNA expression was increased in whole brain lysates following chronic treatment with oxycodone, a mu opioid receptor agonist with a similar mechanism of action to morphine (Hassan et al. 2010). Acute administration of amphetamine (AMPH) (Gonzalez-Nicolini & McGinty 2002), ethanol, morphine, heroin, or methamphetamine (Piechota et al. 2010) increased SGK1 expression in the striatum. Similarly, acute ethanol (Kerns et al. 2005) or lysergic acid diethylamide injection (Nichols & Sanders-Bush 2002) increased SGK1 expression in the prefrontal cortex. These acute effects on SGK1 are consistent with SGK1’s identification as an immediate early gene (Firestone et al. 2003, Webster et al. 1993b, Waldegger et al. 1997), and in fact its pattern of regulation in the striatum was very similar to that of Fos (Piechota et al. 2010).
Despite SGK1 being identified repeatedly by microarray screens in response to either acute or chronic drug treatment, there is surprisingly little data available on whether SGK1 kinase activity is regulated by drugs of abuse. Here, we show robust increases in SGK1 activity in the VTA in response to chronic, but not acute, morphine or cocaine administration (Figs. 2–4). Additionally, this change is time- or concentration-dependent, as increased activity was observed 1 hour, but not 24 hours, after the last chronic injection. In support of this, a morphine pellet paradigm that does not produce nearly as high peak blood morphine concentration, but rather a lower, sustained increase (Fischer et al. 2008) exhibited extremely high levels of SGK1- and NDRG-phosphorylation (Fig. 4A). A similar increase occurred in response to a “binge” cocaine paradigm, where we observed increased responses 4 hours after the last injection compared to 1 hour following the traditional one daily injection paradigm. The fact that morphine and cocaine induce time-dependent changes in SGK1 phosphorylation and activity are in line with data from another AGC kinase family member, AKT. Acute AMPH administration has been shown to increase striatal nuclear AKT phosphorylation 15 minutes but not 2 hours following drug injection and this effect is greater in rats that have previously received chronic AMPH (Shi & McGinty 2007). Interestingly, SGK phosphorylation was decreased in striatal nuclear extracts 15 minutes after acute AMPH, but this effect was absent at 30 minute and 1 hour time-points (McGinty et al. 2008). Given that AKT and SGK1 can both translocate from the cytoplasm to the nucleus, the localization of the kinases also adds complexity to the level and timing of activity changes.
In McGinty et al. (2008), the authors observed an increase in total striatal SGK protein in the nucleus 30 minutes after acute AMPH, a change that was not significant at the 1-hour time-point (although there appears to be a trend for an increase). This time course differed slightly from that observed for AMPH-induced changes in SGK1 mRNA, which was increased at the 1-hour time-point (McGinty et al. 2008, Gonzalez-Nicolini & McGinty 2002). This difference between SGK1 mRNA versus protein levels at a given time-point was also observed in response to acute morphine, where striatal SGK1 mRNA was increased 4 hours after an acute injection, while total SGK1 protein was decreased (Piechota et al. 2010). Similarly, at the 24-hour time-point where we observe an increase in SGK1 mRNA in response to chronic morphine or cocaine (Fig. 1), we do not observe any significant change in total SGK1 protein (Fig. 2B). Together, these results suggest that transcriptional regulation of the SGK1 gene does not predict changes in SGK1 protein or kinase activity, although all of these functions appear to be influenced by exposure to drugs. It is possible that increased transcription of SGK1 mRNA is accompanied by a feedback mechanism that maintains constant protein levels, such as a downregulation in translation or an upregulation in protein degradation, and future studies will undoubtedly focus on these hypotheses.
To determine whether drug-induced changes in VTA SGK1 activity play a role in drug-elicited behaviors, we generated an HSV construct that allows us to overexpress constitutively-active SGK1 (S422D) locally in the VTA. SGK1-CA expression did not affect the initial locomotor response to either morphine or cocaine, although there was a non-significant trend towards an initial increase in cocaine-induced locomotor activity in SGK-CA mice. While we were able to observe locomotor sensitization to cocaine in GFP mice, the effect was absent in SGK1-CA animals, suggesting that SGK-CA either blocked the locomotor sensitizing effects of cocaine, or increased the initial locomotor response to cocaine sufficiently that observation of sensitization was impossible due to a ceiling effect (Fig. 6A). We did not observe any locomotor differences between GFP and SGK-CA to a lower cocaine dose in our CPP experiment (6 mg/kg vs. 7.5 mg/kg), supporting the former hypothesis, but additional studies will be necessary to address this question. Conversely, we found that morphine sensitization was promoted in SGK-CA mice (Fig. 6B), an unexpected result as we predicted a similar behavioral effect with both drugs given their similar regulation of SGK1 expression and activity. We also examined morphine and cocaine CPP and did not observe a significant difference in SGK1-CA mice compared to GFP controls (Fig. 7). These results suggest that increasing VTA SGK1 activity, at least through S422 regulation, does not have a robust effect on morphine or cocaine reward. While we confirmed that SGK1-CA increased NDRG phosphorylation as expected, the drug-induced differences we observed in SGK1 phosphorylation were actually at the S78 site. Unfortunately, we were unable to obtain a reliable phosphorylation signal at the canonical SGK1 phosphorylation sites (S422, T256) using available antibodies (data not shown), a significant hurdle in the literature (Garcia-Martinez & Alessi 2008). Thus, it is possible that overexpression of SGK1-S78D might influence morphine and cocaine behavior through a mechanism separate from NDRG phosphorylation. While S422 and T256 phosphorylation are mediated by mTORC2 and PDK1, respectively, it has recently been shown that MAPK/ERK can phosphorylate the S78 site, and that disrupting S422 versus S78 phosphorylation produces differential behavioral effects (Lee et al. 2006). Specifically, overexpression of SGK1-S422A in the dorsal hippocampus impaired spatial memory performance in the Morris water maze, whereas overexpression of SGK1-S78A did not (Lee et al. 2006). Thus, it is possible that a similar phenomenon occurs with drug effects in the VTA, where S78 phosphorylation, versus S422 phosphorylation, might differentially influence behavioral responses.
