Abstract
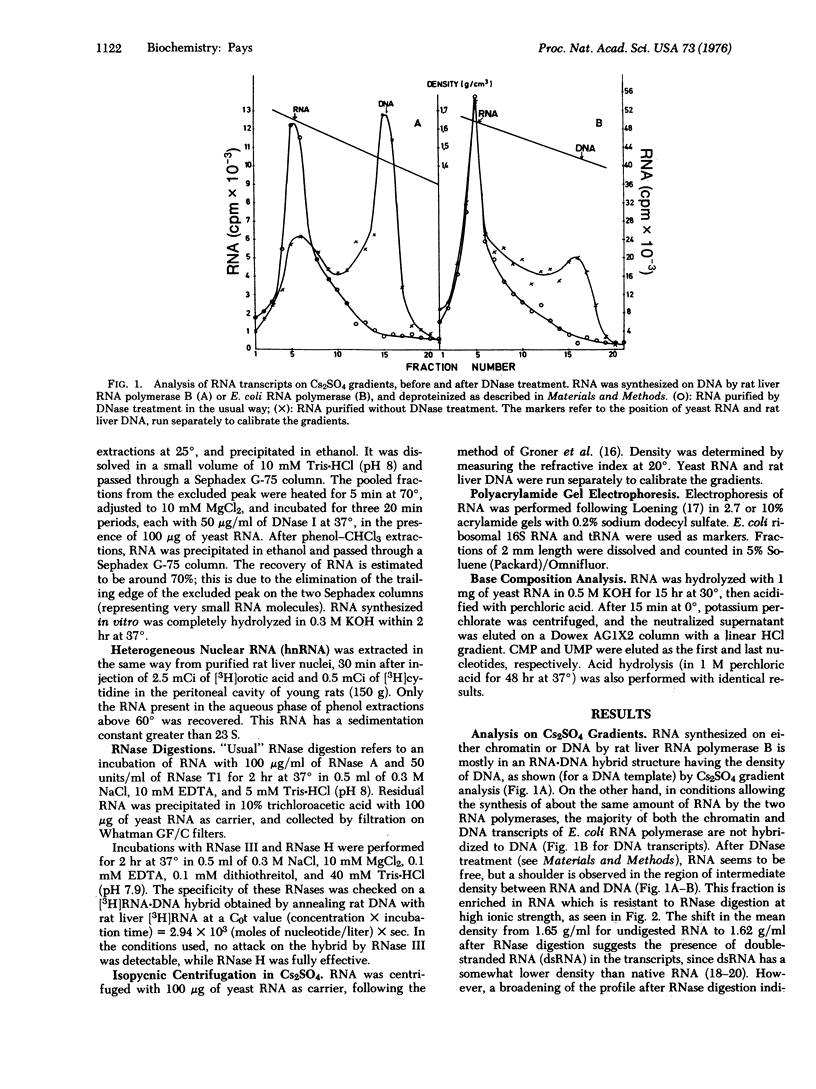
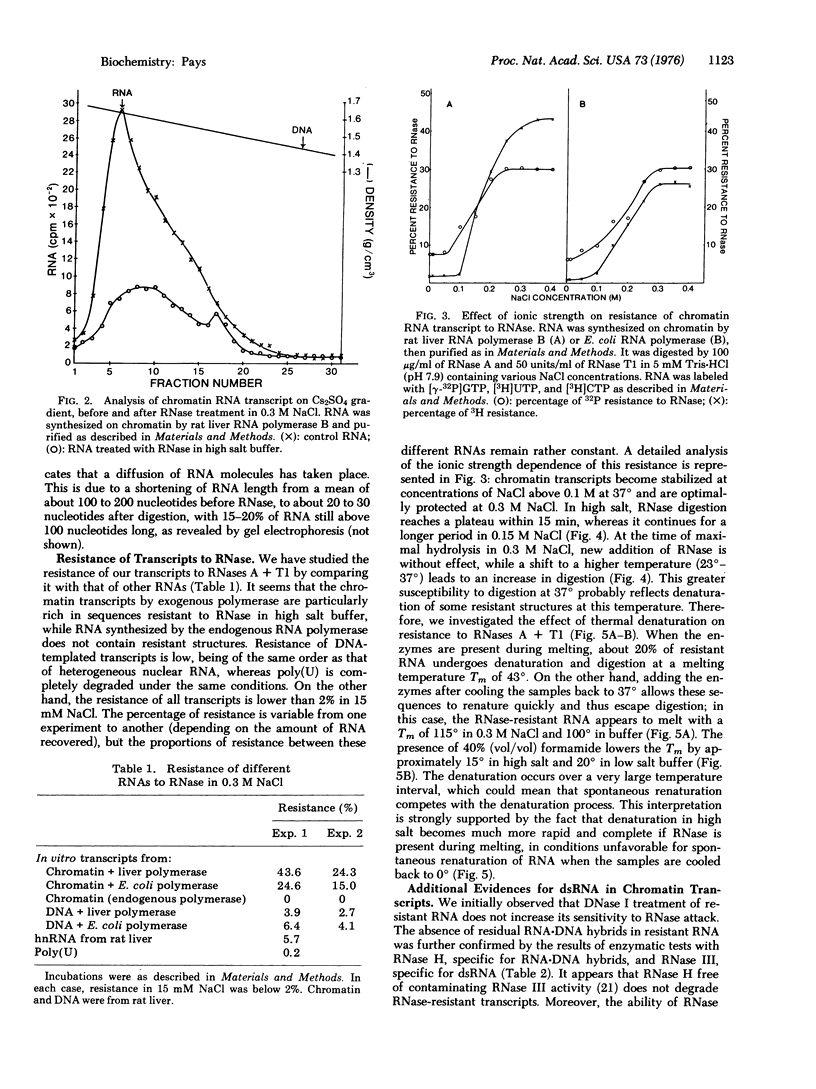
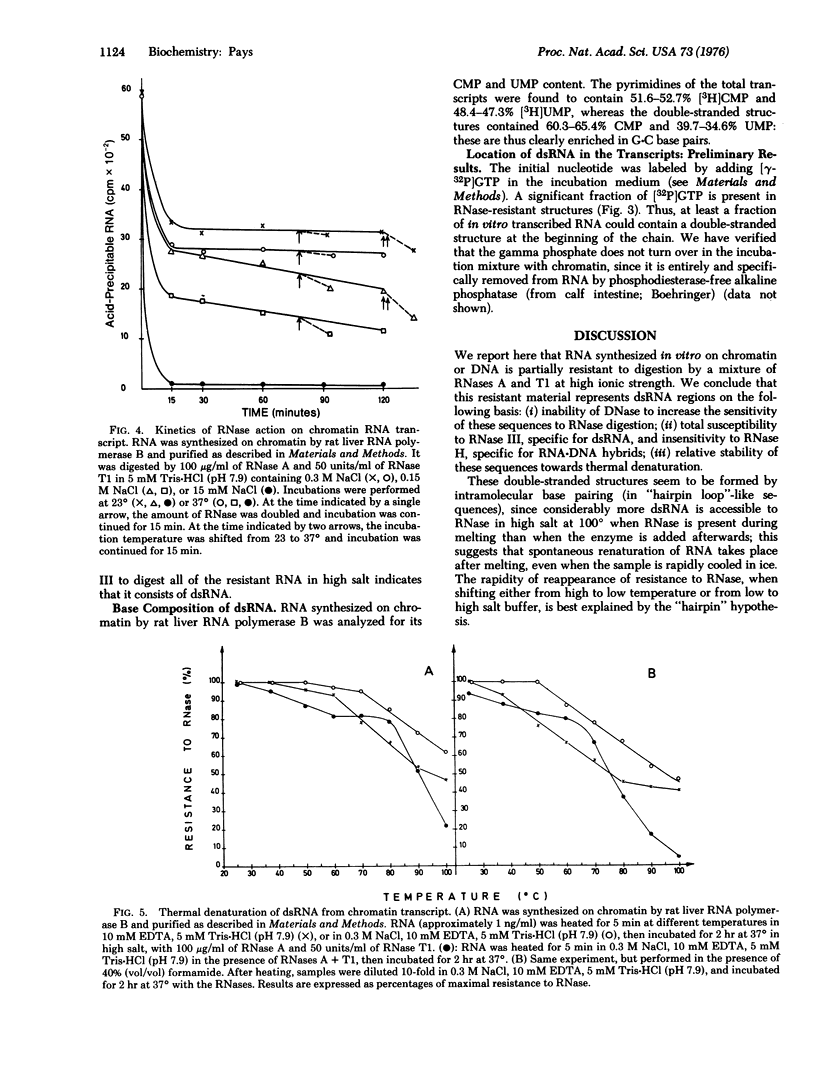
RNA transcribed in vitro at low ionic strength, from either rat liver chromatin or DNA, contains a significant amount of structure resistant to RNase in high salt buffer. This is observed with rat liver (form B polymerase) as well as with Escherichia coli RNA polymerase (RNA nucleotidyltransferase; nucleoside triphosphate: RNA nucleotidyltransferase; EC 2.7.7.6). Treatment with RNases specific for either double-stranded or hybrid RNA indicates that resistance to RNase is due to the presence of double-stranded RNA sequences. Denaturation kinetics in the presence or absence of RNase suggest that these sequences are formed by intramolecular base pairing. Their mean length is about 20 to 30 nucleotides, but 15-20% are more than 100 nucleotides long. They contain 60-65% G-C base pairs. The proportion of double-stranded segments is higher in chromatin transcripts than in DNA-templated RNA, and is higher with homologous RNA polymerase than with the bacterial enzyme. On the other hand, chromatin endogenous RNA polymerase, which is unable to initiate transcription, does not synthesize double-stranded RNA. The problem of the location of these sequences is discussed; preliminary results suggest that the 5' end of the RNA transcripts could be enriched in complementary sequences.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M. In vitro transcription of simian virus 40 sequences in SV3T3 chromatin. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2304–2308. doi: 10.1073/pnas.70.8.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth P. H., Cox R. F., Chesterton C. J. Transcription of mammalian chromatin by mammalian DNA-dependent RNA polymerases. Eur J Biochem. 1971 Nov 11;23(2):229–241. doi: 10.1111/j.1432-1033.1971.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Transcription of high-molecular-weight RNA from hen-oviduct chromatin by bacterial and endogenous form-B RNA polymerases. Eur J Biochem. 1973 Nov 1;39(1):49–61. doi: 10.1111/j.1432-1033.1973.tb03102.x. [DOI] [PubMed] [Google Scholar]