Given that studies of behavioral effects of altered SGK1 activity in the brain are limited, the modest effects in the current study remain impactful. Most results to date are reported in the hippocampus, where SGK1 activity has been implicated in learning and memory (Lee et al. 2006, Lee et al. 2007, Tsai et al. 2002). Differences in SGK1 mRNA levels in the dorsal hippocampus were observed between rats that were slow and fast learners in the Morris water maze (Tsai et al. 2002). Transient overexpression of wild-type SGK1 in the dorsal hippocampus improved water maze performance while expression of a kinase dead SGK1 mutant impaired performance. In addition to SGK1 mRNA changes, phosphorylation of SGK1 at the S78 is regulated in the dorsal hippocampus of rats, where it is increased 30 minutes, 1 hour, and 3 hours after contextual fear training, but not after foot shock alone (Lee et al. 2007). Further, this phosphorylation is behaviorally relevant, as transfection of an S78A mutant construct into the hippocampus impaired contextual fear conditioning, while transfection of a phospho-mimetic S78D construct facilitated fear conditioning. These results suggest that regulation of both SGK1 transcription and kinase activity can play an important role in behavior, and that these effects are not interchangeable. Rather, the specific manner in which SGK1 is regulated appears to be critical for the behavioral output. Thus, it will be important to examine the specific manner in which SGK1 is regulated by drugs of abuse in future studies in order to more fully understand the potential role for this kinase in drug dependence and addiction.
Supplementary Material
Acknowledgements
We would like to thank Dr. Li Shen for help with RNA sequencing analysis and Ezekiel Mouzon for general technical assistance. The authors have no financial interests to disclose. This work was funded by the National Institute on Drug Abuse (R01 DA14133 (EJN) and F32 DA025381 (MSM)) and a Research Starter Grant from the PhRMA foundation (MSM). We would also like to thank the NIDA drug supply program for providing morphine pellets. Author contributions include: EAH, BF, SK, DV, PJK, and MSM designed and completed experiments, RLN generated the viral vectors, EAH and MSM analyzed and interpreted the data, and MSM and EJN wrote the manuscript.
Abbreviations Used
- AMPH
amphetamine
- CA
constitutively-active
- CPP
conditioned place preference
- DA
dopamine
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HSV
herpes simplex virus
- NDRG
N-myc down-regulated gene
- NAc
nucleus accumbens
- SGK
serum- and glucocorticoid-inducible kinase
- VTA
ventral tegmental area
Footnotes
=> if Yes, insert "All experiments were conducted in compliance with the ARRIVE guidelines."
Conflicts of interest: none
References
- Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteaga MF, Canessa CM. Functional specificity of Sgk1 and Akt1 on ENaC activity. Am J Physiol Renal Physiol. 2005;289:F90–F96. doi: 10.1152/ajprenal.00390.2004. [DOI] [PubMed] [Google Scholar]
- Befort K, Filliol D, Ghate A, Darcq E, Matifas A, Muller J, Lardenois A, Thibault C, Dembele D, Le Merrer J, Becker JA, Poch O, Kieffer BL. Mu-opioid receptor activation induces transcriptional plasticity in the central extended amygdala. Eur J Neurosci. 2008;27:2973–2984. doi: 10.1111/j.1460-9568.2008.06273.x. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase), part of the neurotrophin signal transduction cascade, in the rat mesolimbic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pages C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, Hopf FW, Guillory AM, Carelli RM, Chou JK, Bonci A. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone GL, Giampaolo JR, O’Keeffe BA. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- Fischer SJ, Arguello AA, Charlton JJ, Fuller DC, Zachariou V, Eisch AJ. Morphine blood levels, dependence, and regulation of hippocampal subgranular zone proliferation rely on administration paradigm. Neuroscience. 2008;151:1217–1224. doi: 10.1016/j.neuroscience.2007.11.035. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Res Gene Expr Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Hassan HE, Myers AL, Lee IJ, Chen H, Coop A, Eddington ND. Regulation of gene expression in brain tissues of rats repeatedly treated by the highly abused opioid agonist, oxycodone: microarray profiling and gene mapping analysis. Drug Metab Dispos. 2010;38:157–167. doi: 10.1124/dmd.109.029199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Tapping RI, Chao TH, Lo JF, King CC, Yang Y, Lee JD. BMK1 mediates growth factor-induced cell proliferation through direct cellular activation of serum and glucocorticoid-inducible kinase. J Biol Chem. 2001;276:8631–8634. doi: 10.1074/jbc.C000838200. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kerns RT, Ravindranathan A, Hassan S, Cage MP, York T, Sikela JM, Williams RW, Miles MF. Ethanol-responsive brain region expression networks: implications for behavioral responses to acute ethanol in DBA/2J versus C57BL/6J mice. J Neurosci. 2005;25:2255–2266. doi: 10.1523/JNEUROSCI.4372-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Cohen P. Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3-phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem J. 1999;339(Pt 2):319–328. [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010;588:3349–3354. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CT, Ma YL, Lee EH. Serum- and glucocorticoid-inducible kinase1 enhances contextual fear memory formation through down-regulation of the expression of Hes5. J Neurochem. 2007;100:1531–1542. doi: 10.1111/j.1471-4159.2006.04284.x. [DOI] [PubMed] [Google Scholar]
- Lee CT, Tyan SW, Ma YL, Tsai MC, Yang YC, Lee EH. Serum- and glucocorticoid-inducible kinase (SGK) is a target of the MAPK/ERK signaling pathway that mediates memory formation in rats. Eur J Neurosci. 2006;23:1311–1320. doi: 10.1111/j.1460-9568.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- Lee EH, Hsu WL, Ma YL, Lee PJ, Chao CC. Enrichment enhances the expression of sgk, a glucocorticoid-induced gene, and facilitates spatial learning through glutamate AMPA receptor mediation. Eur J Neurosci. 2003;18:2842–2852. doi: 10.1111/j.1460-9568.2003.03032.x. [DOI] [PubMed] [Google Scholar]
- Mazei-Robison MS, Koo JW, Friedman AK, et al. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron. 2011;72:977–990. doi: 10.1016/j.neuron.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ, Zachariou V. Regulation of gene expression by chronic morphine and morphine withdrawal in the locus ceruleus and ventral tegmental area. J Neurosci. 2005;25:6005–6015. doi: 10.1523/JNEUROSCI.0062-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Shi XD, Schwendt M, Saylor A, Toda S. Regulation of psychostimulant-induced signaling and gene expression in the striatum. J Neurochem. 2008;104:1440–1449. doi: 10.1111/j.1471-4159.2008.05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JT, Campbell DG, Morrice N, et al. Exploitation of KESTREL to identify NDRG family members as physiological substrates for SGK1 and GSK3. Biochem J. 2004;384:477–488. doi: 10.1042/BJ20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols CD, Sanders-Bush E. A single dose of lysergic acid diethylamide influences gene expression patterns within the mammalian brain. Neuropsychopharmacology. 2002;26:634–642. doi: 10.1016/S0893-133X(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Niehaus JL, Murali M, Kauer JA. Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur J Neurosci. 2010;32:108–117. doi: 10.1111/j.1460-9568.2010.07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Miller JS, Unterwald EM. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem. 2008;107:570–577. doi: 10.1111/j.1471-4159.2008.05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechota M, Korostynski M, Solecki W, et al. The dissection of transcriptional modules regulated by various drugs of abuse in the mouse striatum. Genome Biol. 2010;11:R48. doi: 10.1186/gb-2010-11-5-r48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, et al. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Shi X, McGinty JF. Repeated amphetamine treatment increases phosphorylation of extracellular signal-regulated kinase, protein kinase B, and cyclase response element-binding protein in the rat striatum. J Neurochem. 2007;103:706–713. doi: 10.1111/j.1471-4159.2007.04760.x. [DOI] [PubMed] [Google Scholar]
- Sklair-Tavron L, Shi WX, Lane SB, Harris HW, Bunney BS, Nestler EJ. Chronic morphine induces visible changes in the morphology of mesolimbic dopamine neurons. Proc Natl Acad Sci U S A. 1996;93:11202–11207. doi: 10.1073/pnas.93.20.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai KJ, Chen SK, Ma YL, Hsu WL, Lee EH. sgk, a primary glucocorticoid-induced gene, facilitates memory consolidation of spatial learning in rats. Proc Natl Acad Sci U S A. 2002;99:3990–3995. doi: 10.1073/pnas.062405399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, et al. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, Goya L, Firestone GL. Immediate-early transcriptional regulation and rapid mRNA turnover of a putative serine/threonine protein kinase. J Biol Chem. 1993a;268:11482–11485. [PubMed] [Google Scholar]
- Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993b;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Nestler EJ, Russell DS. Regulation of neuronal PLCgamma by chronic morphine. Brain Res. 2007;1156:9–20. doi: 10.1016/j.brainres.2007.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf DH, Numan S, Nestler EJ, Russell DS. Regulation of phospholipase Cgamma in the mesolimbic dopamine system by chronic morphine administration. J Neurochem. 1999;73:1520–1528. doi: 10.1046/j.1471-4159.1999.0731520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.