- Darlix J. L. Stimultaneous purification of Escherichia coli termination factor rho, RNAase III and RNAase H. Eur J Biochem. 1975 Feb 21;51(2):369–376. doi: 10.1111/j.1432-1033.1975.tb03937.x. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Britten R. J. Organization, transcription, and regulation in the animal genome. Q Rev Biol. 1973 Dec;48(4):565–613. doi: 10.1086/407817. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner Y., Monroy G., Jacquet M., Hurwitz J. Chromatin as a template for RNA synthesis in vitro. Proc Natl Acad Sci U S A. 1975 Jan;72(1):194–199. doi: 10.1073/pnas.72.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquet M., Groner Y., Monroy G., Hurwitz J. The in vitro synthesis of avian myeloblastosis viral RNA sequences. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3045–3049. doi: 10.1073/pnas.71.8.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Molloy G., Fernandez-Munoz R., Salditt M., Darnell J. E. Secondary structure in heterogeneous nuclear RNA: involvement of regions from repeated DNA sites. J Mol Biol. 1974 Jan 25;82(3):361–370. doi: 10.1016/0022-2836(74)90597-x. [DOI] [PubMed] [Google Scholar]
- Keshgegian A. A., Garibian G. S., Furth J. J. Transcription of chromatin. Initial and terminal nucleotides of ribonucleic acid synthesized by calf thymus and Escherichia coli ribonucleic acid polymerases. Biochemistry. 1973 Oct 23;12(22):4337–4342. doi: 10.1021/bi00746a006. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac M., Chambon P. Animal DNA-dependent RNA polymerases. Initiation sites on calf-thymus DNA. Eur J Biochem. 1973 Jun 15;35(3):454–463. doi: 10.1111/j.1432-1033.1973.tb02859.x. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S. Organ-specific restriction of transcription in mammalian chromatin. J Mol Biol. 1968 Jul 14;34(2):305–316. doi: 10.1016/0022-2836(68)90255-6. [DOI] [PubMed] [Google Scholar]
- Pays E., Ronsse A. Effects of deoxyribonucleases I and II on rat-liver-chromatin transcription in vitro. Eur J Biochem. 1973 Dec 3;40(1):119–131. doi: 10.1111/j.1432-1033.1973.tb03175.x. [DOI] [PubMed] [Google Scholar]
- Pays E., Ronsse A. Interspersion of repetitive sequences in rat liver DNA. Biochem Biophys Res Commun. 1975 Feb 17;62(4):862–867. doi: 10.1016/0006-291x(75)90402-7. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Yenikolopov G. N., Limborska S. A. Complementary regions of the nuclear precursor of messenger RNA. FEBS Lett. 1974 Oct 1;47(1):98–102. doi: 10.1016/0014-5793(74)80434-5. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Khoury G., Martin M. A. In vitro transcription of the viral-specific sequences present in the chromatin of cells transformed by simian virus 40. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3506–3510. doi: 10.1073/pnas.70.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. D., Church R. B., McCarthy B. J. Template specificity of isolated chromatin. Biochemistry. 1969 Nov;8(11):4271–4277. doi: 10.1021/bi00839a007. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Steggles A. W., Wilson G. N., Kantor J. A., Picciano D. J., Falvey A. K., Anderson W. F. Cell-free transcription of mammalian chromatin: transcription of globin messenger RNA sequences from bone-marrow chromatin with mammalian RNA polymerase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1219–1223. doi: 10.1073/pnas.71.4.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Griensven L. J., van Kammen A., Rezelman G. Characterization of the double-stranded RNA isolated from cowpea mosaic virus-infected Vigna leaves. J Gen Virol. 1973 Mar;18(3):359–367. doi: 10.1099/0022-1317-18-3-359. [DOI] [PubMed] [Google Scholar]


